Abstract
The transport characteristics of six fluoroquinolones (FQs) with various lipophilicities were compared in a Calu-3 cell model. For each FQ, an active polarized transport was observed in the direction of the apical side. However, the apparent permeability of FQs resulted from active transport and passive diffusion that were highly variable between compounds and mainly governed by lipophilicity. Therefore, active transport was predominant for compounds with relatively low lipophilicity but minor for FQs with higher lipophilicity.
Fluoroquinolones (FQs) have been shown to be substrates of efflux transport systems, including P-glycoprotein (P-gp), in various tissues and cell lines (1). We have recently shown that P-gp is expressed in Calu-3 lung epithelial cells and that it is responsible for an active efflux transport of moxifloxacin (MXF) as a representative FQ (2). The objective of this new study was to compare the diffusion characteristics of several FQs with various lipophilicities, differentiating between passive diffusion and active transport, and to determine their relative contributions to the overall diffusion through Calu-3 cells.
Ciprofloxacin (CIP) hydrochloride and moxifloxacin (MXF) hydrochloride were kindly supplied by Bayer Healthcare (Leverkusen, Germany); grepafloxacin (GRX) hydrochlorate was supplied by Otsuka Pharmaceutical Co. (Tokyo, Japan); and PSC-833 was supplied by Novartis (Basel, Switzerland). Commercial solutions of levofloxacin (LVX) and pefloxacin (PFX) from, respectively, Sanofi Aventis (Paris, France) and Rhône-Poulenc-Rorer (Antony, France) were used. Norfloxacin (NOR) was purchased from Sigma-Aldrich. Other chemicals and reagents had the same origins as previously described (2). Calu-3 cell culture, transport, and inhibition studies as well as tight junction integrity control were done as previously described (2), with FQ concentrations set at 50 μM, corresponding to a third of the apparent Km for MXF transport (2), and chosen to limit the risk of efflux transporter saturation. FQ partition coefficients between octanol and pH 7.4 buffered solution (log D) were taken from the literature: NOR, −1.16, and CIP, −0.93 (10); LVX, −0.41, and GRX, 0.03 (11); MXF, −0.28 (5); and PFX, 0.25 (6). FQs were assayed by high-performance liquid chromatography with fluorescence detection using the same method as for MXF (2), except that excitatory and emission wavelengths were set at 285 and 490 nm, respectively, for MXF and LVX and at 285 and 444 nm, respectively, for GRX, PFX, CIP, and NOR and that the flow rate was 0.5 ml/min. The precisions intra- and interday for FQs were satisfactory, with CV values between 0.3 and 6.7%. Apparent permeability (Papp) values were obtained according to the following equation: Papp = Q/(T × A × C0), where Q is the amount of drug in μg that appeared in the acceptor compartment, T is the incubation time of 60 min, A is the semipermeable membrane surface area of 4.67 cm2, and C0 is the initial concentration of FQ in the donor compartment in μg/cm−3. The efflux ratio (ER) was determined by dividing the Papp in the secretory direction (BL-AP) by the Papp in the absorptive direction (AP-BL). All values are presented as means ± one standard error of the mean (SEM). The statistical evaluation of the data was performed with one-way or two-way analysis of variance followed by Bonferroni's post-hoc tests with a significance level of less than 0.05 (P < 0.05). Linear regression was performed to analyze the relationship between the Papp values of FQs in the presence of PSC-833 and their log D values. The statistics were done with GraphPad Prism version 4.03 for Windows.
Papp estimates in the secretory direction were always significantly higher than those in the absorptive direction (Table 1), attesting to an active efflux transport through Calu-3 cells. Efflux ratio values varied in a range of around 3-fold between the lowest and highest values for PFX and CIP, respectively. The dissymmetry between absorptive and secretory Papp values was always totally abolished in the presence of PSC-833, suggesting that P-gp is likely to be predominantly responsible for this active transport, as was previously shown with MXF (2). Efflux ratio values became equal or close to 1 in the presence of PSC-833 at 3 μM, suggesting that under these conditions Papp reflects passive diffusion. These estimated passive Papp values varied by more than 10-fold between the lowest and highest values estimated for NOR and PFX, respectively (Table 1). Indeed, a significant linear relationship was found between passive Papp estimates and previously reported log D values within the −1.2 to 0.25 range (Fig. 1), indicating that for these compounds with relatively close sizes and molecular weights, lipophilicity is the major determinant of passive permeability.
TABLE 1.
Permeability (Papp, 10−6 cm·s−1) for the six FQs in the absence and presence of PSC-833
| Drug | Log D | In the absence of PSC-833 (total Papp) |
In the presence of PSC-833 (passive Papp) |
PSC-833-sensitive (active Papp) differenceb |
% of active contribution (BL-AP secretory) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AP-BL absorptive | BL-AP secretorya | Efflux ratio | AP-BL absorptive | BL-AP secretory | Efflux ratio | AP-BL absorptive | BL-AP secretory | |||
| CIP | −0.93 | 0.7 ± 0.02 | 2.7 ± 0.07** | 4 | 0.8 ± 0.03 | 0.7 ± 0.01 | 0.9 | 0.1 | 2.0 | 73 |
| NOR | −1.16 | 0.6 ± 0.05 | 2.0 ± 0.04* | 3.3 | 0.7 ± 0.03 | 0.6 ± 0.01 | 0.8 | 0.1 | 1.4 | 70 |
| LVX | −0.41 | 2.4 ± 0.10 | 6.2 ± 0.12** | 2.6 | 4.2 ± 0.05 | 4.3 ± 0.10 | 1.0 | 1.8 | 1.9 | 30 |
| MXF | −0.28 | 5.0 ± 0.20 | 10.4 ± 0.20** | 2.1 | 8.3 ± 0.12 | 7.8 ± 0.09 | 0.9 | 3.3 | 2.6 | 25 |
| GRX | 0.03 | 3.1 ± 0.12 | 11.8 ± 0.78** | 3.8 | 7.3 ± 0.26 | 7.2 ± 0.28 | 1.0 | 4.2 | 4.6 | 40 |
| PFX | 0.25 | 7.1 ± 0.28 | 9.8 ± 0.19** | 1.4 | 8.7 ± 0.36 | 8.7 ± 0.20 | 1.0 | 1.6 | 1.1 | 11 |
*, P < 0.01, **, P < 0.001.
Calculated as the absolute difference between Papp in the presence and absence of PSC-833. Data are expressed as the means ± SEM of results of three experiments.
FIG. 1.
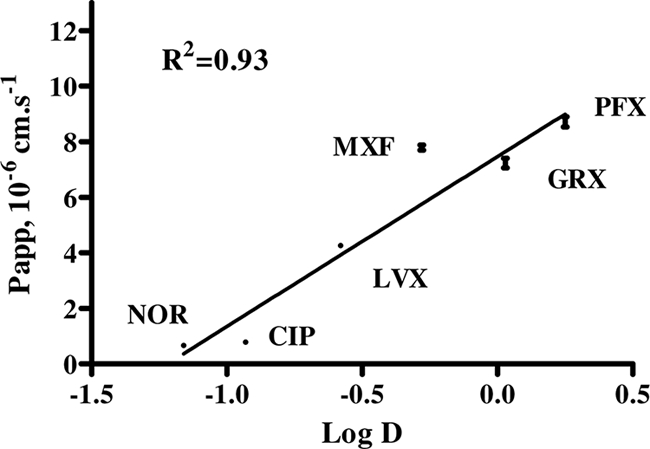
Linear relationship between the apparent permeability of FQs in the presence of PSC-833 and the log D. The linear regression fitted the data with the following equation: Papp = 6.1 log D + 7.5. Data are expressed as mean ± SEM (n = 6).
Active Papp values, assessed from the difference between Papp values obtained in the absence and in the presence of PSC-833, were virtually similar in the absorptive and secretory directions except for CIP and NOR (Table 1). Transport direction-dependent differences in P-gp-mediated efflux activity have been reported for digoxin, rhodamine 123, and fexofenadine in Caco-2 cells (7, 9). In Calu-3 and human alveolar epithelial cells, P-gp is predominantly located at the apical membrane (3, 4) and may therefore be exposed to higher FQ concentrations when drugs are applied at the basolateral side, especially for compounds such as CIP and NOR, with the lowest passive Papp due to lowest log D, that may have more difficulties in reaching efflux transporter binding sites in this lipophilic environment. Therefore, the low active Papp values in the AP-BL direction for these two FQs could reflect a limited accessibility to efflux pumps. In fact, the ability of FQs to passively diffuse across the apical membrane in the AP-BL direction should rate limit the PSC-833-inhibited active efflux. Hence, active Papp values determined in the BL-AP direction were used for the assessment of the differences between compounds in their affinity for transporters.
Active Papp values were less variable among the six FQs tested than passive Papp values, since with the exception of GRX (active Papp = 4.6 × 10−6 cm·s−1), active Papp estimates ranged between 1.1 × 10−6 cm·s−1 and 2.6 × 10−6 cm·s−1 (Table 1). However, the relative contribution of the active transport to the total process was much more variable between compounds, essentially due to differences in passive permeabilities. Active Papp values corresponded to 70% and 73% of total Papp values for NOR and CIP, respectively, the two FQs with the lowest passive Papp, and to only 11% for PFX (Table 1). Intermediate results (39%) were observed for GRX, the compound with the highest active Papp. Therefore, inhibition of FQ active transport, such as in the presence of competition with other efflux transporter substrates, should have a relatively limited effect on the most lipophilic compounds. Active transport in the BL-AP direction would correspond to drug secretion from blood to lung, resulting in intra-alveolar unbound drug concentrations higher than those in the blood. Since pulmonary delivery of FQs using aerosols has recently been proposed (8), it is interesting to note that low passive permeability and high active efflux permeability should favor high concentrations in the alveolar compartment. Yet, because of the nature of the Calu-3 cells, direct extrapolation of these in vitro data to the in vivo situation should be done with caution. Indeed, the cancerous origin of the Calu-3 cells may affect the amount of P-gp present at the apical membrane, which may be overexpressed compared with alveolar epithelial cells. It would be interesting to evaluate the transport of FQs in an in vitro model of human primary alveolar cells.
In conclusion, this study has demonstrated that the six FQs tested are all actively transported in Calu-3 cells by systems that are virtually totally inhibited by PSC-833, consistent with our previous demonstration that MXF was subject to P-gp-mediated active efflux transport in this model (2). Furthermore, the variability between compounds with active Papp is relatively limited compared with that with passive Papp, which is mainly determined by lipophilicity. Yet although Calu-3 constitutes a popular model of the bronchoalveolar barrier, it remains to be demonstrated that active efflux transport systems, including P-gp, are also expressed in non-tumor cells, such as pneumocytes, which constitute most lung cells.
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Alvarez, A. I., M. Perez, J. G. Prieto, A. J. Molina, R. Real, and G. Merino. 2008. Fluoroquinolone efflux mediated by ABC transporters. J. Pharm. Sci. 97:3483-3493. [DOI] [PubMed] [Google Scholar]
- 2.Brillault, J., W. V. De Castro, T. Harnois, A. Kitzis, J. C. Olivier, and W. Couet. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob. Agents Chemother. 53:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endter, S., U. Becker, N. Daum, H. Huwer, C. M. Lehr, M. Gumbleton, and C. Ehrhardt. 2007. P-glycoprotein (MDR1) functional activity in human alveolar epithelial cell monolayers. Cell Tissue Res. 328:77-84. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton, K. O., E. Topp, I. Makagiansar, T. Siahaan, M. Yazdanian, and K. L. Audus. 2001. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol. Exp. Ther. 298:1199-1205. [PubMed] [Google Scholar]
- 5.Langlois, M.-H., M. Montagut, J.-P. Dubost, J. Grellet, and M.-C. Saux. 2005. Protonation equilibrium and lipophilicity of moxifloxacin. J. Pharm. Biomed. Anal. 37:389-393. [DOI] [PubMed] [Google Scholar]
- 6.Montay, G., C. Jacquot, J. Bariety, and R. Cunci. 1985. Pharmacokinetics of pefloxacin in renal insufficiency. Eur. J. Clin. Pharmacol. 29:345-349. [DOI] [PubMed] [Google Scholar]
- 7.Petri, N., C. Tannergren, D. Rungstad, and H. Lennernas. 2004. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm. Res. 21:1398-1404. [DOI] [PubMed] [Google Scholar]
- 8.Sabet, M., C. E. Miller, T. G. Nolan, K. Senekeo-Effenberger, M. N. Dudley, and D. C. Griffith. 2009. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:3923-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troutman, M. D., and D. R. Thakker. 2003. Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm. Res. 20:1200-1209. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji, A., H. Sato, Y. Kume, I. Tamai, E. Okezaki, O. Nagata, and H. Kato. 1988. Inhibitory effects of quinolone antibacterial agents on gamma-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob. Agents Chemother. 32:190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi, H., I. Yano, H. Saito, and K. Inui. 2001. Transport characteristics of grepafloxacin and levofloxacin in the human intestinal cell line Caco-2. Eur. J. Pharmacol. 431:297-303. [DOI] [PubMed] [Google Scholar]


