Abstract
Fungal biofilms produce a small number of persister cells which can tolerate high concentrations of fungicidal agents. Persisters form upon attachment to a surface, an important step in the pathogenesis of Candida strains. The periodic application of antimicrobial agents may select for strains with increased levels of persister cells. In order to test this possibility, 150 isolates of Candida albicans and C. glabrata were obtained from cancer patients who were at high risk for the development of oral candidiasis and who had been treated with topical chlorhexidine once a day. Persister levels were measured by exposing biofilms growing in the wells of microtiter plates to high concentrations of amphotericin B and plating for survivors. The persister levels of the isolates varied from 0.2 to 9%, and strains isolated from patients with long-term carriage had high levels of persisters. High-persister strains were isolated from every patient with Candida carriage of more than 8 consecutive weeks but from no patients with transient carriage. All of the high-persister isolates had an amphotericin B MIC that was the same as that for the wild type, indicating that these strains were drug-tolerant rather than drug-resistant mutants. Biofilms of the majority of high-persister strains also showed an increased tolerance to chlorhexidine and had the same MIC for this antimicrobial as the wild type. This study suggests that persister cells are clinically relevant, and antimicrobial therapy selects for high-persister strains in vivo. The drug tolerance of persisters may be a critical but overlooked component responsible for antimicrobial drug failure and relapsing infections.
Candida species are opportunistic pathogens that are typically present in the oral cavities of healthy individuals (2, 14, 24). In immunocompromised patients, the severity of Candida infection can range from a superficial annoyance to a life-threatening systemic infection of the organs and sepsis. While superficial infections of the oral or vaginal mucosa are easily treatable with azoles, 5 to 8% resist therapy, producing relapses (11, 30). Systemic invasive fungal infections, characterized by the hyphal growth of Candida albicans, are the cause of high rates of morbidity and mortality, which approach 40% (8). Difficult-to-treat Candida infections also occur on prosthetic devices, such as catheters and heart valves, and an infected prosthesis requires device removal to avoid systemic infection (22).
Candida forms biofilms on the surfaces of mucosal tissues and prostheses that are highly tolerant to antifungal agents (18, 26). The recalcitrance of biofilm infections to antimicrobials is not obvious, since planktonic populations of disease-causing strains can be highly susceptible to antifungals, including azoles, echinocandins, and amphotericin B (AMB) (16).
We reported that upon attachment, C. albicans forms a small subpopulation (∼1%) of persister cells that are completely tolerant to the currently used systemic antifungals (16) and resemble well-characterized dormant persisters formed by pathogenic bacteria (12, 13, 18, 27, 28, 31, 32). The concentration-dependent killing of a C. albicans biofilm with a fungicidal agent such as AMB shows a sharply biphasic pattern, with the bulk of the cells rapidly dying and a small plateau of surviving persisters being present. Vital staining shows live persister cells present in a biofilm killed by exposure to a high level of AMB, and these cells can be sorted out from the bulk. Surviving persisters produce a new biofilm with a similarly small population of persisters, indicating that these cells are not classical resistant mutants but phenotypic variants of the wild type. Persisters exhibit multidrug tolerance, which is a hallmark of a biofilm infection.
Attachment to a surface is an important step in fungal pathogenesis, including pathogenesis resulting in vaginitis, oral thrush, and catheter biofilm infections (6, 10, 15). It seems that persisters, which form upon attachment of the pathogen to a surface, may play an important role in the tolerance of Candida infections to antifungals.
In Escherichia coli, the periodic application of a high concentration of a bactericidal antibiotic in vitro leads to the selection of high-persister (hip) mutants (20, 21). Importantly, these mutants have the same MIC as the wild type, but they produce considerably more persister cells. While the persister phenotype itself is not due to a mutation, high-persister strains carry mutations that cause an increased incidence of persisters. One of these mutants was mapped to an allele of a hipA gene coding for a toxin of the hipBA toxin/antitoxin module (13, 21). The mechanistic basis of HipA-dependent persister formation was recently identified (27). HipA is a protein kinase (3) that phosphorylates elongation factor EF-Tu, which leads to the inhibition of protein synthesis. This creates a dormant, persister state. The HipA7 allele, which causes the high persistence of the hip mutant, apparently has decreased binding to the antitoxin HipB, which leads to increased persister production.
We reasoned that a similar selection for high-persister mutants is likely to occur in vivo, especially in cases of recalcitrant infections, where pathogens are periodically exposed to high levels of antimicrobial compounds. With this in mind, we tested a collection of 150 clinical isolates of Candida species for their persister levels. The strains were obtained from cancer patients who received daily topical chlorhexidine (CHX) treatment. We report that the strains isolated from patients with long-term Candida carriage had increased levels of surviving persisters.
MATERIALS AND METHODS
Patient selection.
Forty-six cancer patients, including 4 patients with hematological malignancies and 42 patients with solid tumors, from two affiliated teaching hospitals and a cancer hospital of the Shandong University Medical School were selected for this study and sampled for oral Candida carriage. The patients' ages ranged from 21 to 76 years, and the average age was 48.8 years. There were 29 male patients and 17 female patients. The inclusion criteria for the study were a previous diagnosis of advanced cancer for more than 1 year and treatment by chemotherapy. The exclusion criteria for this study were significant physical defects, the wearing of dentures, uncontrolled diabetes mellitus, and the use of systemic antifungal medication or steroids within the previous 2 weeks. The patients with head and neck cancer receiving radiation therapy were excluded. This study was approved by the Ethics Committee of Shandong University (approval no. 2008-03) and an institutional review board at the Northeastern University Division of Research Integrity (institutional review board no. 09-01-02).
Sampling methods.
All patients received regular oral hygiene care that included 0.2% CHX daily. The oral cavities of the patients were sampled for Candida carriage, and the specimens were obtained 8 h following regular oral hygiene care. The specimens were collected by passing a sterile swab across mucosal surface of the inner cheek five times. The procedure was repeated a total of three times within a half-hour interval. The swabs were transferred to a sterile test tube containing 500 μl sterile phosphate-buffered saline (PBS). The tubes containing the swabs were vigorously vortexed for 30 s to suspend the samples. After 30 s, the swabs were removed and the samples were pelleted by centrifugation and concentrated by resuspension in 50 μl buffer. This suspension was spread plated on CHROMagar Candida differential medium and incubated for 24 to 48 h (24). Colonies were identified as Candida by CHROMagar on the basis of a color change and the colony morphology on this selective and differential medium (23). The Candida strains were confirmed to be members of the genus Candida by use of the API 20C system (BioMerieux, Marcy l'Etoile, France), based on carbon source utilization and other differential biochemical assays, according to the manufacturer's directions (33). Candida albicans species were additionally confirmed by observing by microscopy the induction of germ tube formation by serum (19). If multiple Candida colonies were present, a single isolate was chosen for further analysis for each patient and time point. Patients were sampled for Candida carriage once per week, until an oral Candida yeast was not isolated or the patient left the hospital after chemotherapy.
Antifungal drug susceptibility testing.
Stock solutions (10 mg/ml) of AMB (Fisher Scientific) and CHX (Sigma-Aldrich) were prepared in dimethyl sulfoxide. The MICs for the clinical isolates were determined by broth microdilution on the basis of the CLSI guidelines described in document M27-A2 (29). Briefly, cells from an overnight culture grown in yeast-peptone-dextrose (YPD) medium were harvested by centrifugation, washed twice in sterile PBS, and resuspended to 0.5 × 103 to 2.5 × 103 cells/ml in RPMI 1640 medium (Gibco) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma-Aldrich). The cells were dispensed into a 96-well microtiter plate at 100 μl per well, and the antifungals were added by making a series of twofold dilutions at 100 μl per well in RPMI 1640 medium. The plates were incubated at 37°C for 48 h. The MICs of AMB and CHX were determined visually and were defined as the lowest concentration of drug that resulted in no growth.
Candida persister determination.
Twenty-four-hour biofilms were formed by standard methods, as described by Ramage et al. (25). Briefly, cells were obtained from an overnight culture grown at 30°C in YPD liquid medium. Cells were harvested by centrifugation, washed twice in sterile PBS (pH 7.4), and resuspended in RPMI 1640 medium with l-glutamine and 0.165 M MOPS (BioWhittaker). The optical density at 600 nm of the suspension was adjusted to 0.1, or approximately 1 × 106 cells/ml. One hundred microliters of this suspension was aliquoted into the wells of a flat-bottom microtiter plate (Costar 3370; Corning). The plates were incubated for 24 h at 37°C on a microtiter plate shaker (model 4625; Lab-Line Instruments) at approximately 100 rpm. For persister quantification, the method of LaFleur et al. (16) was followed. Briefly, the biofilms were washed twice with sterile PBS to remove nonadherent cells and were challenged with 100 μg/ml AMB or 200 μg/ml CHX dissolved in fresh RPMI 1640 medium for 48 h at 37°C. At these concentrations, regular cells are killed and only persisters survive. The biofilms were then washed twice with PBS; and the surviving persisters were quantified by scraping the biofilm, vigorously vortexing the bioflim, serially diluting the suspension, and plating the suspension. The percentage of persisters was calculated by comparing the number of persisters with the total number of cells in an untreated 24-h biofilm control.
RESULTS
Candida carriage in the oral cavities of cancer patients.
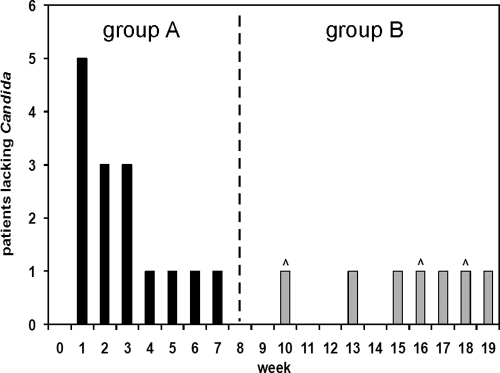
Candida was isolated from the oral cavities of patients receiving chemotherapy for cancer treatment in 22 of 46 cases. The frequency of oral Candida carriage was 47.8%, slightly less than in studies which estimated the occurrence of Candida in the oral cavity of cancer patients to be from 66% to 70% (4, 5). Patients with Candida carriage were sampled once per week until an oral yeast was not isolated. For each patient, the week in which Candida was no longer isolated is shown in Fig. 1. The patients were divided into two groups: those with long-term carriage and those with transient carriage. Fifteen patients and 42 isolates were classified as group A, in which Candida was isolated for less than 8 weeks. Candida carriage was present for an average of 2.8 weeks in group A patients. Seven patients and 108 isolates were classified as group B, in which Candida was isolated for more than 8 weeks. On average, Candida was isolated for 15.4 consecutive weeks from group B patients. Three group B patients left the study with active Candida carriage when chemotherapy had concluded, including one patient at week 10. A majority of isolates were Candida albicans (n = 131), and a few C. glabrata strains (n = 19) were isolated from each group. The average number of weeks of Candida carriage and the numbers of patients and strains isolated are summarized in Table 1.
FIG. 1.
Oral Candida carriage in patients undergoing chemotherapy. Cancer patients were sampled weekly for oral Candida carriage, and the first week in which Candida was no longer isolated was plotted. Carets above the bars indicate patients who left the study after chemotherapy concluded. The patients were divided into two groups. In group A, Candida was isolated for less than 8 consecutive weeks, and in group B, Candida was isolated for more than 8 weeks.
TABLE 1.
Candida strains isolated from patients with resolving and chronic carriage
| Group | Avg time of carriage (wk) | No. of patients | Total no. of Candida isolates recovereda |
|---|---|---|---|
| A | 2.8 | 15 | 42 (39/3) |
| B | 15.4 | 7 | 108 (92/16) |
| Total | 22 | 150 (131/19) |
The values in parentheses indicate the number of C. albicans isolates recovered/number of C. glabrata isolates recovered.
Persisters in Candida clinical isolates.
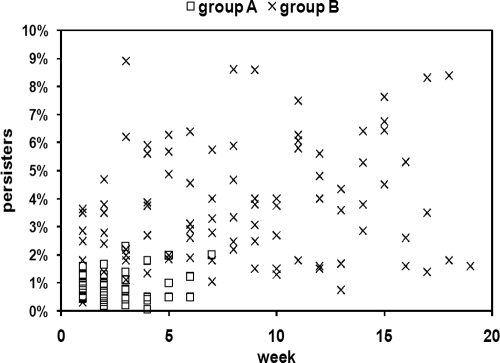
We reasoned that selection for high-persister strains may occur in vivo, especially in the oral cavity, where pathogens are periodically exposed to high levels of antimicrobial compounds and recurrent infections are common. With this in mind, we tested a collection of 150 clinical isolates of Candida for their persister levels. These strains were obtained from patients who were at high risk for the development of oral candidiasis as a result of cancer chemotherapy. The patients were treated daily with topical CHX (0.2%). The number of persisters among the Candida clinical isolates was determined by growing biofilms in vitro, challenging the biofilms with high concentrations of AMB, and plating for survivors (1, 16). All 19 C. glabrata strains had low levels of persisters, averaging 0.19%, and were excluded from further analysis. The levels of surviving persisters for each C. albicans strain isolated in a given week is shown in Fig. 2. The mean level of persisters tolerant to AMB among all isolates obtained from group A patients, which maintained Candida carriage for an average of 2.8 weeks, was 0.90% ± 0.55% (n = 39), which is comparable to the frequency of persisters among laboratory strains. The mean persister frequency of all strains isolated from group B patients, which maintained Candida carriage for an average of 15.4 weeks, was significantly higher at 3.73% ± 2.67% (n = 92), according to Student's t test (P ≤ 0.01). This result indicates that there was an apparent difference in persister levels between strains from patients with long-term Candida carriage and strains from patients exhibiting transient carriage. However, as it is likely that several clonal (not independent) Candida strains were repeatedly isolated from individual patients over several weeks, an additional t test was performed to control for this potential lack of independence. For this test, we treated each patient (n) as a single data point, in effect, assuming that each patient harbored a single strain. To accomplish this, the persister levels for all isolates from a single patient at all time points were averaged together into a single value. A t test comparing the values for group A patients with the values for group B patients was then performed. The persister levels in group B patients were again found to be significantly higher than those in group A patients, 3.82% ± 0.88% and 0.72% ± 0.28%, respectively (P ≤ 0.01), confirming our result that there are more persisters in patients with long-term carriage than in patients with transient carriage.
FIG. 2.
Levels of C. albicans persisters in clinical isolates. Persisters were determined by exposing biofilms of a panel of clinical isolates to 100 μg/ml AMB for 48 h. The number of weeks denotes the time of C. albicans carriage in a given patient from which a particular strain was isolated. The biofilms were washed, disrupted, diluted, and plated for determination of colony counts. The percentage of persisters was determined by comparing the number of cells surviving AMB treatment to the number of cells of the untreated control for each strain. Each strain was tested in triplicate.
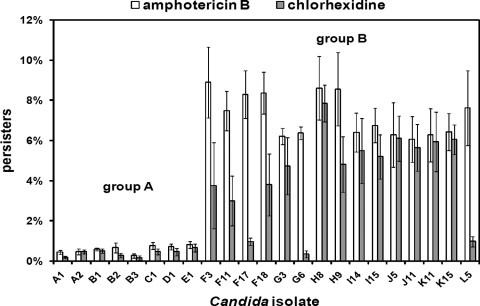
Fifteen hip isolates (of 131 total C. albicans strains) with more than 6% surviving persisters tolerant to 100 μg/ml AMB were identified from group B patients. High-persister strains were isolated from every group B patient but none of the group A patients. The highest frequency of persisters from any single group A isolate was 2.3%, whereas that value was 8.9% for group B strains. Twelve of the 15 hip strains also exhibited increased survival in the presence of 200 μg/ml CHX, while 3 strains showed increased survival in the presence of AMB alone. The survival of the 15 hip isolates from group B and representative susceptible isolates from group A in the presence of AMB or CHX is shown in Fig. 3. It is important to note that high persistence was determined for each strain in three independent consecutive experiments in which a single colony from each culture was inoculated. This suggests that the hip phenotype is caused by an underlying genetic change.
FIG. 3.
C. albicans high-persister strains isolated from cancer patients. The level of surviving persister cells was determined by exposing the biofilms of a panel of clinical isolates to 100 μg/ml AMB or 200 μg/ml CHX for 48 h. After antifungal challenge, the biofilms were washed, disrupted, diluted, and plated for determination of colony counts. A total of 131 C. albicans strains were tested for survival in the presence of AMB, and representative strains were also tested for survival in the presence of CHX. Clinical isolates from patients A to E are representatives from group A in which Candida was isolated for less than 8 consecutive weeks (n = 39). Clinical isolates from patients F to L are hip mutants from group B patients, who had long-term Candida carriage (n = 92). The number following each patient indicates the week that each strain was isolated. The data represent the average of three independent experiments performed with biological duplicates, and the error bars are standard deviations.
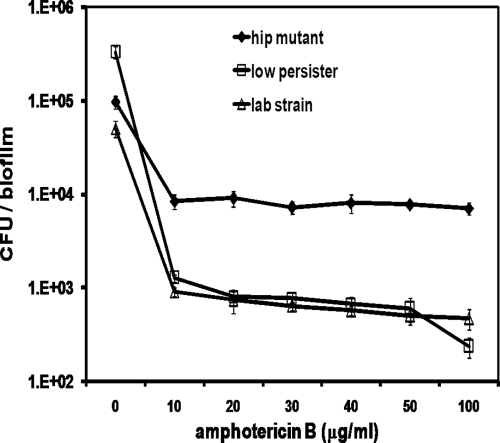
In order to verify that hip mutants had an increased incidence of persisters over a range of concentrations, we performed a concentration-dependent killing experiment with a hip mutant and compared its survival to that of a laboratory strain, strain CAF2-1 (9), and to that of a low-persister clinical isolate from group A. All strains showed biphasic killing characteristic of persisters, and the hip mutant had an increased tolerance to AMB over a range of concentrations, from 10 to 100 μg/ml (Fig. 4).
FIG. 4.
Dose-dependent killing of C. albicans biofilms by AMB. Biofilms were cultured in RPMI 1640 medium for 24 h and exposed to AMB for 48 h. After the antifungal challenge, the biofilms were washed, disrupted, diluted, and plated for determination of colony counts. The data represent the average of three independent biological replicates for each strain, and the error bars are standard deviations.
MICs for high-persister strains.
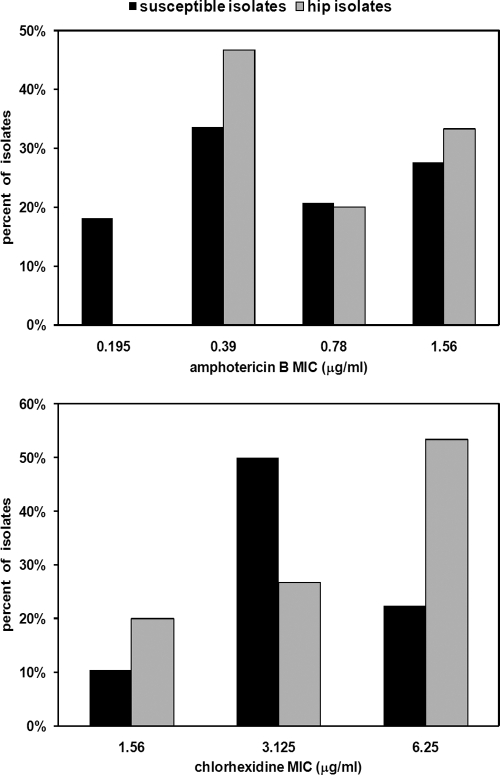
Although clinical resistance to AMB or CHX is extremely rare, we wanted to test the MICs for the hip mutants. The MICs for the 131 C. albicans clinical isolates from this study ranged from 0.2 to 1.5 μg/ml for AMB and from 1.5 to 6 μg/ml for CHX (Fig. 5). Importantly, the MICs for the 15 hip mutants closely overlapped the range of MICs for susceptible isolates (Fig. 5). In fact, three hip isolates were among the group with the lowest CHX MIC (1.5 μg/ml) and seven hip isolates had an AMB MIC of 0.4 μg/ml. The hip mutants did not appear to be resistant but were strains that had increased levels of drug-tolerant persisters compared to the number among the susceptible isolates. These findings suggest that persisters contribute to the recalcitrance of pathogens to antimicrobials.
FIG. 5.
Susceptibility of Candida albicans isolates to AMB and CHX. The MICs for the hip mutants and susceptible C. albicans strains isolated from cancer patients were determined by broth microdilution assay. The data for the isolates are expressed as the percentage of all strains in each group: n = 116 and n = 15 for susceptible strains and hip strains, respectively.
DISCUSSION
The discovery of persister cells in bacteria and fungi raises the possibility that drug tolerance may be an overlooked contributor to the therapeutic failure of antimicrobials (17). The present study suggests that Candida persisters are clinically relevant. High-persister C. albicans isolates were obtained exclusively from a group of cancer patients with chronic carriage. Persister cells apparently contribute to the maintenance of Candida carriage in the oral cavity of cancer patients treated with prophylactic CHX.
The high-persister Candida isolates formed increased levels of these drug-tolerant cells in biofilms, but they did not exhibit increased AMB or CHX MICs. Considering that resistance to high concentrations of AMB or CHX is extremely rare, this result was not surprising. Our results raise the intriguing possibility that persister cells may serve as an indicator of therapeutic failure for fungicidal agents. In contrast, the standard MIC detection does not differentiate between cases of rapidly resolving infections and chronic infections. Elevated MICs for azole antifungals are known to correlate with previous antifungal therapy and recurrent oropharyngeal candidiasis (11). However, the correlation between an increased MIC and recurrent infection does not appear to be true for fungicidal agents, such as AMB and CHX.
This study indicates the significance of persisters in oral candidiasis. The formation of hip mutants may be a general feature of recalcitrant fungal infections, although it is likely that persisters from both wild-type strains and hip mutants contribute to drug tolerance and the survival of the pathogen. It must be noted that a recent study reproducing our previous findings reported persisters in biofilms of some, but not all, isolates of C. albicans and C. glabrata (1). This conclusion was based on the complete eradication of the population of some strains by AMB at 100 μg/ml. However, the same experiments showed biphasic killing, suggesting the presence of cells with elevated tolerance, or persisters. Importantly, after initial killing of the bulk of the cells in the presence of low drug concentrations, surviving persisters of each strain were detected in the presence of AMB at concentrations as high as 50 μg/ml. Apparently, the level and the degree of drug tolerance of persisters vary among different strains, as well as among growth types (such as biofilm versus planktonic cell growth). In the present study, each clinical isolate produced measurable levels of persisters tolerant to 100 μg/ml AMB, although high-persister strains were identified only from the C. albicans isolates and not the C. glabrata isolates.
The strains in this study were isolated from patients with Candida carriage who were at high risk for the development of candidiasis but who did not exhibit signs of infection. Thus, there is no direct evidence suggesting that persisters contribute to relapsing infections. However, Candida carriage has been shown to correlate with an increased incidence of systemic candidiasis in solid-organ transplant patients (7). Future studies will test whether the elevated levels of persister cells in clinical isolates of cancer patients contribute to relapsing infections. The isolation of hip mutants could lead to the identification of the hip genes by whole-genome sequencing or other comparative approaches. Additional studies are needed to determine the molecular basis and clinical relevance of persisters of pathogenic fungi.
Acknowledgments
This work was supported by National Institute of General Medical Sciences grant 2R01GM061162-09. The clinical isolate collection was supported by an NSFC grant (grant 30400498, China) to Q.Q.
We thank Eric Stewart, Iris Keren, Bronwyn Williams, and Brown Williams for helpful discussions, suggestions, and mathematical insight.
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Al-Dhaheri, R. S., and L. J. Douglas. 2008. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob. Agents Chemother. 52:1884-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon, R. D., A. R. Holmes, A. B. Mason, and B. C. Monk. 1995. Oral Candida: clearance, colonization, or candidiasis? J. Dent. Res. 74:1152-1161. [DOI] [PubMed] [Google Scholar]
- 3.Correia, F. F., A. D'Onofrio, T. Rejtar, L. Li, B. L. Karger, K. Makarova, E. V. Koonin, and K. Lewis. 2006. Kinase activity of overexpressed hipA is required for growth arrest and multidrug tolerance in Escherichia coli. J. Bacteriol. 188:8360-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies, A. N., S. Brailsford, K. Broadley, and D. Beighton. 2002. Oral yeast carriage in patients with advanced cancer. Oral Microbiol. Immunol. 17:79-84. [DOI] [PubMed] [Google Scholar]
- 5.Davies, A. N., S. R. Brailsford, and D. Beighton. 2006. Oral candidosis in patients with advanced cancer. Oral Oncol. 42:698-702. [DOI] [PubMed] [Google Scholar]
- 6.De Bernardis, F., H. Liu, R. O'Mahony, R. La Valle, S. Bartollino, S. Sandini, S. Grant, N. Brewis, I. Tomlinson, R. C. Basset, J. Holton, I. M. Roitt, and A. Cassone. 2007. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J. Infect. Dis. 195:149-157. [DOI] [PubMed] [Google Scholar]
- 7.Dongari-Bagtzoglou, A., P. Dwivedi, E. Ioannidou, M. Shaqman, D. Hull, and J. Burleson. 2009. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol. Immunol. 24:249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 9.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum, M. A., and S. S. Radwan. 1990. The adherence process, p. 106-154. In Candida adherence to epithelial cells. CRC Press, Boca Raton, FL.
- 11.Hamza, O. J., M. I. Matee, M. J. Moshi, E. N. Simon, F. Mugusi, F. H. Mikx, W. H. Helderman, A. J. Rijs, A. J. van der Ven, and P. E. Verweij. 2008. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, S., K. Lewis, and M. Vulić. 2008. Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli. Antimicrob. Agents Chemother. 52:2718-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keren, I., D. Shah, A. Spoering, N. Kaldalu, and K. Lewis. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186:8172-8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinegger, C. L., S. R. Lockhart, K. Vargas, and D. R. Soll. 1996. Frequency, intensity, species, and strains of oral Candida vary as a function of host age. J. Clin. Microbiol. 34:2246-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumamoto, C. A., and M. D. Vinces. 2005. Alternative Candida albicans lifestyles: growth on surfaces. Annu. Rev. Microbiol. 59:113-133. [DOI] [PubMed] [Google Scholar]
- 16.LaFleur, M. D., C. A. Kumamoto, and K. Lewis. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis, K. 2005. Persister cells and the riddle of biofilm survival. Biochemistry (Mosc) 70:267-274. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48-56. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie, D. W. 1962. Serum tube identification of Candida albicans. J. Clin. Pathol. 15:563-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyed, H. S., and K. P. Bertrand. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 155:768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyed, H. S., and S. H. Broderick. 1986. Molecular cloning and expression of hipA, a gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 166:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee, P. K., G. Zhou, R. Munyon, and M. A. Ghannoum. 2005. Candida biofilm: a well-designed protected environment. Med. Mycol. 43:191-208. [DOI] [PubMed] [Google Scholar]
- 23.Odds, F. C., and R. Bernaerts. 1994. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J. Clin. Microbiol. 32:1923-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi, Q. G., T. Hu, and X. D. Zhou. 2005. Frequency, species and molecular characterization of oral Candida in hosts of different age in China. J. Oral Pathol. Med. 34:352-356. [DOI] [PubMed] [Google Scholar]
- 25.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramage, G., B. L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42-44. [PubMed] [Google Scholar]
- 27.Schumacher, M. A., K. M. Piro, W. Xu, S. Hansen, K. Lewis, and R. G. Brennan. 2009. Molecular mechanisms of hipA-mediated multidrug tolerance and its neutralization by hipB. Science 323:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah, D., Z. Zhang, A. Khodursky, N. Kaldalu, K. Kurg, and K. Lewis. 2006. Persisters: a distinct physiological state of E. coli. BMC Microbiol. 6:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silici, S., and A. N. Koc. 2006. Comparative study of in vitro methods to analyse the antifungal activity of propolis against yeasts isolated from patients with superficial mycoses. Lett. Appl. Microbiol. 43:318-324. [DOI] [PubMed] [Google Scholar]
- 30.Sobel, J. D. 1985. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 152:924-935. [DOI] [PubMed] [Google Scholar]
- 31.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spoering, A. L., M. Vulić, and K. Lewis. 2006. glpD and plsB participate in persister cell formation in Escherichia coli. J. Bacteriol. 188:5136-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu, J., B. C. Millar, J. E. Moore, R. McClurg, M. J. Walker, J. Evans, S. Hedderwick, and R. McMullan. 2002. Comparison of API20C with molecular identification of Candida spp isolated from bloodstream infections. J. Clin. Pathol. 55:774-777. [DOI] [PMC free article] [PubMed] [Google Scholar]