Abstract
We present a simple assay to examine effects of compounds on virulence gene expression in the human pathogen Staphylococcus aureus. The assay employs transcriptional reporter strains carrying lacZ fused to central virulence genes. Compounds affecting virulence gene expression and activity of the agr locus are scored based on color change in the presence of a chromogenic β-galactosidase substrate. The assay can be used to screen for novel antivirulence compounds from many different sources, such as fungi, as demonstrated here.
Staphylococcus aureus is a significant human pathogen that causes a variety of diseases ranging from minor skin infections to life-threatening endocarditis and septicemia (2). A contributing factor to the severity of staphylococcal infections is the ability of the organism to acquire resistance to numerous antibiotics (22). The growing problems of antibiotic-resistant microorganisms have necessitated alternative therapeutic strategies, including antivirulence therapy (3, 6). The aim of antivirulence therapy is to silence virulence gene expression, allowing the host immune system time to act and eradicate the pathogen. In S. aureus, the ability to cause disease relies on the timely production of an impressive collection of virulence factors. During exponential growth, the cell surface-located virulence factors are expressed, including spa, encoding protein A, while upon entry into stationary phase, transcription of hla, encoding α-hemolysin, and other genes encoding extracellular factors are induced (15). This regulation is mediated partly by the agr quorum sensing system, composed of a two-component system and a regulatory RNA molecule, RNAIII, that is synthesized in response to increasing concentrations of autoinducer peptide (AIP), also encoded by the agr locus (17, 18). In order to search for new agents that interfere with S. aureus virulence gene expression, we have developed a simple plate assay that allows an easy screen of compounds from a diverse range of biological sources.
Results and Discussion.
In order to examine the influence of novel compounds on hla and spa gene expression in S. aureus, we incorporated the 8325-4 (16)-derived hla::lacZ (PC322; Eryr) (5) and spa::lacZ (PC203; Eryr) (5) fusion strains in tryptic soy agar (TSA) (Oxoid) plates containing erythromycin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Briefly, 1 ml of 10−3-diluted overnight culture (tryptone soya broth [TSB]; Oxoid) was placed in a petri dish to which 25 ml of TSA (∼40°C) containing 150 μg ml−1 X-Gal and 5 μg·ml−1 erythromycin was added and mixed by careful whirling. The plates were dried, and wells were formed with a sterile drill. When incubated at 37°C for optimized periods of time, the plates containing PC203 (spa::lacZ) became light blue while those containing PC322 (hla::lacZ) became intensely blue, consistent with the Agr-dependent reciprocal regulation of cell-associated and secreted virulence determinants (15) (Fig. 1).
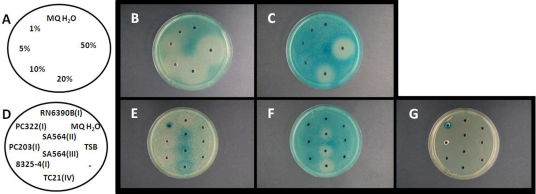
FIG. 1.
Effect of glucose and AIPs on expression of hla and spa. To agar plates containing 8325-4-derived strain PC203 (spa::lacZ; agr group I) (B and E), PC322 (hla::lacZ; agr group I) (C and F), or no cells (G), 30 μl of either a glucose solution (B and C) with the indicated concentrations (A) or spent supernatants (E and F) from the agr groups indicated (D) were added. The plates were incubated at 11 h (F), 19 h (B and C), or 26 h (E and G) at 37°C.
To verify that the assay can visualize established effects of known compounds on virulence gene expression, we added 30 μl of 1%, 5%, 10%, 20%, and 50% glucose solutions to the wells. In three independent experiments, we found that glucose markedly reduced the expression of hla as shown by a large white zone surrounding the well (Fig. 1B and C), confirming previous observations (21). The decreased expression of both spa and hla supports recent findings that glucose affects virulence gene expression independently of agr (24).
Another well-known mode of virulence regulation is the cross talk between different agr specificity classes of S. aureus strains, of which 4 different classes are known (10, 11). Hereby the autoinducing peptide of one class induces agr of strains belonging to the same class but inhibits agr of other classes (11). To visualize this phenomenon, we prepared spent supernatants of RN6390B and 8325-4 (AIP group I), AIP group II and III derivatives of SA564, and TC21 (AIP group IV; kindly provided by P. Hill). When spent supernatants (collected from overnight cultures in TSB) were added to the wells, we repeatedly observed that the supernatants of S. aureus strains expressing AIP groups II, III, and IV reduced β-galactosidase expression of the hla reporter strain PC322 (Fig. 1F) and increased expression of the spa reporter strain PC203 (Fig. 1E), correlating with both reporter strains being derivatives of the AIP group I strain (5). Spent supernatant of PC322 revealed a strong blue inner zone surrounding the well, resulting from β-galactosidase present in the culture supernatant, as verified from an agar plate containing only X-Gal but no reporter strain (Fig. 1E and G).
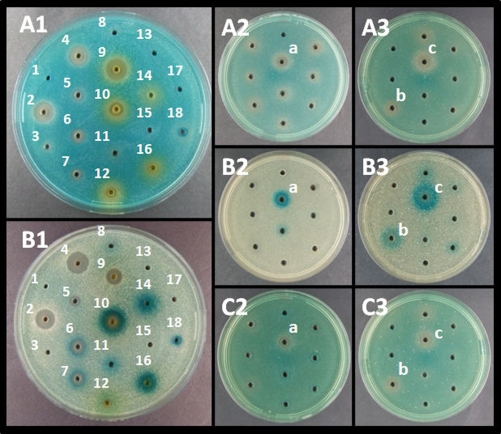
In order to use the developed plate assay for identification of compounds that modulate S. aureus virulence gene expression, we prepared extracts from various fungi within the genera Penicillium, Aspergillus, and Trichoderma (12). Extracts were made from 7- to 9-day-old cultures grown at 25°C as 3 point inoculations on either yeast extract sucrose agar, potato dextrose agar, Czapek yeast extract agar, malt extract agar, or oatmeal agar (8, 23). Mycelium together with agar was transferred to stomacher bags with 30 ml extraction mixture (ethyl acetate, dichloromethane, methanol [3:2:1 vol/vol] plus 1% formic acid), subjected to stomacher treatment for 2 min, and left for 30 min. The extract was filtered through Whatmann 4 filter paper, evaporated to dryness using N2, and dissolved in 0.6 ml 80% ethanol (ETOH) per plate. The ability of the extracts to modulate hla and spa expression was evaluated (Fig. 2A1 and B1). One extract from Penicillium algidum (IBT 24414) grown on yeast extract sucrose agar, which showed pronounced agr inhibitory activity as visualized by enhanced spa expression and decreased hla expression (Fig. 2A2 and B2), also repressed expression of RNAIII as monitored by an 8325-4-derived rnaIII::lacZ fusion strain (9) (Fig. 2C2). In order to identify the active constituents, this extract was fractionated by semipreparative high-performance liquid chromatography (HPLC) using a Phenomenex Luna C18 (2) column (250 mm by 10 mm, 5 μm; Torrance, CA). A 3.0-mg amount of extract was injected into H2O-MeCN (80:20) and collected into 25 fractions (each of 1 min) running a gradient at 4 ml/min up to 100% MeCN in 20 min with a holding time of 5 min (19). Fractions 13 and 15 proved to carry Agr inhibitory activity (Fig. 2,A3 B3, and C3).
FIG. 2.
Effect of fungal extracts on virulence gene expression. To agar plates containing PC322 hla::lacZ (A1 to A3), PC203 spa::lacZ (B1 to B3), or SH101F7 rnaIII::lacZ (C2 and C3) were added 30 μl of either crude fungal extracts (A1 and B1) or extract fractions (A2 through C3). Crude fungal extract wells included sterile water (well 1); Trichoderma asperellum (wells 2 to 8); Aspergillus niger extracts isolated from yeast extract sucrose agar (wells 9 to 11), Czapek yeast extract agar (wells 12 to 13), oatmeal agar (wells 14 to 15), and malt extract agar (wells 16 and 17). Well 18 contained spent supernatant from PC322. A crude extract was obtained from Penicillium algidum isolated from yeast extract sucrose agar (well a) that upon further fractionation displayed activity in fraction 13 (well b) and fraction 15 (well c). The plates were incubated for 12 h (A2, A3, C2, and C3), 28 h (A1), 40 h (B3), 60 h (B2), or 86 h (B1) at 37°C.
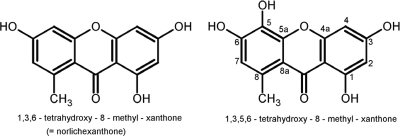
Fractions 13 and 15 were analyzed by analytical HPLC-UV/visible-diode array detection-high-resolution mass spectrometry (HPLC-DAD-HRMS) with positive electrospray ionization (13, 14). Detected peaks in UV and/or mass spectrometry were matched against the Antibase 2008 database (John Wiley and Sons, Inc.) (13). HPLC-DAD-HRMS analysis revealed that fraction 13 contained a single compound and that fraction 15 contained two compounds. One of the compounds in fraction 15 was easily recognized as griseofulvin due to its characteristic UV spectrum and characteristic isotope pattern (14, 20). The UV spectrum of the other major compound in fraction 15 and the single major compound in fraction 13 proved to be practically identical, displaying the characteristics of xanthones as reported in Antibase. Mass spectrometry analysis of the xanthone in fraction 15 indicated it to be norlichexanthone, which was confirmed by analysis of an authentic standard. When tested, pure commercial griseofulvin (Sigma) did not show any activity in the assay (data not shown), indirectly proving that norlichexanthone is the active component of fraction 15. The elemental composition of the xanthone (C14H10O6) in fraction 13 showed an additional oxygen atom, which along with the earlier retention time indicated a norlichexanthone analogue with an additional hydroxyl group. A search in Antibase revealed that two xanthones with this formula are known where the additional hydroxyl group is either placed in the 5 position (Fig. 3) (4) or in the 7 position (1). Further purification by preparative liquid chromatography generated 1.5 mg of the more-polar xanthone for nuclear magnetic resonance (NMR) studies (500 MHz). NMR analysis of the proton (1H), heteronuclear multiple quantam coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) data proved the hypothesis of a further hydroxyl group since only three aromatic protons could be detected. The additional hydroxy group could be assigned to the 5 position, since a singlet proton at 6.62 ppm was assigned to position 7 due to clear HMBC correlations to C5, C8a, and 8-CH3 in accordance with the work of Belofsky et al., who also reported that 1,3,5,6-tetrahydroxy-8-methyl-xanthone has antibacterial activity against Staphyloccocus aureus (4).
FIG. 3.
Structures of 1,3,6-trihydroxy-8-methyl-xanthone (norlichexanthone) and 1,3,5,6-trihydroxy-8-methyl-xanthone, shown to be antivirulence compounds against Staphylococcus aureus.
Collectively, we have established a simple, inexpensive assay to screen compounds for their ability to interfere with S. aureus virulence gene expression. The assay allowed us to examine how complex mixtures of compounds at multiple concentrations affect virulence gene expression. From P. algidum, we obtained two fractions containing hydroxyxanthones that increased spa and decreased hla expression and to a minor extent reduced rnaIII expression. Our results suggest that hydroxyxanthones interfere with S. aureus virulence gene expression, possibly through modulation of Agr activity. However, additional experimentation will be required to assess the clinical potential of the identified compounds.
Acknowledgments
We thank Charlotte H. Gotfredsen for NMR data aquisition, S. Foster and P. Hill for kindly providing strains, and the Research Council for Technology and Production for supporting A. Nielsen.
Footnotes
Published ahead of print on 16 November 2009.
REFERENCES
- 1.Abdel-Lateff, A., C. Klemke, G. M. König, and A. D. Wright. 2003. Two new xanthone derivatives from algicolous marine fungus Wardomyces anomalus. J. Nat. Prod. 66:706-708. [DOI] [PubMed] [Google Scholar]
- 2.Archer, G. L. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179-1181. [DOI] [PubMed] [Google Scholar]
- 3.Balaban, N., T. Goldkorn, R. T. Nhan, L. B. Dang, S. Scott, R. M. Ridgley, A. Rasooly, S. C. Wright, J. W. Larrick, R. Rasooly, and J. R. Carlson. 1998. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science 280:438-440. [DOI] [PubMed] [Google Scholar]
- 4.Belofsky, G. N., K. B. Gloer, J. B. Gloer, D. T. Wicklow, and P. F. Dowd. 1998. New p-terphenyl and polyketide metabolites from the sclerotia of Penicillium raistrickii. J. Nat. Prod. 61:1115-1119. [DOI] [PubMed] [Google Scholar]
- 5.Chan, P. F., and S. J. Foster. 1998. The role of environmental factors in the regulation of virulence-determinant expression in Staphylococcus aureus 8325-4. Microbiology 144:2469-2479. [DOI] [PubMed] [Google Scholar]
- 6.Clatworthy, A. E., E. Pierson, and D. T. Hung. 2007. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3:541-548. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Frisvad, J. C., J. Smedsgaard, T. O. Larsen, and R. A. Samson. 2004. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 49:201-241. [Google Scholar]
- 9.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarraud, S., G. J. Lyon, A. M. Figueiredo, L. Gerard, F. Vandenesch, J. Etienne, T. W. Muir, and R. P. Novick. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182:6517-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 12.Larsen, T. O., J. Smedsgaard, K. F. Nielsen, M. E. Hansen, and J. C. Frisvad. 2005. Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Nat. Prod. Rep. 22:672-695. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen, K. F., T. Graefenhan, D. Zafari, and U. Thrane. 2005. Trichothecene production by Trichoderma brevicompactum. J. Agric. Food Chem. 53:8190-8196. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen, K. F., and J. Smedsgaard. 2003. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A 1002:111-136. [DOI] [PubMed] [Google Scholar]
- 15.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 16.Novick, R. P., and S. I. Morse. 1967. In vivo transmission of drug resistance factors between strains of Staphylococcus aureus. J. Exp. Med. 125:45-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rank, C., R. K. Phipps, P. Harris, J. C. Frisvad, C. H. Gotfredsen, and T. O. Larsen. 2006. epi-Aszonalenins A, B, and C from Aspergillus novofumigatus. Tetrahedron Lett. 47:6099-6102. [Google Scholar]
- 20.Rebacz, B., T. O. Larsen, M. H. Clausen, M. H. Rønnest, H. Löffler, A. D. Ho, and A. Krämer. 2007. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 67:6342-6350. [DOI] [PubMed] [Google Scholar]
- 21.Regassa, L. B., R. P. Novick, and M. J. Betley. 1992. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect. Immun. 60:3381-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakoulas, G., and R. C. Moellering, Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin. Infect. Dis. 46(Suppl. 5):S360-S367. [DOI] [PubMed] [Google Scholar]
- 23.Samson, R. A., E. S. Hoekstra, J. C. Frisvad, and O. Filtenborg. Introduction to food- and airborne fungi. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 24.Seidl, K., M. Stucki, M. Ruegg, C. Goerke, C. Wolz, L. Harris, B. Berger-Bachi, and M. Bischoff. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]