Abstract
The feasibility, safety, and efficacy of prolonged, continuous, intravenous clindamycin therapy were retrospectively evaluated for 70 patients treated for bone and joint infections, 40% of whom were treated as outpatients. The median treatment duration was 40 days, the median daily clindamycin dose was 2,400 mg, and three moderate-grade adverse events occurred. The median serum clindamycin concentrations on days 3 to 14 and days 8 to 28 were 5 and 6.2 mg/liter, respectively; the median concentration was significantly lower (P < 0.02) in patients treated with rifampin (5.3 mg/liter) than in those not treated with rifampin (8.9 mg/liter). Among 53 patients with a median follow-up of 30 months (range, 24 to 53 months), 49 (92%) were considered cured (1 patient had a relapse, and 3 patients had reinfections).
The optimization of antibiotic regimens remains a major issue in the management of bone and joint infections, because no consensus guidelines are currently available (15, 26). Clindamycin (7-chloro-7-deoxy-lincomycin) is a valuable option, because this lincosamide antibiotic is active against staphylococci, streptococci, and anaerobic bacteria (25); has high levels of joint and bone penetration (7, 9, 11, 21, 22, 23, 27); inhibits biofilm formation and bacterial adherence (6, 18, 19); and is well tolerated (11, 12). Its efficacy has been established in several experimental models (13, 16), but only a few series on the clindamycin treatment of human bone and joint infections have been reported (10-12).
In our Referral Center for the Treatment of Bone and Joint Infections, clindamycin is a drug of choice for the treatment of susceptible staphylococcal, streptococcal, and gram-positive anaerobic bacterial infections. To optimize the efficacy of the drug (14), we administer clindamycin via continuous intravenous infusion, because its antibacterial activity is time dependent (1, 5).
The aim of this study was to evaluate retrospectively the feasibility, tolerability, and efficacy of prolonged administration of continuous intravenous clindamycin in our cohort of patients and to determine the serum clindamycin concentrations. Because serum clindamycin concentrations were frequently low in patients also receiving rifampin, we compared the serum concentrations of patients receiving combined therapy with and without rifampin.
MATERIALS AND METHODS
Patients.
This retrospective cohort study included all the patients treated in our center for a bone and/or joint infection with continuous intravenous clindamycin for ≥1 week and for whom at least one clindamycin concentration determination was performed. All the patients gave written informed consent before inclusion.
All the pathogens were clindamycin susceptible, as determined by the standard disk diffusion method of the Société Française de Microbiologie (MIC < 2 mg/liter), and none of them had inducible resistance to clindamycin. All the Staphylococcus strains were erythromycin susceptible.
Drug administration.
Clindamycin, administered intravenously through a central venous catheter, was initiated in our referral center with a 600-mg loading dose infused over 60 min, followed immediately by the continuous infusion of 30 to 40 mg/kg of body weight/day. For the continuous infusion, clindamycin was dissolved in 50 ml of 5% dextrose and was administered over a 12-h period twice daily via an infusion pump. All but one patient received two antibiotics; one patient received clindamycin alone.
For patients receiving parenteral outpatient therapy, antibiotic therapy was administered twice daily by a nurse through an infusion pump. Intravenous therapy was followed by an oral regimen so that the patients completed 12 weeks of treatment for prosthetic joint infections, osteomyelitis, and osteoarthritis and 6 to 8 weeks of treatment for spondylodiscitis or acute septic arthritis.
The patients were monitored for treatment and adverse events (AEs), as described previously (30).
Blood sampling and drug analysis.
The first blood sample was obtained between days 3 and 14 of clindamycin administration. For 47 patients, a second sample was obtained between days 8 and 28, with a minimum of 5 days between the times of collection of the two samples. Samples were assayed as described before (30). Multiresistant Staphylococcus aureus SJ11617 (rifampin MIC, >128 mg/liter; fosfomycin MIC, >512 mg/liter; fluoroquinolone MICs, >64 mg/liter; fusidic acid MIC, >256 mg/liter) was used as the indicator organism. Penicillins and cephalosporins were inactivated by β-lactamase or cephalosporinase; aminoglycosides were inactivated by cellulose-phosphate powder. The limit of quantification was 0.2 mg/liter, and the variation range was 5 to 11%.
The target serum steady-state concentration was 5 to 8 mg/liter, with lower and higher values being considered to be underdosing and overdosing, respectively; the patients' daily clindamycin doses were increased or decreased (25% of the daily dose) accordingly.
Outcome.
The outcome analysis considered only patients treated with continuous intravenous clindamycin for ≥2 weeks. Patients were assessed for follow-up at 6, 12, 24, and 52 weeks and then once a year; for patients who had not been seen for >1 year, they or their general practitioners were contacted by phone. The following events, defined previously (30), were recorded: relapse, reinfection, and death.
Statistical analysis.
Two patient groups were established: those who received clindamycin with rifampin or those who received clindamycin without rifampin. Student's t test was used to compare quantitative variables, with P values of <0.05 considered significant.
RESULTS
Patient and treatment characteristics.
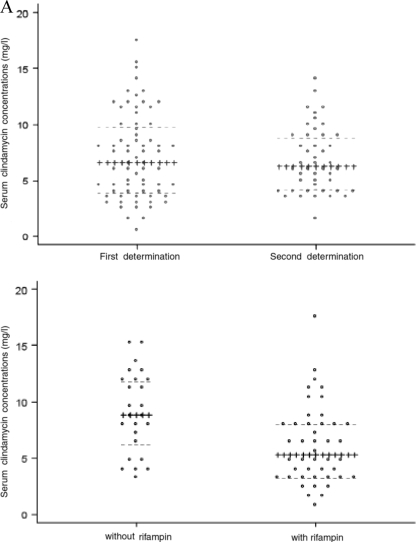
Seventy consecutive patients hospitalized between August 2004 and May 2007 were included in the study; 67 underwent surgery for their bone and/or joint infections. Their demographic and clinical characteristics and creatinine clearances are given in Table 1, the infection site and the pathogen(s) isolated are given in Table 2, the clindamycin treatment characteristics are given in Table 3, and the serum steady-state concentrations are given in Fig. 1A.
TABLE 1.
Main demographic and clinical characteristics of the 70 patients treated with continuous intravenous clindamycin
| Characteristic | Value |
|---|---|
| Demographic | |
| Median (range) age (yr) | 62 (18-88) |
| No. of males | 37 |
| Clinical | |
| No. of patients with American Society of | |
| Anesthesiology score of ≥3a | 11 |
| Median (range) wt (kg) | 74 (45-114) |
| Median (range) creatinine clearance (ml/min) | 101 (10-200) |
| No. of patients with the following comorbidities: | 34b |
| Cardiovascular disease | 11 |
| Diabetes mellitus | 10 |
| Chronic inflammatory rheumatic disease | 6 |
| Malignancy | 3 |
| Obesity (body mass index ≥ 30) | 4 |
| Chronic viral hepatitis/cirrhosis | 1/1 |
| Neuropsychiatric impairment | 6 |
| Other | 4 |
See reference 8.
Thirteen patients had two comorbidities.
TABLE 2.
Type of infection in and pathogens isolated from the 70 patients treated with continuous intravenous clindamycin
| Characteristic | No. of patients |
|---|---|
| Type of infection | |
| Joint arthroplasty infection | 44 |
| Chronic osteomyelitis | 11 |
| Septic arthritis/osteoarthritis | 11 |
| Spondylodiscitis | 2 |
| Miscellaneous | 2 |
| Pathogen isolated | |
| Staphylococcus aureus | 25 |
| Coagulase-negative staphylococci | 25 |
| Streptococcus spp. | 2 |
| Gram-positive anaerobic bacteriaa | 5 |
| Polymicrobial | 9 |
| Undetermined | 4 |
Propionibacterium acnes and others.
TABLE 3.
Main therapeutic characteristics of the 70 patients treated with continuous intravenous clindamycin
| Therapeutic characteristic | Value |
|---|---|
| Median (range) daily dose (mg) | 2,400 (1,200-3,600) |
| Median (range) duration of treatment (days) | 40 (7-63) |
| No. of patients receiving the following antibiotic in combination with clindamycin: | |
| Gentamicin followed by rifampin | 32 |
| Rifampin | 24 |
| Othera | 14 |
| No. of patients receiving a dose adaptation | 33b |
| Increase | 17 |
| Decrease | 20 |
| No. of patients receiving outpatient parenteral | |
| antibiotic therapy | 28 |
| No. of patients with adverse events | 3 |
Other antibiotics combined with clindamycin: a beta-lactam for five patients, minocycline for three patients, gentamicin or pefloxacin for two patients each, and fusidic acid or no other antibiotic for one patient each.
The daily dose was increased and decreased for four patients.
FIG. 1.
Individual (points) and median (horizontal lines) clindamycin steady-state concentrations at the first determination (days 3 to 14; median concentration, 6.6 mg/liter; first and third quartile concentrations, 4 and 9.8 mg/liter, respectively) and the second determination (days 8 to 28; median concentration, 6.2 mg/liter; first and third quartile concentrations, 4.2 and 8.9 mg/liter, respectively) (A) and in patients treated with clindamycin without rifampin (median concentration, 8.9 mg/liter; first and third quartile concentrations, 6.2 and 11.9 mg/liter, respectively) and clindamycin with rifampin (median concentration, 5.3; first and third quartile concentrations, 3.2 and 8.1 mg/liter, respectively) (P < 0.02) (B).
Three patients experienced clindamycin-related AEs, all of which were classified as Common Terminology Criteria of Adverse Events grade 2. These AEs were an allergic rash, non-Clostridium difficile-related diarrhea, and cytolytic hepatitis. After the withdrawal of clindamycin, their AEs disappeared.
Outcomes.
Sixty patients received continuous intravenous clindamycin for ≥2 weeks. Among them, three died from unrelated causes (cardiac insufficiency, septic shock after transurethral resection of the prostate, pancreatic cancer) within 2 years. Four other patients received long-term suppressive antibiotic therapy. The data for these seven patients were excluded from the outcome analysis.
Among the 53 remaining patients, the median duration of follow-up was 30 months (range, 24 to 53 months). One patient's prosthetic knee infection relapsed with the same methicillin-resistant S. aureus strain that had become clindamycin resistant. No cause of relapse was identified, and the serum clindamycin concentrations were within the target range. Three patients with knee arthroplasty infections developed prosthesis reinfections. No infection or treatment-related death occurred.
Overall, 49 (92%) patients were considered to be cured.
Statistical analysis.
The median clindamycin steady-state concentration was significantly lower with combined therapy with clindamycin and rifampin than with clindamycin without rifampin (Fig. 1B).
DISCUSSION
The results of our study of 70 consecutive patients treated with clindamycin as a prolonged continuous intravenous infusion showed that this mode of administration is feasible, convenient, well tolerated, and safe. To the best of our knowledge, this is the first report on the use of this way of administration and one of the largest cohorts to have received clindamycin for the treatment of bone and joint infections. Only three reports of retrospective studies of clindamycin treatment for these infections have been published to date (10-12). Two studies, published >30 years ago, gave clindamycin monotherapy to 48 children (11) and to 54 adults (12). More recently, El Samad et al. (10) described 61 patients treated with combination antibiotic therapy, including clindamycin. In those three studies, clindamycin (20 to 30 mg/kg/day) was intermittently infused for several days, and this was followed by oral intake for several weeks. Considering only cure of the initial infection, our patients' outcomes were better than theirs (1/53 versus 5/56 relapses), but comparison of these very different studies and affirmation of one regimen's superiority over the other in the absence of a comparative randomized trial are not possible. In our opinion, oral administration raises several uncertainties, including uncertainties related to the observation of drug dosing, drug absorption, and gastrointestinal tolerance. As noted by El Sayad et al. (10), their patients reported frequent nausea, vomiting, and diarrhea. These AEs can lead to drug non- or malabsorption and treatment failure, especially during the first weeks, when a high degree of efficacy is required.
We chose to administer clindamycin by continuous infusion, as the pattern of in vitro bactericidal activity for clindamycin is time dependent (1, 5). However, the pharmacokinetic and pharmacodynamic parameters that correlate with antibacterial efficacy in animal models is the 24-h area under concentration-time curve/MIC (1), which can be considered a combination of time and concentration dependence. By using continuous infusion, we maintained high serum clindamycin concentrations, i.e., concentrations 50 times greater than the MIC of susceptible S. aureus strains, throughout the treatment duration. To determine the target range (5 to 8 mg/liter), we considered (i) an in vitro MIC of 0.1 mg/liter for methicillin-susceptible staphylococcal strains (25) and a minimal bactericidal concentration of >32 mg/liter; (ii) the demonstration by Weinstein et al. that peak serum bactericidal titers of ≥1/16 and trough titers of ≥1/4 predicted cure of chronic osteomyelitis, whereas respective titers of <1/16 and <1/2 predicted failure (29); (iii) reported clindamycin bone penetration rates of 30 to 50% (7, 9, 11); (iv) that a bone concentration/MIC ratio of 5 is required for antibiotics with time-dependent killing (5); and (v) our observation of no clindamycin toxicity when concentrations are continuously <10 mg/liter. Even though our approach to the determination of target serum clindamycin levels can be discussed, fundamental pharmacokinetic-pharmacodynamic parameters (5) were applied to go beyond published medical findings to try get the upper hand over these difficult-to-treat infections.
We observed that serum clindamycin concentrations were significantly lower in patients treated with rifampin than in those not treated with rifampin (Fig. 1B). Because rifampin is a potent hepatic cytochrome P-450 inducer (20, 24), its interactions with other drugs are well-known. However, we found no previous report on this specific and significant drug interaction. As shown in the present study, the rifampin treatment-associated lower serum clindamycin steady-state levels were still >10 times greater than the MIC of susceptible staphylococci. In our experience, comparable clindamycin levels were not obtained with the oral clindamycin-rifampin combination; indeed, we observed very low trough levels (<1 mg/liter) and peak levels (<2.5 mg/liter) (data not shown).
Continuous intravenous clindamycin infusion has potential benefits in managing these difficult-to-treat infections, but it also a practical advantage, as it avoids the need for repeated intermittent injections because the drug is stable over 24 h (17). The latter is a major advantage for parenteral outpatient therapy, which is a recognized modality for the treatment of patients receiving prolonged intravenous regimens (3, 28). Importantly, all our patients had a portable electronic infusion pump rather than an elastomeric infusion system to ensure maximum flow regulation-related safety; this precaution was justified by two case reports of the induction of cardiac arrest by the rapid intravenous injection of clindamycin (2, 4).
Our study has several limitations. First, the retrospective design, the heterogeneity of the population in terms of the type of infection, the surgical treatment performed and the pathogen(s) isolated, and the lack of a control group treated with a more conventional mode, such as intermittent intravenous administration, limit our ability to draw definitive conclusions concerning drug efficacy. Furthermore, the concomitant use of another active antibiotic in nearly all the patients once again limits the ability to analyze the efficacy of the drug. Second, in this retrospective study, we might have missed some AEs. However, all our patients were managed according to standardized local protocols, were visited daily by a physician or nurse, and had blood tests performed at least once a week. Finally, our data on serum clindamycin concentrations and clindamycin-rifampin interactions are rather crude, and more consistent data on the serum concentrations obtained after oral intake are lacking.
In conclusion, combination therapy with continuous intravenous clindamycin is a valid alternative treatment for joint and bone infections due to susceptible gram-positive cocci and anaerobic bacteria. To confirm these preliminary findings, prospective clinical studies are needed to evaluate drug efficacy and pharmacological studies are needed to describe more precisely these drug interactions and the serum clindamycin levels obtained by the use of the intravenous and oral routes.
Acknowledgments
We thank Janet Jacobson for editorial assistance.
No financial support was received.
None of us has a conflict of interest to declare concerning this report.
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Aucoin, P., R. R. Beckner, and N. M. Gantz. 1982. Clindamycin-induced cardiac arrest. South. Med. J. 75:768. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, L., L. El-Hajj, B. Pron, A. Lotthé, V. Gleizes, F. Signoret, P. Denormandie, J. L. Gaillard, and C. Perrone. 2001. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J. Clin. Pharm. Ther. 26:445-451. [DOI] [PubMed] [Google Scholar]
- 4.Brandi, L. S., M. Grana, and M. Carmellini. 1983. Cardiac arrest induced by intravenous administration of clindamycin phosphate. Description of a clinical case. Minerva Anestesiol. 49:535-537. [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antimicrobial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Dall, L., M. Keilhofner, B. Herndon, W. Barnes, and J. Lane. 1990. Clindamycin effect on glycocalyx production in experimental viridans streptococcal endocarditis. J. Infect. Dis. 161:1221-1224. [DOI] [PubMed] [Google Scholar]
- 7.Dornbusch, K., A. Carlström, H. Hugo, and A. Lidströlm. 1977. Antibacterial activity of clindamycin and lincomycin in human bone. J. Antimicrob. Chemother. 3:153-160. [DOI] [PubMed] [Google Scholar]
- 8.Dripps, R. D., A. Lamont, and J. E. Eckenhoff. 1961. The role of anaesthesia in surgical mortality. JAMA 178:261-266. [DOI] [PubMed] [Google Scholar]
- 9.Duckworth, C., J. F. Fisher, S. A. Carter, C. L. Newman, C. Cogburn, and R. R. Nesbit. 1993. Tissue penetration of clindamycin in diabetic foot infections. J. Antimicrob. Chemother. 31:581-584. [DOI] [PubMed] [Google Scholar]
- 10.El Samad, Y., E. Havet, H. Bentayeb, B. Olory, B. Canarelli, J. F. Lardanchet, Y. Douadi, F. Rousseau, F. X. Lescure, P. Mertl, F. Eb, and J. L. Schmit. 2008. Treatment of osteoarticular infections with clindamycin in adults. Med. Mal. Infect. 38:465-470. [DOI] [PubMed] [Google Scholar]
- 11.Feigin, R. D., L. K. Pickering, D. Anderson, R. E. Keeney, and P. G. Shakleford. 1975. Clindamycin treatment of osteomyelitis and septic arthritis in children. Pediatrics 55:213-223. [PubMed] [Google Scholar]
- 12.Geddes, A. M., N. S. Dwyer, A. P. Ball, and R. S. Amos. 1977. Clindamycin in bone and joint infections. J. Antimicrob. Chemother. 3:501-507. [DOI] [PubMed] [Google Scholar]
- 13.Gisby, J., A. S. Beale, J. E. Bryant, and C. D. Toseland. 1994. Staphylococcus osteomyelitis—a comparison of co-amoxiclav with clindamycin and flucloxacillin in an experimental rat model. J. Antimicrob. Chemother. 34:755-764. [DOI] [PubMed] [Google Scholar]
- 14.Kasiakou, S. K., G. J. Sermaides, A. Michalopoulos, E. S. Soteriades, and M. E. Falagas. 2005. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect. Dis. 5:581-589. [DOI] [PubMed] [Google Scholar]
- 15.Lazzarini, L., B. A. Lipsky, and J. T. Mader. 2005. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int. J. Infect. Dis. 9:127-138. [DOI] [PubMed] [Google Scholar]
- 16.Mader, J. T., K. Adams, and L. Morrison. 1989. Comparative evaluation of cefazolin and clindamycin in the treatment of experimental Staphylococcus aureus osteomyelitis in rabbits. Antimicrob. Agents Chemother. 33:1760-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansur, J. M., P. W. Abramowitz, S. A. Lerner, R. B. Smith, and R. J. Townsend. 1985. Stability and cost analysis of clindamycin-gentamicin admixtures given every eight hours. Am. J. Hosp. Pharm. 42:332-335. [PubMed] [Google Scholar]
- 18.Mayberry-Carson, K. J., W. R. Mayberry, B. K. Tober-Meyer, J. W. Costerton, and D. W. Lambe. 1986. An electron microscopic study of the effect of clindamycin on adherence of Staphylococcus aureus to bone surface. Microbios 45:21-32. [PubMed] [Google Scholar]
- 19.Mayberry-Carson, K. J., B. Tober-Meyer, D. W. Lambe, and J. W. Costerton. 1986. An electron microscopic study of the effect of clindamycin therapy on bacterial adherence and glycocalyx formation in experimental Staphylococcus aureus osteomyelitis. Microbios 48:189-206. [PubMed] [Google Scholar]
- 20.Niemi, M., J. T. Backman, M. F. Fromm, P. J. Neuvonen, and K. T. Kivistö. 2003. Pharmacokinetic interactions with rifampin: clinical relevance. Clin. Pharmacokinet. 42:819-850. [DOI] [PubMed] [Google Scholar]
- 21.Panzer, J. D., D. C. Brown, W. L. Epstein, R. L. Lipson, H. W. Mahaffey, and W. H. Atkinson. 1972. Clindamycin levels in various body tissues and fluids. J. Clin. Pharmacol. 12:259-262. [DOI] [PubMed] [Google Scholar]
- 22.Plott, M. A., and H. Roth. 1970. Penetration of clindamycin into synovial fluid. Clin. Pharmacol. Ther. 11:577-580. [DOI] [PubMed] [Google Scholar]
- 23.Schurman, D. J., B. L. Johnson, G. Finerman, and H. C. Amstutz. 1975. Antibiotic bone penetration. Concentrations of methicillin and clindamycin phosphate in human bone taken during total hip replacement. Clin. Orthop. Rel. Res. 111:142-146. [PubMed] [Google Scholar]
- 24.Sousa, M., A. Pozniak, and M. Boffito. 2008. Pharmacokinetics and pharmacodynamics of drug interactions involving rifampicin, rifabutin and antimalarial drugs. J. Antimicrob. Chemother. 62:872-878. [DOI] [PubMed] [Google Scholar]
- 25.Steigbigel, N. H. 2000. Macrolides and clindamycin, p. 366-382. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, PA.
- 26.Stengel, D., K. Bauwens, J. Sehouli, A. Ekkernkamp, and F. Porzsolt. 2001. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect. Dis. 1:175-188. [DOI] [PubMed] [Google Scholar]
- 27.Summersgill, J. T., L. G. Schupp, and M. J. Raff. 1982. Comparative penetration of metronidazole, clindamycin, chloramphenicol, cefoxitin, ticarcillin, and moxalactam into bone. Antimicrob. Agents Chemother. 21:601-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tice, A. D., S. J. Rehm, J. R. Dalovisio, J. S. Bradley, L. P. Martinelli, D. R. Graham, R. B. Gainer, M. J. Kunkel, R. W. Yancey, and D. N. Williams. 2004. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin. Infect. Dis. 38:1651-1672. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein, M. P., C. W. Stratton, H. B. Hawley, A. Ackley, and L. B. Reller. 1987. Multicenter collaborative evaluation of standardized serum bactericidal test as a predictor of therapeutic efficacy in acute and chronic osteomyelitis. Am. J. Med. 83:218-222. [DOI] [PubMed] [Google Scholar]
- 30.Zeller, V., F. Durand, M. D. Kitzis, L. Lhotellier, J. M. Ziza, P. Mamoudy, and N. Desplaces. 2009. Prolonged continuous intravenous cefazolin to treat bone and joint infections: feasibility, tolerance, efficacy, serum and bone concentrations. Antimicrob. Agents Chemother. 53:883-887. [DOI] [PMC free article] [PubMed] [Google Scholar]