Abstract
B virus infection of humans results in high morbidity and mortality in as many as 80% of identified cases. The main objective of this study was to conduct a comparative analysis of conventional and experimental antiviral drug susceptibilities of B virus isolates from multiple macaque species and zoonotically infected humans. We used a plaque reduction assay to establish the effective inhibitory doses of acyclovir, ganciclovir, and vidarabine, as well as those of a group of experimental nucleoside analogs with known anti-herpes simplex virus activity. Four of the experimental drugs tested were 10- to 100-fold more potent inhibitors of B virus replication than conventional antiviral agents. Drug efficacies were similar for multiple B virus isolates tested, with variations within 2-fold of the median effective concentration (EC50) for each drug, and each EC50 was considerably lower than those for B virus thymidine kinase (TK) mutants. We observed no differences in the viral TK amino acid sequence between B virus isolates from rhesus monkeys and those from human zoonoses. Differences in the TK protein sequence between cynomolgus and pigtail macaque B virus isolates did not affect drug sensitivity except in the case of one compound. Taken together, these data suggest that future B virus zoonoses will respond consistently to conventional antiviral treatment. Further, the considerably higher potency of FEAU (2′-fluoro-5-ethyl-Ara-U) than of conventional antiviral drugs argues for its compassionate use in advanced human B virus infections.
In its natural host, macaque monkeys, B virus (Macacine herpesvirus 1; Simplexvirus, Herpesviridae) causes lesions on epithelial surfaces (2, 33) and establishes reactivatable latent infection in sensory neurons (30, 37), like herpes simplex virus (HSV) in humans. B virus often results in severe pathogenesis, including paralysis, encephalitis, and in many cases, rapid death, following infection of humans (reviewed in reference 22). Nearly all reported cases of B virus zoonosis have been associated with individuals handling macaques during the course of research or technology development (33). Five fatalities, along with at least 23 cases in which the patient survived, have occurred in the past 20 years, underscoring that zoonotic infections remain a problem in the laboratory animal environment (7-9).
The CDC's B Virus Working Group currently recommends treatment of confirmed zoonotic infections with herpesvirus-specific antiviral drugs, including acyclovir (ACV) and ganciclovir (GCV) (10). Both agents in this class of compounds are phosphorylated to active form by virus-encoded thymidine kinase (TK). The resulting nucleoside triphosphate analog inhibits viral DNA replication by termination of chain elongation and by direct inhibition of herpesvirus DNA polymerase (24). While ACV is effective against B virus both in cell culture and in animal models (6), the dose required for 50% plaque reduction is more than 10-fold higher for B virus than for HSV type 1 (HSV-1) (38). GCV is twice as potent as ACV in B virus plaque reduction, yet the median effective concentration (EC50) of GCV for B virus is almost 10 times higher than that for HSV-1 (38). In some, but not all, cases of zoonotic B virus infection, acyclovir and ganciclovir have proven to be effective at curtailing disease progression (7, 8). Zoonotic infections that have progressed to extensive central nervous system (CNS) involvement, including respiratory arrest, appear to be refractory to conventional antiviral intervention (12, 15; J. Hilliard, unpublished data).
Vidarabine (9-β-d-arabinofuranosyladenine [ara-A, or VDB]), an antiviral agent effective against HSV, may be useful for the treatment of early stages of zoonotic B virus infections, but it has not been used alone in previous cases. Prior to the use of ACV for treatment of HSV, intravenous VDB was used for treatment of encephalitic infections (34). In cell culture, it has been shown to be equally potent against B virus and HSV-1 (5). VDB does not require selective phosphorylation by the viral TK for activity (4); however, since VDB can be converted to its active form by host enzymes, it has potential toxicity in humans (29).
Experimental drugs effective in cell culture against HSV include the β-d-2′-fluoro-5-substituted arabinosyl pyrimidines FMAU (2′-fluoro-5-methyl-Ara-U) and FEAU (2′-fluoro-5-ethyl-Ara-U), both of which require phosphorylation by viral thymidine kinase for activation (19). FMAU and FEAU have potencies similar to that of ACV for inhibition of HSV-1 in cell culture, but FMAU has 10-fold greater potency than ACV against HSV-2 (19). Unfortunately, FMAU has been reported to be toxic to humans at elevated doses used for cancer chemotherapy (1, 13); FMAU can be incorporated into uninfected cell DNA by host DNA polymerase, suggesting the basis of its toxicity in vivo (11). FEAU has been shown to be a selective inhibitor of HSV DNA synthesis in cell culture (7), but its toxicity in humans has not been investigated. Other β-d-2′-fluoro-5-substituted arabinosyl pyrimidines, such as FMAC (2′-fluoro-5-methyl-Ara-C) and FBrAC (2′-fluoro-5-bromyl-Ara-C), have been synthesized and tested against HSV (17, 32; R. F. Schinazi, unpublished results). No information on FBrAC toxicity is known.
The goal of this study was to determine the general variability of drug susceptibility and the efficacy of a class of experimental antiviral agents by using a panel of B virus isolates from multiple macaque species and humans. The results presented here suggest that B virus isolates in the wild are susceptible to antiviral agents that require the viral TK for activity. Further, our findings that specific experimental nucleoside analogs are appreciably more effective than conventionally used antiviral agents at blocking B virus replication suggest that these drugs may benefit the treatment of high-morbidity human cases.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (Vero) cells (ATCC CCL-81) were obtained from the American Type Culture Collection (Manassas, VA) and propagated in Dulbecco's modified Eagle's medium (DMEM; Mediatech) supplemented with 7% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Infections were done using DMEM supplemented with 1% FBS. All B virus plaque reduction assays were performed under biosafety level 3 conditions acceptable at the time of these experiments performed in compliance with the 4th edition of the Biosafety in Microbiological and Biomedical Laboratories. B virus infections for viral DNA purification and virus stock production were done under biosafety level 4 conditions, as mandated by the 5th edition of Biosafety in Microbiological and Biomedical Laboratories (28a) for propagating and handling B virus.
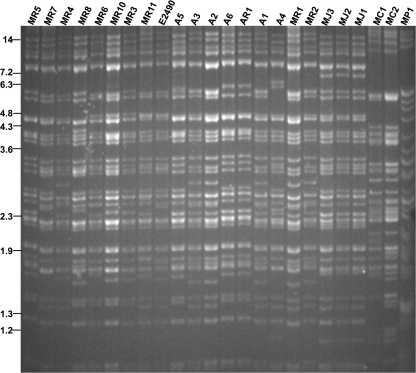
The E2490 reference strain of rhesus macaque B virus was originally obtained from R. N. Hull, Eli Lilly Research Laboratories (Indianapolis, IN), and passage 72 was used. All other wild-type viruses used in this study were diagnostic isolates obtained from the NIH's NCRR-supported National B Virus Resource Center, Georgia State University, Atlanta, GA. Restriction endonuclease digestions of these isolates' viral genomes are shown in Fig. 1.
FIG. 1.
Confirmation of B virus isolate genotype by RFLP. Viral DNA was digested with the SalI restriction enzyme and was separated on a 0.8% agarose gel. MR, rhesus macaque isolate; E2490, prototype laboratory strain; A, human isolate; MJ, Japanese macaque isolate; MC, cynomolgous macaque isolate; MP, pigtail macaque isolate. Size markers are indicated in kilobase pairs and reflect the positions of BstEII-digested lambda DNA fragments.
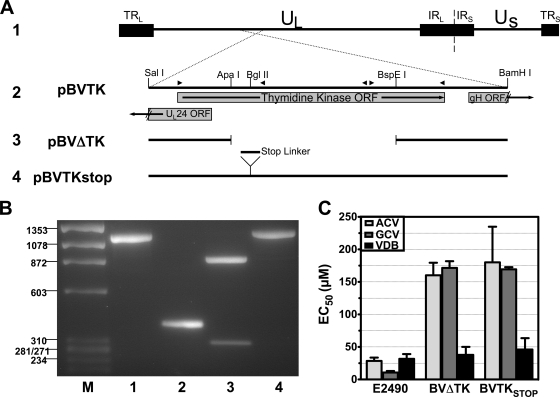
The TK mutants were generated by cotransfection of NaI gradient-purified E2490 viral DNA with plasmids containing the TK gene with either a deletion (pBVΔTK) or a premature stop codon (pBVTKstop) into rabbit skin cells (ATCC CCL-68) (Fig. 2A) (technique described in reference 27). For pBVTKstop, a SpeI linker containing stop codons in all frames (catalog no. 1061; New England Biolabs) was inserted into the BglII site of pBVTK. Dilutions of the subsequent transfection stocks were plated on Vero cells, and individual plaques were screened by PCR and Southern hybridization. After three (BVΔTK) or four (BVTKstop) rounds of plaque purification, one plaque with no evidence of wild-type virus was chosen as the representative mutant of each virus for preparation of stocks.
FIG. 2.
Design and confirmation of recombinant B virus harboring mutations in thymidine kinase. (A) Overview of TK mutant construction: 1, orientation of the B virus genome; 2, BamHI-SalI fragment containing the complete TK open reading frame; 3, ApaI-BspEI collapse of pBVTK, deleting 754 amino acids from the center of TK; 4, stop codon linker insertion into the BglII site to prematurely stop TK translation. (B) Confirmation of mutants by PCR. Lanes: M, φX174/HaeIII marker; 1, E2490; 2, BVΔTK; 3, BVTKstop PCR product cut with SpeI; 4, BVTKstop (uncut). (C) Antiviral sensitivities of E2490, BVΔTK, and BVTKstop versus acyclovir, ganciclovir, and vidarabine. Error bars indicate standard deviations of three replicates.
The DNA sequence of both viral mutants was confirmed by sequencing. Confirmation of the deletion and stop codon insertion using PCR is shown in Fig. 2B. The parent virus PCR amplimer is 1,143 bp (Fig. 2B, lane 1). The deletion from ApaI to BspEI removes a 759-bp fragment, resulting in a 384-bp amplimer (lane 2). The insertion of the stop codon linker into the BglII site results in a 1,157-bp amplimer (lane 4) that, when cut with SpeI, yields 856-bp and 301-bp fragments (lane 3). Manipulation of these drug-resistant TKnull viruses is restricted to a biosafety level 4 laboratory in accordance with the Georgia State University Institutional Biosafety Committee and the Office of Biotechnology Activities at the National Institutes of Health.
Antiviral compounds.
Acyclovir (ACV), ganciclovir (GCV), and vidarabine (VDB) were purchased from Sigma (St. Louis, MO). The compounds 1-(2-fluoro-5-methyl-beta-d-arabinofuranosyl)uracil (FMAU), 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-ethyluracil (FEAU), 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-bromylcytosine (FBrAC), and 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-methylcytosine (FMAC) were synthesized in one of our laboratories (R.F.S.).
Plaque reduction assay.
Confluent monolayers of Vero cells in 12-well microplates were infected with approximately 200 PFU of virus and incubated at 37°C with twofold serial dilutions of antiviral drug. After 1 h, the inoculum was removed and replaced with medium containing 1% methylcellulose and antiviral drug. Control wells did not contain antiviral drugs. The cells were fixed in 100% methanol 40 h after infection and were stained with Giemsa stain. Plaques were visually inspected and counted using a dissecting microscope. The number of plaques at each drug concentration was plotted versus the log of the drug concentration, and the slope of the regression line in the linear range was determined. The amount of drug required to reduce the number of plaques by 50% from those in the control wells (EC50) was calculated from the equation of the regression line. Each drug was tested against each isolate at least twice in replicate wells, and the EC50 was calculated as an average value. Cytotoxicity in Vero cells for all the compounds was determined previously using a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. None of the compounds were cytotoxic when evaluated up to 10 μM (data not shown).
Viral DNA analysis.
Viral DNA was isolated from infected cells by the method of Poffenberger and Roizman (23). Restriction digests were separated on 0.8% agarose gels. Each PCR amplification of the TK gene from rhesus, Japanese macaque, cynomolgus, and human B virus isolates was performed with primers 5′-CCAACGCTCCGTAAAACCG-3′ and 5′-ACCATCTTTATTGCGCCAGG-3′. Amplimers were purified after extraction from 1% agarose gels, and both strands were sequenced using an automated sequencer, model 377 (Applied Biosystems), with the primers given above and three internal primers, 5′-AAGATCGTGTCCTCGATCTC-3′, 5′-GAGATCGAGGACACGATCTT-3′, and 5′-CACCTGAGCGCTGGCCATTG-3′. Because PCR amplification of the pigtail macaque B virus TK gene was not possible with the rhesus macaque-based PCR primers, restriction fragments containing the pigtail macaque B virus TK gene were cloned. These plasmids were subcloned, and the pigtail B virus TK gene was then sequenced using vector-specific primers and two internal gene-specific primers, 5′-AAGATCGTGTCCTCGATCTC-3′ and 5′-GAGATCGAGGACACGATCTT-3′. Sequence alignment was performed using MegAlign, version 4.03 (DNAStar, Madison, WI), with the Jotun Hein algorithm.
RESULTS
Characterization of the virus panel.
One of the goals of this work was to examine the variability of drug potency and susceptibility for multiple B virus isolates from macaques and humans. We chose a large panel of B virus isolates from various species of macaques, as well as human isolates; many of the viruses were isolated recently and have been maintained at 3 or fewer passages. To verify the genotype and observe the genetic variability of the B virus isolates, we performed restriction fragment length polymorphism (RFLP) analysis with SalI restriction endonuclease (Fig. 1). Numerous polymorphisms were observed, similar to those noted in a previous study of multiple isolates from cynomolgus macaques (31). It is also noted that the seven B virus isolates from human zoonoses were genotyped as rhesus B virus. B virus isolates from cynomolgus monkeys and pigtail macaques had restriction profiles distinct from those of isolates from rhesus macaques, as previously reported by others (25). Recently, sequencing and RFLP analysis of PCR amplimers demonstrated specific characteristics that distinguish B virus isolates from Japanese and rhesus macaques (21). Our results show that Japanese macaque B virus RFLP patterns were very similar to those from rhesus macaque virus isolates, yet these isolates had distinct RFLPs generated by digestion with SalI (Fig. 1) and other restriction endonucleases (data not shown).
In order to determine if a B virus isolate was resistant to ACV or GCV, we created two thymidine kinase-defective B viruses as controls (Fig. 2). These viruses were 6-fold more resistant to ACV and 16-fold more resistant to GCV than the wild-type parental strain.
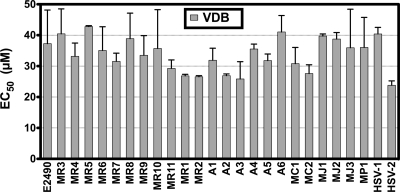
Sensitivity of B virus isolates to vidarabine.
As previously reported (5), B virus and HSV-1 have similar sensitivities to VDB in cell culture, suggesting the hypothesis that the reason B virus is less sensitive to ACV and GCV than HSV is due to variation in the viral TKs, not the viral DNA polymerases. To detect differences in the B virus DNA polymerases, the sensitivities of the B virus isolates to VDB were determined by a plaque reduction assay. The rationale for the use of VDB was to determine whether mutations affecting the viral DNA polymerase might be present, since these mutations could potentially result in an observed resistance to drugs that require phosphorylation by the viral TK and may be mistakenly attributed to a partial or total loss of TK function.
We found that all rhesus isolates had very similar sensitivities to VDB (mean EC50, 34.3 μM; range, 26.5 to 40.4 μM) (Fig. 3). As expected, the mean VDB sensitivities of the BVΔTK and the BVTKstop mutants were similar to those of the rhesus isolates (37.8 μM and 46.1 μM, respectively) (Fig. 2C). While the possibility of mutations in the viral DNA polymerase that do not affect the sensitivity of VDB cannot be completely ruled out, these results indicate that the DNA polymerase genes in these isolates are similar, suggesting that the large differences observed with TK-activated antiviral agents would likely be due to variations in the viral TK.
FIG. 3.
Vidarabine sensitivities of B virus isolates. Plaque reduction assays were performed, and the 50% effective concentration (EC50) of the drug against each isolate was determined. Each bar corresponds to the mean EC50; error bars, standard deviations. Each isolate was tested against the drug in at least two independent experiments.
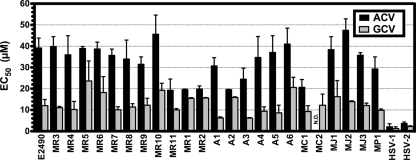
Sensitivities of B virus isolates to acyclovir and ganciclovir.
Fatalities following zoonotic infections from 1987 to 1997 occurred despite pharmacologic intervention with ACV and/or GCV. To rule out the possibility that the fatality-associated viruses were strain variants that were “naturally” resistant to these antiviral agents, we determined the sensitivities of our panel of B virus isolates to these antivirals using plaque reduction assays. As controls, the TKnull mutants were used to determine a standard for completely resistant virus.
Our results for ACV and GCV (Fig. 4) were similar to those of previously published studies for rhesus B virus (38). The EC50 for each drug against each isolate was within a 2-fold difference of the mean sensitivity of all isolates from rhesus monkeys and humans (33.2 μM; range, 19.3 to 45.7 μM). The sensitivities of Japanese macaque, cynomolgus monkey, and pigtail macaque B virus isolates to ACV and GCV were very similar to those of the human and rhesus B virus isolates, suggesting that future human infections with these B virus genotypes could be treated with these antivirals. Because the drug sensitivities of the isolates did not approach the levels of the TKnull mutants (Fig. 2C), our results indicate that the isolates in this panel have wild-type antiviral drug sensitivity; however, it is possible that the variability observed in this assay could be due to minor differences in the TK genes.
FIG. 4.
Sensitivities of B virus isolates to conventional antivirals. Plaque reduction assays were performed, and the 50% effective concentration (EC50) of each drug against each isolate was determined. Each bar corresponds to the mean EC50; error bars, standard deviations. Each isolate was tested against each drug in at least two independent experiments. N.D., not done.
Experimental drugs.
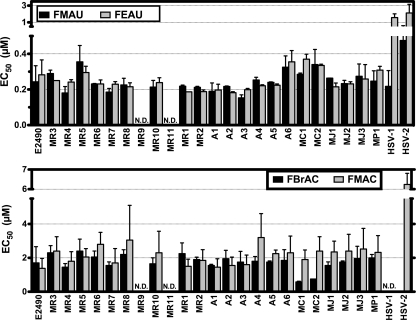
While ACV and GCV have been shown to be effective in some cases of B virus zoonosis, the prognosis for patients is bleak once clinical signs indicate entry of B virus into the CNS. In order to discover more-efficacious B virus antiviral agents, we tested a panel of experimental compounds with known activity against HSV. FMAU and FEAU were found to be approximately 100 times more potent than conventional drugs (Fig. 5, upper panel). FMAC and FBrAC had approximately 10 times greater potency than ACV (Fig. 5, lower panel). Each of these agents requires the viral TK for activation, indicating that either they are activated with higher efficiency by TK or their phosphorylated forms are recognized by the viral DNA polymerase with higher affinity than the triphosphate forms of ACV or GCV. These data demonstrate that a more-efficacious panel of B virus antiviral agents exists within the class of 2′-fluoro-5-substituted arabinosyl pyrimidines.
FIG. 5.
Sensitivities of B virus isolates to experimental antivirals. Plaque reduction assays were performed, and the 50% effective concentration (EC50) of each drug against each isolate was determined. Each bar corresponds to the mean EC50; error bars, standard deviations. Each isolate was tested against each drug in at least two independent experiments. N.D., not done.
Thymidine kinase heterogeneity.
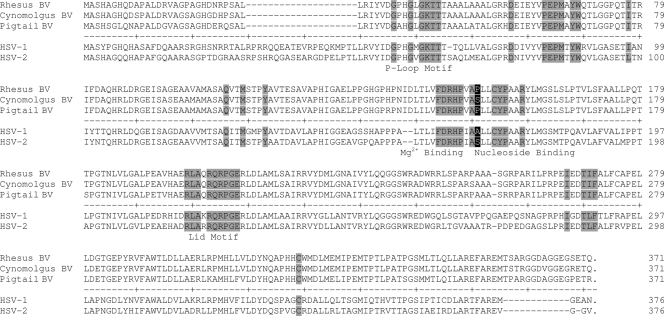
Most of the B virus isolates had similar sensitivities to each drug, with a range less than 2-fold from the mean. It is possible that the observed variation between these isolates is due to minor differences at the amino acid level in the viral proteins that can confer resistance to nucleoside analogs (TK or DNA polymerase). To determine whether apparent functional mutations existed in TK, the TK open reading frame (ORF) was sequenced from rhesus, Japanese macaque, cynomolgus monkey, pigtail macaque, and human B virus isolates.
Striking conservation between B virus TK ORFs from rhesus macaque, Japanese macaque, and human isolates was observed; one nucleic acid substitution was present in 8 of 22 of these isolates, but the difference did not affect the amino acid sequence. Cynomolgus monkey-derived B virus isolates have seven unique TK amino acid differences from rhesus and pigtail macaque B virus isolates (Fig. 6), and these differences may underlie the increased sensitivity of the cynomolgus isolates to FBrAC (Fig. 5, lower panel). While 27 unique amino acid differences were present in the pigtail macaque B virus isolate relative to rhesus and cynomolgus monkey B virus TK (Fig. 6), the drug sensitivity of the pigtail macaque B virus fell into the range of the rhesus B virus isolates with all tested antiviral agents. We hypothesize that the observed amino acid differences are likely in domains that are not functional with respect to drug interaction, or alternatively, they are in functional domains that are sufficiently conserved to retain function.
FIG. 6.
Amino acid alignment of thymidine kinase sequences from three B virus genotypes, HSV-1, and HSV-2. Thymidine kinase open reading frames from the B virus strains E2490 (Rhesus BV), MC1 (Cynomolgus BV), and MP1 (Pigtail BV) were aligned using the Jotun Hein method. The thymidine kinase open reading frame from HSV-1 strain F and HSV-2 strain 333 were included for comparison. Shaded amino acids indicate identity with known functional domains.
Included in these studies were isolates from three humans and two rhesus macaques sampled during a cluster of zoonotic infections in Pensacola, FL (8). The three human isolates and two monkey isolates are designated A1, A2, A3, MR1, and MR2, respectively. While there were minor differences in drug sensitivity among these isolates, the DNA sequences of the TK ORF were identical (data not shown). Taken together, the similar drug sensitivity and identical TK sequence suggest that replication in the human host did not result in antiviral resistance in these B virus isolates.
DISCUSSION
These data provide support for the value of and choices for antiviral intervention against zoonotic B virus infections, extending data originally demonstrating the efficacy of conventional drugs that target viral DNA replication. The potential of 2′-fluoroarabinofuranosyl 5-substituted pyrimidine nucleosides is demonstrated for the first time; these data may serve as the basis of compassionate use when the onset of severe morbidity signals unlikely success with conventional therapeutic approaches, as learned from the five fatal cases in recent years. Our data further suggest that there is no immediate host pressure that induces drug susceptibility-related changes in B virus during human infection. Each isolate, whether isolated from a macaque or from a human, was similarly sensitive to the antiviral agents used in these studies, suggesting that time to treatment is a major factor in human survival.
The TKnull mutants had VDB sensitivities similar to those of the wild-type isolate panel, supporting the hypothesis that the increased requirements of ACV and GCV for B virus plaque reduction are due to the differences between B virus and HSV TKs. While most of the known functional domains of TK are identical between the rhesus B virus and HSV, it is tempting to speculate that the substitution of Pro150 in the B virus TK would place a kink in the nucleoside-binding domain that is not present in the same position in HSV TK (Ala168). Indeed, a change in the homologous amino acid in HSV-2 (Ser169) is responsible for the resistance of that virus to brivudin (BVdU) (35), and the same amino acid variation in the binding pocket is present in cynomolgus monkey B virus TK (Ser150). Zwartouw and colleagues (38) demonstrated the increased resistance of cynomolgus monkey B virus to BVdU almost 20 years ago. Site-directed mutagenesis and a structure-activity relationship analysis of B virus TK would be required to determine the roles of individual amino acids in the relative resistance of B virus to ACV and GCV.
B virus isolates from recent zoonotic infections demonstrated no variation in TK genes relative to isolates from rhesus macaques, and all appear similar to those from rhesus monkeys by restriction endonuclease profiles. These data validate a rhesus origin of B virus associated with these specific zoonoses. However, this is a small sample set, and it was noted that affected individuals had been working specifically with rhesus macaques. Thus, our data do not rule out the possibility that B virus from macaque species other than Macaca mulatta may share its pathogenic potential (25). Even so, B virus can be transmitted to nonmacaque monkeys (18, 36), and other macaques can harbor B virus acquired from rhesus monkeys (21, 25). Thus, regardless of the monkey species, if a monkey has been in contact with macaques, B virus should be presumed to be a hazard. Few cases previously reported in the literature have been associated with specific macaques (22); in fact, many affected individuals had histories of contact with multiple macaque species.
In each of the recent symptomatic cases of B virus zoonosis, treatment has consisted of acyclovir and/or ganciclovir (3, 9, 12, 16, 20). Of 11 infected individuals, 6 survived. The data presented here suggest that variable infection outcomes of antiviral therapy were not associated with virus mutations. It is possible that each individual had different host-dependent susceptibilities to B virus infection. Alternatively, since four of the five fatally infected individuals were not treated until severe neurological symptoms were detected, whereas three of the six survivors were hospitalized prior to the onset of acute neurological symptoms, the time for effective intervention may have been limited. This would underscore the importance of early identification of zoonotic disease, which can be generally accomplished early after infection for less than $300 worth of laboratory evaluations.
Zwartouw and colleagues (38) compared ACV and GCV against B virus in experimentally infected rabbits, observing 100% survival for the GCV-treated group for 5 months, compared to 33% survival for those treated with ACV. Interestingly, one of the patients from the Michigan cluster of human infections with B virus was given ganciclovir (5 mg/kg of body weight intravenously) twice daily, following a lack of success with ACV used at 15 mg/kg for 3 days, resulting in abatement of neurological symptoms and stabilization of cerebrospinal fluid antibody levels (12). In four other human cases, however, GCV was unable to reverse the fatal progression of disease (9, 17, 20), substantiating the need for more-efficacious antivirals for late-stage infection.
During the preparation of this article, a biochemical analysis of B virus TK was published, including some overlap with the present study (14). Our somewhat different approach was to determine if variability existed among wild isolates of B virus, hence our inclusion of many more virus strains, instead of just three strains. Instead of characterizing the biochemical properties of B virus TK, we sought out compounds that had significantly greater efficacy in cell culture against a wide scope of B virus isolates, so that infectious-disease physicians would have more-powerful choices to treat cases of advanced human B virus infection. The creation of the TKnull mutant viruses as opposed to the production of recombinant protein allowed us to examine TK-mediated drug resistance in the context of viral infection. The authors of the previous study could not detect the phosphorylation of ACV or GCV with recombinant B virus TK; however, the increased resistance of the TK mutants to these drugs suggests that in cell culture infections, B virus TK does activate ACV and GCV.
Our studies indicate that a new, improved group of antiviral agents, 2′-fluoroarabinofuranosyl 5-substituted pyrimidine nucleosides, should be considered for therapeutic use to combat zoonotic B virus infection. Comprehensive analyses examining the cellular toxicity and effectiveness of a number of these compounds against HSV have been reported. FEAU has been shown to be the most selective anti-HSV antiviral in this class; it was not incorporated into uninfected cell DNA (17) and was 10-fold less toxic than ACV in cell culture (19). Other laboratories have demonstrated the in vivo safety of FEAU; no negative effects were reported in a monkey following six intravenous doses of FEAU at 30 mg/kg (7). Further work has demonstrated the in vivo safety and effectiveness of FEAU against simian varicella virus in African green monkeys (26), and analysis of FEAU in both a mouse model of HSV-2 (19) and a rabbit model of HSV-1 (28) has also demonstrated safety and effectiveness. In view of the impressively low doses required to inhibit B virus replication, FEAU should be considered as a compassionate-use treatment for human cases in which there is progressive or severe morbidity.
Acknowledgments
We thank David Katz and Ludmila Perelygina for helpful comments during the preparation of the manuscript.
This work was supported in part by NIH grants P40 RR05162 (J.K.H.) and 2P30-AI-50409 (R.F.S.), the Georgia Research Alliance, and the Department of Veterans Affairs.
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Abbruzzese, J. L., S. Schmidt, M. N. Raber, J. K. Levy, A. M. Castellanos, S. S. Legha, and I. H. Krakoff. 1989. Phase I trial of 1-(2′-deoxy-2′-fluoro-1-beta-d-arabinofuranosyl)-5-methyluracil (FMAU) terminated by severe neurologic toxicity. Invest. New Drugs 7:195-201. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. C., R. B. Swenson, J. L. Orkin, S. S. Kalter, and H. M. McClure. 1994. Primary Herpesvirus simiae (B-virus) infection in infant macaques. Lab. Anim. Sci. 44:526-530. [PubMed] [Google Scholar]
- 3.Artenstein, A. W., C. B. Hicks, B. S. Goodwin, Jr., and J. K. Hilliard. 1991. Human infection with B virus following a needlestick injury. Rev. Infect. Dis. 13:288-291. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., and E. De Clercq. 1990. 9-Beta-d-arabinofuranosyladenine 5′-monophosphate (araAMP) is converted directly to its antivirally active 5′-triphosphate form by 5-phosphoribosyl-1-pyrophosphate (PRPP) synthetase. Biochem. Biophys. Res. Commun. 173:781-787. [DOI] [PubMed] [Google Scholar]
- 5.Boulter, E. A., and D. P. Grant. 1977. Latent infection of monkeys with B virus and prophylactic studies in a rabbit model of this disease. J. Antimicrob. Chemother. 3(Suppl. A):107-113. [DOI] [PubMed] [Google Scholar]
- 6.Boulter, E. A., B. Thornton, D. J. Bauer, and A. Bye. 1980. Successful treatment of experimental B virus (Herpesvirus simiae) infection with acyclovir. Br. Med. J. 280:681-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. 1989. B virus infections in humans—Michigan. MMWR Morb. Mortal. Wkly. Rep. 38:453-454. [PubMed] [Google Scholar]
- 8.Centers for Disease Control. 1987. B-virus infection in humans—Pensacola, Florida. MMWR Morb. Mortal. Wkly. Rep. 36:289-290, 295-296. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1998. Fatal Cercopithecine herpesvirus 1 (B virus) infection following a mucocutaneous exposure and interim recommendations for worker protection. MMWR Morb. Mortal. Wkly. Rep. 47:1073-1076, 1083. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1995. Publication of guidelines for the prevention and treatment of B virus infections in exposed persons. MMWR Morb. Mortal. Wkly. Rep. 44:96-97. [PubMed] [Google Scholar]
- 11.Chou, T. C., X. B. Kong, M. P. Fanucchi, Y. C. Cheng, K. Takahashi, K. A. Watanabe, and J. J. Fox. 1987. Synthesis and biological effects of 2′-fluoro-5-ethyl-1-beta-d-arabinofuranosyluracil. Antimicrob. Agents Chemother. 31:1355-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport, D. S., D. R. Johnson, G. P. Holmes, D. A. Jewett, S. C. Ross, and J. K. Hilliard. 1994. Diagnosis and management of human B virus (Herpesvirus simiae) infections in Michigan. Clin. Infect. Dis. 19:33-41. [DOI] [PubMed] [Google Scholar]
- 13.Fanucchi, M. P., B. Leyland-Jones, C. W. Young, J. H. Burchenal, K. A. Watanabe, and J. J. Fox. 1985. Phase I trial of 1-(2′-deoxy-2′-fluoro-1-beta-d-arabinofuranosyl)-5-methyluracil (FMAU). Cancer Treat. Rep. 69:55-59. [PubMed] [Google Scholar]
- 14.Focher, F., A. Lossani, A. Verri, S. Spadari, A. Maioli, J. J. Gambino, G. E. Wright, R. Eberle, D. H. Black, P. Medveczky, M. Medveczky, and D. Shugar. 2007. Sensitivity of monkey B virus (Cercopithecine herpesvirus 1) to antiviral drugs: role of thymidine kinase in antiviral activities of substrate analogs and acyclonucleosides. Antimicrob. Agents Chemother. 51:2028-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes, G. P., L. E. Chapman, J. A. Stewart, S. E. Straus, J. K. Hilliard, and D. S. Davenport. 1995. Guidelines for the prevention and treatment of B-virus infections in exposed persons. The B Virus Working Group. Clin. Infect. Dis. 20:421-439. [DOI] [PubMed] [Google Scholar]
- 16.Holmes, G. P., J. K. Hilliard, K. C. Klontz, A. H. Rupert, C. M. Schindler, E. Parrish, D. G. Griffin, G. S. Ward, N. D. Bernstein, T. W. Bean, et al. 1990. B virus (Herpesvirus simiae) infection in humans: epidemiologic investigation of a cluster. Ann. Intern. Med. 112:833-839. [DOI] [PubMed] [Google Scholar]
- 17.Kong, X. B., A. C. Scheck, R. W. Price, P. M. Vidal, M. P. Fanucchi, K. A. Watanabe, J. J. Fox, and T. C. Chou. 1988. Incorporation and metabolism of 2′-fluoro-5-substituted arabinosyl pyrimidines and their selective inhibition of viral DNA synthesis in herpes simplex virus type 1 (HSV-1)-infected and mock-infected Vero cells. Antiviral Res. 10:153-166. [DOI] [PubMed] [Google Scholar]
- 18.Loomis, M. R., T. O'Neill, M. Bush, and R. J. Montali. 1981. Fatal herpesvirus infection in patas monkeys and a black and white colobus monkey. J. Am. Vet. Med. Assoc. 179:1236-1239. [PubMed] [Google Scholar]
- 19.Mansuri, M. M., I. Ghazzouli, M. S. Chen, H. G. Howell, P. R. Brodfuehrer, D. A. Benigni, and J. C. Martin. 1987. 1-(2-Deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-ethyluracil. A highly selective antiherpes simplex agent. J. Med. Chem. 30:867-871. [DOI] [PubMed] [Google Scholar]
- 20.Nanda, M., V. T. Curtin, J. K. Hilliard, N. D. Bernstein, and R. D. Dix. 1990. Ocular histopathologic findings in a case of human herpes B virus infection. Arch. Ophthalmol. 108:713-716. [DOI] [PubMed] [Google Scholar]
- 21.Ohsawa, K., D. H. Black, R. Torii, H. Sato, and R. Eberle. 2002. Detection of a unique genotype of monkey B virus (Cercopithecine herpesvirus 1) indigenous to native Japanese macaques (Macaca fuscata). Comp. Med. 52:555-559. [PubMed] [Google Scholar]
- 22.Palmer, A. E. 1987. B virus, Herpesvirus simiae: historical perspective. J. Med. Primatol. 16:99-130. [PubMed] [Google Scholar]
- 23.Poffenberger, K. L., and B. Roizman. 1985. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J. Virol. 53:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reardon, J. E. 1989. Herpes simplex virus type 1 and human DNA polymerase interactions with 2′-deoxyguanosine 5′-triphosphate analogues. J. Biol. Chem. 264:19039-19044. [PubMed] [Google Scholar]
- 25.Smith, A. L., D. H. Black, and R. Eberle. 1998. Molecular evidence for distinct genotypes of monkey B virus (herpesvirus simiae) which are related to the macaque host species. J. Virol. 72:9224-9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soike, K. F., T. C. Chou, J. J. Fox, K. A. Watanabe, and C. A. Gloff. 1990. Inhibition of simian varicella virus infection of monkeys by 1-(2-deoxy-2-fluoro-1-beta-d-arabinofuranosyl)-5-ethyluracil (FEAU) and synergistic effects of combination with human recombinant interferon-beta. Antiviral Res. 13:165-174. [DOI] [PubMed] [Google Scholar]
- 27.Tognon, M., E. M. Cattozzo, S. Bianchi, and M. G. Romanelli. G. 1996. Enhancement of HSV-DNA infectivity, in Vero and RS cells, by a modified calcium-phosphate transfection technique. Virus Genes 12:193-197. [DOI] [PubMed] [Google Scholar]
- 28.Trousdale, M. D., J. L. Law, F. A. Yarber, K. A. Watanabe, and J. J. Fox. 1992. Evaluation of 1-(2′-deoxy-2′-fluoro-beta-d-arabinofuranosyl)-5-ethyluracil in a rabbit model of herpetic keratitis. Antiviral Res. 17:157-167. [DOI] [PubMed] [Google Scholar]
- 28a.U.S. Department of Health and Human Services Public Health Service, Centers for Disease Control and Prevention, and National Institutes of Health. 2007. Biosafety in microbiological and biomedical laboratories, 5th ed. Government Printing Office, Washington, DC. http://www.cdc.gov/OD/OHS/biosfty/bmbl5/BMBL_5th_Edition.pdf.
- 29.Van Etta, L., J. Brown, A. Mastri, and T. Wilson. 1981. Fatal vidarabine toxicity in a patient with normal renal function. JAMA 246:1703-1705. [DOI] [PubMed] [Google Scholar]
- 30.Vizoso, A. D. 1975. Recovery of herpes simiae (B virus) from both primary and latent infections in rhesus monkeys. Br. J. Exp. Pathol. 56:485-488. [PMC free article] [PubMed] [Google Scholar]
- 31.Wall, L. V., H. T. Zwartouw, and D. C. Kelly. 1989. Discrimination between twenty isolates of herpesvirus simiae (B virus) by restriction enzyme analysis of the viral genome. Virus Res. 12:283-296. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe, K. A., U. Reichman, K. Hirota, C. Lopez, and J. J. Fox. 1979. Nucleosides. 110. Synthesis and antiherpes virus activity of some 2′-fluoro-2′-deoxyarabinofuranosylpyrimidine nucleosides. J. Med. Chem. 22:21-24. [DOI] [PubMed] [Google Scholar]
- 33.Weigler, B. J. 1992. Biology of B virus in macaque and human hosts: a review. Clin. Infect. Dis. 14:555-567. [DOI] [PubMed] [Google Scholar]
- 34.Whitley, R. J., and C. A. Alford. 1981. Antiviral agents: clinical status report. Hosp. Pract. (Off. Ed.) 16:109-121. [DOI] [PubMed] [Google Scholar]
- 35.Wild, K., T. Bohner, G. Folkers, and G. E. Schulz. 1997. The structures of thymidine kinase from herpes simplex virus type 1 in complex with substrates and a substrate analogue. Protein Sci. 6:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson, R. B., M. A. Holscher, T. Chang, and J. R. Hodges. 1990. Fatal Herpesvirus simiae (B virus) infection in a patas monkey (Erythrocebus patas). J. Vet. Diagn. Invest. 2:242-244. [DOI] [PubMed] [Google Scholar]
- 37.Zwartouw, H. T., and E. A. Boulter. 1984. Excretion of B virus in monkeys and evidence of genital infection. Lab. Anim. 18:65-70. [DOI] [PubMed] [Google Scholar]
- 38.Zwartouw, H. T., C. R. Humphreys, and P. Collins. 1989. Oral chemotherapy of fatal B virus (herpesvirus simiae) infection. Antiviral Res. 11:275-283. [DOI] [PubMed] [Google Scholar]