Abstract
In vitro activity of fosfomycin was evaluated against 68 blaKPC-possessing Klebsiella pneumoniae (KpKPC) isolates, including 23 tigecycline- and/or colistin-nonsusceptible strains. By agar dilution, 93% of the overall KpKPC were susceptible (MIC50/90 of 16/64 μg/ml, respectively). The subgroup of 23 tigecycline- and/or colistin-nonsusceptible strains showed susceptibility rates of 87% (MIC50/90 of 32/128 μg/ml, respectively). Notably, 5 out of 6 extremely drug-resistant (tigecycline and colistin nonsusceptible) KpKPC were susceptible to fosfomycin. Compared to agar dilution, disk diffusion was more accurate than Etest.
The worldwide spread of blaKPC-possessing Klebsiella pneumoniae (KpKPC) isolates represents a serious threat to our health care systems (20). KpKPC isolates are responsible for hospital outbreaks in the United States, Israel, and Greece with mortality rates of approximately 35% (14, 18, 23, 26).
KpKPC isolates are nonsusceptible (NS) in vitro to all β-lactams, including β-lactam/β-lactamase inhibitor combinations and carbapenems, quinolones, and frequently, aminoglycosides (10, 22). Thus, our therapeutic options against infections due to KpKPC are often limited to tigecycline and colistin. Unfortunately, an increasing number of colistin- and/or tigecycline-NS KpKPC isolates have been observed recently, primarily in the New York City area (1, 15). KpKPC isolates that are both colistin and tigecycline NS are defined as “extremely drug-resistant” (XDR) strains because all available standard antibiotics are ineffective in vitro (22). The spread of these XDR K. pneumoniae isolates may have devastating effects on patient outcomes (7).
As a result of this urgency, new therapeutic strategies against KpKPC isolates need to be rapidly devised and implemented. Thus far, there are a few novel compounds in development that promise to be active (9, 11). However, these new drugs are currently in clinical trials and it will take a few years to see if their promise holds true in the clinic.
Fosfomycin is an “old” antimicrobial that inhibits the first step of peptidoglycan synthesis and shows potent bactericidal action against many Gram-negative and Gram-positive pathogens (17). Fosfomycin tromethamine, an oral formulation, is approved in the United States and other countries for the treatment of uncomplicated urinary tract infections (UTIs) caused by Escherichia coli or Enterococcus faecalis (21, 27). In some European countries and Japan, fosfomycin disodium is also available for parenteral use. The drug shows little toxicity, and very high peak levels can be achieved in serum and urine (12, 13). Interestingly, fosfomycin rapidly penetrates tissues (12, 24), a property that is highly desirable in the treatment of serious infections. Unfortunately, resistance develops rapidly when fosfomycin is used as monotherapy (12, 19). Since fosfomycin shows synergistic action with other antimicrobials (2), it is used in combination to treat a wide range of infections, including pneumonia and septicemia. Overall, cure rates of >80% are observed (12, 13). Surprisingly, data regarding in vitro and in vivo activity of fosfomycin against KpKPC isolates are lacking.
In the present work, we analyzed the in vitro activity of fosfomycin against a collection of 68 KpKPC clinical isolates. Forty-two isolates were previously characterized strains collected in the Eastern United States (8, 10, 11), whereas the remaining 26 were recently (January to July 2009) isolated in institutions located in New York City (n = 17) and Cleveland, OH (n = 9).
MICs for tigecycline and colistin were obtained using the Etest method (AB bioMérieux) on Mueller-Hinton agar (MHA; BBL, Becton Dickinson). The results for tigecycline were interpreted according to the U.S. FDA criteria, whereas those for colistin were interpreted according to Clinical and Laboratory Standards Institute (CLSI) criteria established for organisms that are not members of the Enterobacteriaceae (i.e., susceptibility of ≤2 μg/ml for both antimicrobials) (5).
Susceptibility to fosfomycin was determined using three methods: agar dilution (AD), disk diffusion (DD), and Etest. AD was performed using fosfomycin disodium salt (Sigma-Aldrich Co.) on MHA containing 25 μg/ml of glucose-6-phosphate (G6P; Roche Diagnostics), employing a Steers replicator that delivered 104 CFU/10-μl spot (4, 5). Disk diffusion was carried out on MHA with disks (BBL, Becton Dickinson) containing 200 μg of fosfomycin and 50 μg of G6P (5). Etest was performed on MHA containing G6P, following the manufacturer's instructions. Since CLSI criteria to evaluate fosfomycin susceptibility in K. pneumoniae are not available, results were interpreted according to guidelines approved for Escherichia coli in UTIs (i.e., susceptible at MICs of ≤64 μg/ml or with zones of ≥16 mm) (5); these breakpoints have been used by authors of similar studies (6, 16, 17, 25). ATCC strains Escherichia coli 25922 and Pseudomonas aeruginosa 27853 were used as controls for all experiments.
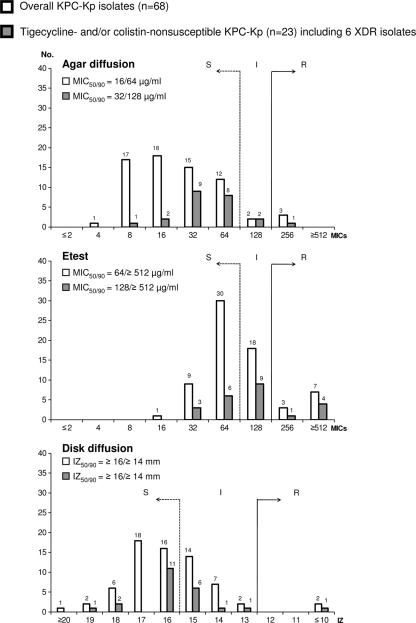
Among the 68 KpKPC isolates analyzed, 23 were tigecycline and/or colistin NS (i.e., 5 tigecycline NS, 12 colistin NS, and 6 XDR). In Fig. 1, we present the susceptibility results for fosfomycin obtained with the AD, Etest, and DD. By AD, an overall susceptibility of 92.6% (MIC50 and MIC90 of 16 and 64 μg/ml, respectively) was found. The subgroup of tigecycline- and/or colistin-NS isolates showed susceptibility rates of 87.0% (MIC50 and MIC90 of 32 and 128 μg/ml, respectively). Notably, fosfomycin was active in vitro against five of the six XDR KpKPC isolates (i.e., two with MICs of 32 μg/ml, three with MICs of 64 μg/ml, and one with a MIC of 256 μg/ml). By Etest and DD, overall susceptibility rates of approximately 60% were recorded (Fig. 1).
FIG. 1.
MIC distributions for fosfomycin were obtained using agar diffusion and Etest (MICs were adjusted up to the next highest doubling concentration, e.g., from 48 to 64 μg/ml). Inhibition zone (IZ) diameter distribution was obtained with disk diffusion. Results were interpreted according to CLSI criteria for E. coli (5). The number of KpKPC isolates that had each result is presented above the bars. Dashed line, susceptible cutoff; solid line, resistant cutoff; S, susceptible; I, intermediate; R, resistant.
The results of Etest and DD were compared with those obtained from the AD, used as the reference method, to establish their ability to characterize fosfomycin susceptibility. Essential agreement (EA), categorical agreement (CA), minor errors (MiE), major errors (MaE), and very major errors (VME) were calculated (see definitions in Table 1). CLSI recommends that <10% MiE, <3% MaE, and <1.5% VME should be obtained to approve the performance of susceptibility tests (3).
TABLE 1.
Agreement of the Etest and disk diffusion methods with agar dilution in testing susceptibility to fosfomycin
| Method | No. (%) of isolates with indicated resulta |
|||||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | EA | CA | MiE | MaE | VME | |
| Etest | 42 (61.8) | 16 (23.5) | 10 (14.7) | 25 (36.8) | 46 (67.6) | 16 (23.5) | 7 (16.7) | 0 (0.0) |
| DD | 43 (63.2) | 23 (33.8) | 2 (2.9) | NA | 45 (66.2) | 23 (33.8) | 0 (0.0) | 0 (0.0) |
S, susceptible; I, intermediate; R, resistant; NA, not applicable; EA, essential agreement (MICs of Etest equal to or within ±1 dilution of the agar dilution [AD] value); CA, categorical agreement (AD and Etest or disk diffusion [DD] agree using the interpretative CLSI criteria); MiE, minor errors (Etest or DD are S or R and AD is I; alternatively, Etest or DD are I and AD is S or R); MaE, major errors (Etest or DD are R and AD is S; the percentage of major errors was calculated only for S isolates); VME, very major errors (Etest or DD are S and AD is R; the percentage of very major errors was calculated only for R isolates).
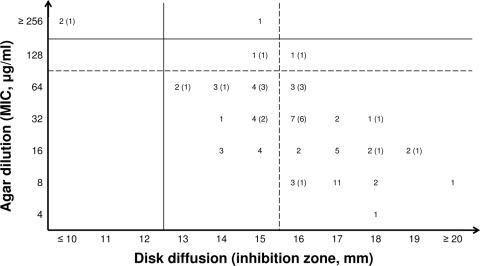
By Etest, VME were not observed, but 23.5% MiE and 16.7% MaE were found (Table 1). We concluded that the Etest is not a reliable method to test fosfomycin MICs against KpKPC strains. These findings are consistent with those reported for fosfomycin against extended-spectrum β-lactamase-producing K. pneumoniae isolates (16). Using DD, MaE and VME were not found. However, 33.8% MiE were recorded. As shown in Fig. 2, this phenomenon is primarily due to numerous KpKPC isolates (n = 21) that have inhibitory diameters of 15 (upper end of the intermediate range), 14, or 13 mm by DD but were susceptible by AD.
FIG. 2.
Comparison of the phenotypic test results obtained with the two methods suggested by the CLSI (i.e., disk diffusion and agar dilution). The overall number of KpKPC isolates that had each result is presented. Results for the subgroup of tigecycline- and/or colistin-NS isolates are reported in parentheses. Dashed lines, susceptibility cutoffs; solid lines, resistance cutoffs.
In conclusion, fosfomycin demonstrates in vitro activity against contemporary KPC-producing K. pneumoniae isolates, representing a possible alternative to tigecycline and colistin. An important consideration is that fosfomycin may be a “salvage” therapy for the growing number of infections due to XDR KpKPC isolates. However, we note that the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org) has established fosfomycin clinical breakpoints for Enterobacteriaceae that are lower (i.e., susceptible at MICs of ≤32 μg/ml and resistant at MICs of ≥64 μg/ml) than those of CLSI. These different cutoffs could drive the overall susceptibility of our collection to 75%, but our results still demonstrate that fosfomycin is a possible option in our therapeutic armamentarium against infections due to KpKPC isolates. Since AD is a time-intensive method, DD seems to be the most practical system to evaluate fosfomycin susceptibility among KpKPC isolates. However, inhibitory diameters in the intermediate ranges should be confirmed with the AD method. Efforts are under way to define the pharmacokinetics of fosfomycin against KpKPC isolates and the optimum partner to pair with this antimicrobial in order to further enhance activity and suppress resistant mutants.
Acknowledgments
This work was supported in part by the Veterans Affairs Merit Review Program (to R.A.B.), the National Institutes of Health (grants RO1-AI063517 and RO3-AI081036 to R.A.B. and grant RO1-AI045626 to L.B.R.), and the Geriatric Research Education and Clinical Center VISN 10 (to R.A.B.).
Footnotes
Published ahead of print on 9 November 2009.
REFERENCES
- 1.Bratu, S., P. Tolaney, U. Karumudi, J. Quale, M. Mooty, S. Nichani, and D. Landman. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128-132. [DOI] [PubMed] [Google Scholar]
- 2.Cai, Y., Y. Fan, R. Wang, M. M. An, and B. B. Liang. 2009. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J. Antimicrob. Chemother. 64:563-566. [DOI] [PubMed] [Google Scholar]
- 3.CLSI. 2008. Development of in vitro susceptibility testing criteria and quality control parameters, 3rd ed. Approved guidelines; CLSI document M23-A3. CLSI, Wayne, PA.
- 4.CLSI. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Approved standard; CLSI document M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.CLSI. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.de Cueto, M., L. Lopez, J. R. Hernandez, C. Morillo, and A. Pascual. 2006. In vitro activity of fosfomycin against extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob. Agents Chemother. 50:368-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elemam, A., J. Rahimian, and W. Mandell. 2009. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin. Infect. Dis. 49:271-274. [DOI] [PubMed] [Google Scholar]
- 8.Endimiani, A., L. L. Carias, A. M. Hujer, C. R. Bethel, K. M. Hujer, F. Perez, R. A. Hutton, W. R. Fox, G. S. Hall, M. R. Jacobs, D. L. Paterson, L. B. Rice, S. G. Jenkins, F. C. Tenover, and R. A. Bonomo. 2008. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob. Agents Chemother. 52:2680-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endimiani, A., Y. Choudhary, and R. A. Bonomo. 2009. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob. Agents Chemother. 53:3599-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endimiani, A., A. M. Hujer, F. Perez, C. R. Bethel, K. M. Hujer, J. Kroeger, M. Oethinger, D. L. Paterson, M. D. Adams, M. R. Jacobs, D. J. Diekema, G. S. Hall, S. G. Jenkins, L. B. Rice, F. C. Tenover, and R. A. Bonomo. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J. Antimicrob. Chemother. 63:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endimiani, A., K. M. Hujer, A. M. Hujer, E. S. Armstrong, Y. Choudhary, J. B. Aggen, and R. A. Bonomo. 2009. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 53:4504-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falagas, M. E., K. P. Giannopoulou, G. N. Kokolakis, and P. I. Rafailidis. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 46:1069-1077. [DOI] [PubMed] [Google Scholar]
- 13.Falagas, M. E., A. C. Kastoris, D. E. Karageorgopoulos, and P. I. Rafailidis. 2009. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int. J. Antimicrob. Agents 34:111-120. [DOI] [PubMed] [Google Scholar]
- 14.Kitchel, B., J. K. Rasheed, J. B. Patel, A. Srinivasan, S. Navon-Venezia, Y. Carmeli, A. Brolund, and C. G. Giske. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53:3365-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, J., G. Patel, S. Huprikar, D. P. Calfee, and S. G. Jenkins. 2009. Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J. Clin. Microbiol. 47:1611-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Cerero, L., M. de Cueto, M. A. Diaz-Guerrero, C. Morillo, and A. Pascual. 2007. Evaluation of the Etest method for fosfomycin susceptibility of ESBL-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 59:810-812. [DOI] [PubMed] [Google Scholar]
- 17.Maraki, S., G. Samonis, P. I. Rafailidis, E. K. Vouloumanou, E. Mavromanolakis, and M. E. Falagas. 2009. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob. Agents Chemother. 53:4508-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navon-Venezia, S., A. Leavitt, M. J. Schwaber, J. K. Rasheed, A. Srinivasan, J. B. Patel, and Y. Carmeli. 2009. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob. Agents Chemother. 53:818-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson, A. I., O. G. Berg, O. Aspevall, G. Kahlmeter, and D. I. Andersson. 2003. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 47:2850-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordmann, P., G. Cuzon, and T. Naas. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228-236. [DOI] [PubMed] [Google Scholar]
- 21.Patel, S. S., J. A. Balfour, and H. M. Bryson. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637-656. [DOI] [PubMed] [Google Scholar]
- 22.Paterson, D. L., and Y. Doi. 2007. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin. Infect. Dis. 45:1179-1181. [DOI] [PubMed] [Google Scholar]
- 23.Pournaras, S., E. Protonotariou, E. Voulgari, I. Kristo, E. Dimitroulia, D. Vitti, M. Tsalidou, A. N. Maniatis, A. Tsakris, and D. Sofianou. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 64:348-352. [DOI] [PubMed] [Google Scholar]
- 24.Schintler, M. V., F. Traunmuller, J. Metzler, G. Kreuzwirt, S. Spendel, O. Mauric, M. Popovic, E. Scharnagl, and C. Joukhadar. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J. Antimicrob. Chemother. 64:574-578. [DOI] [PubMed] [Google Scholar]
- 25.Schito, G. C., K. G. Naber, H. Botto, J. Palou, T. Mazzei, L. Gualco, and A. Marchese. 2009. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 34:407-413. [DOI] [PubMed] [Google Scholar]
- 26.Schwaber, M. J., and Y. Carmeli. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911-2913. [DOI] [PubMed] [Google Scholar]
- 27.Warren, J. W., E. Abrutyn, J. R. Hebel, J. R. Johnson, A. J. Schaeffer, and W. E. Stamm. 1999. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin. Infect. Dis. 29:745-758. [DOI] [PubMed] [Google Scholar]