Abstract
Nemonoxacin (TG-873870) is a novel nonfluorinated quinolone with broad-spectrum activities against Gram-positive and Gram-negative aerobic, anaerobic, and atypical pathogens, as well as against methicillin-resistant Staphylococcus aureus, vancomycin-resistant S. aureus, and multiple-resistant bacterial pathogens. We conducted a randomized, double-blind, placebo-controlled, dose-escalating study to ascertain the safety, tolerability, and pharmacokinetics of nemonoxacin. We enrolled 46 healthy volunteers and used a once-daily oral-dosing range of 75 to 1,000 mg for 10 days. Additionally, the food effect was evaluated in subjects in the 500-mg cohort. Nemonoxacin was generally safe and well tolerated, with no significant changes in the clinical laboratory tests or electrocardiograms. Adverse effects, including headache, contact dermatitis, and rash, were mild and resolved spontaneously. Nemonoxacin was rapidly absorbed within 2 h postdosing, and generally, a steady state was reached after 3 days. The maximum plasma concentration and the area under the plasma concentration-time curve were dose proportional over the dosing range. The elimination half-life was approximately 7.5 h and 19.7 h on days 1 and 10, respectively. Approximately 37 to 58% of the drug was excreted in the urine. Food affected the pharmacokinetics, with decreases in the maximum plasma concentration and area under the plasma concentration-time curve of 46% and 27%, respectively. However, the free AUC/MIC90 of nemonoxacin was more than 100 under both the fasting and fed conditions, predicting the efficacy of nemonoxacin against most of the tested pathogens. In conclusion, the results support further clinical investigation of once-daily nemonoxacin administration for antibiotic-sensitive and antibiotic-resistant bacterial infections.
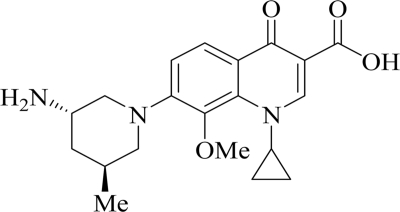
Nemonoxacin (TG-873870) (31) is a novel nonfluorinated quinolone developed from a series of 8-methoxy nonfluorinated quinolones (25). It lacks the characteristic fluorine at the sixth position in fluoroquinolones (FQs) (Fig. 1). Nemonoxacin is a potent antibacterial agent with broad-spectrum activity against clinical isolates and reference strains in both in vitro studies and experimental infections in animals. It is active against Gram-positive, Gram-negative, and atypical pathogens, including penicillin- and quinolone-resistant Streptococcus pneumoniae (PRSP and QRSP), methicillin-resistant Staphylococcus aureus (MRSA), quinolone-resistant MRSA (QR-MRSA), and vancomycin-resistant enterococci (4, 17). The nemonoxacin MIC90 range is 0.06 to 1 μg/ml for MRSA clinical isolates and 0.03 to 1 μg/ml for PRSP clinical isolates from North America and Taiwan (17, 24, 33). For QR-MRSA clinical isolates, the MIC90 range is 1 to 2 μg/ml; this is 4- to 64-fold lower than the MIC90s of FQs in parallel testing, including ciprofloxacin, levofloxacin, and moxifloxacin (17, 24). There is increasing concern about vancomycin-intermediate S. aureus (VISA) among clinical isolates. Nemonoxacin showed an MIC90 of 2 μg/ml against heterogeneous VISA, VISA, and vancomycin-resistant S. aureus clinical isolates from the United States (24). Moreover, nemonoxacin is less prone to develop resistance than other FQs. The drug requires mutations in three different bacterial genes, gyrA, gyrB, and parC, rather than the usual two genes needed for FQs to develop significant resistance (16).
FIG. 1.
Structure of nemonoxacin (TG-873870).
The notable adverse effects (AEs) of FQs include phototoxicity, hepatotoxicity, and prolongation of cardiac repolarization (3, 19, 23, 29). In preclinical toxicology studies, nemonoxacin was found to lack these side effects and was considered safe (7, 8, 14, 15). Prior to this study, a double-blind, placebo-controlled, single-dosing study was conducted in 56 healthy subjects. The safety, tolerability, and pharmacokinetics (PK) of nemonoxacin were studied over a dosing range of 25 to 1,500 mg in this phase 1a study (18). Nemonoxacin was found to have a good safety profile with no clinically relevant changes in vital signs, hematology, blood chemistry, or electrocardiograms (ECGs). No serious AEs were observed. The drug was tolerated well up to the maximum tolerated dose of 1,500 mg. The most frequent AEs included contact dermatitis associated with ECG electrode application in the chest area, pruritus, and erythema; of these, four events of pruritus were assessed as moderate in severity and associated with higher-dose groups. The relatively long half-life of 9 to 14 h in the single-dose PK study supported the once-daily oral-dosing schedule used in the present study.
The present study was designed to evaluate the safety and tolerability of multiple daily doses of nemonoxacin in the range of 75 to 1,000 mg for 10 days by using healthy adult subjects and to determine the multiple-dose PK parameters following oral administration of the drug.
(The preliminary data were presented at the 45th Annual Meeting of the Infectious Diseases Society of America, abstr. 440, 2007.)
MATERIALS AND METHODS
Subjects.
Healthy male and surgically sterile or postmenopausal female subjects between the ages of 18 and 45 years were eligible to participate in the study. They were screened over a 21-day period prior to study admission and were qualified to participate in the study when the baseline assessments on day −2 and day −1 met the criteria. The baseline assessment included medical history, physical examination, clinical laboratory tests, and ECG studies. Eligible subjects were those with no evidence of diseases of the major organs, diabetes, chronic viral hepatitis, HIV infection, or prolonged ECG QTc interval and no clinically significant abnormalities of chemistry, hematology, or urinalysis data. Additionally, the body mass index of the subjects was within 10 to 30 kg/m2. Those with a history of hypersensitivity to any drugs, alcohol or controlled drug abuse, or investigational drug use within 30 days prior to dosing were excluded. All study subjects provided informed consent to participate in the study.
Study design.
A randomized, double-blind, placebo-controlled, multiple- and ascending-dose study was conducted at a single study site (Parexel Clinical Research Unit, Harbor Hospital Center, Baltimore, MD). The study consisted of two study periods. In the first study period, each subject received a once-daily oral dose of nemonoxacin or a placebo at five dose levels (75, 250, 500, 750, and 1,000 mg) during a 10-day dosing period, with subsequent follow-up. Dosing was carried out in ascending dose cohorts by administering nemonoxacin and the placebo at a ratio of 6:2 for the 75- and 250-mg cohorts and a ratio 8:2 for the 500-, 750-, and 1,000-mg cohorts. The subjects received nemonoxacin in the morning after a 12-h fast (fasting state). Food intake was not allowed for 4 h after drug administration, but water was allowed ad libitum. All subjects stayed at the study site from the evening 2 days before dosing initiation to 96 h after administration of the last dose. In the second study period, the food effect was studied with the 500-mg dosing cohort, which received an additional dose after a high-fat meal on day 16. These subjects stayed at the study site until day 18 for observation.
Safety assessment.
All 46 subjects (36 in the nemonoxacin group and 10 in the placebo group) were included in the safety analysis. Assessment of vital signs, physical examinations, AE monitoring, and clinical laboratory tests—including clinical chemistry, hematology, urinalysis, and 12-lead ECG studies—were performed according to predefined schedules. Baseline assessments were performed 1 and 2 days before dosing (i.e., days −2 and −1) for pairwise comparison at corresponding time points. ECG studies were performed at the time of screening; 0 h (predose); 1, 2, 4, 8, 12, and 24 h on days −1, 1 (first dose), and 10 (10th dose); and 2 h after dosing on days 3, 5, and 7. Blood samples were collected at 0 (predose) and 0.5, 1, 1.5, 2, 4, 6, 8, 12, 16, and 24 h after dosing on days 1 and 10. Additional blood samples were collected at 36, 48, 72, and 96 h after the last dose on day 10 to calculate the terminal-phase elimination half-life (t1/2) and plasma concentration-time curves. Trough blood samples were also collected prior to dosing on days 3, 5, 8, and 9. Blood was collected from the 500-mg fed cohort after dosing on day 16 at the same time points as in the fasting cohort studies and at 36 and 48 h after dosing. The blood samples were collected in vials containing sodium heparin as an anticoagulant and centrifuged to obtain plasma. The total urine output was collected over the intervals −3 to 0 (predose), 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after dosing on days 1 and 10. Additional urine samples were collected over the intervals 24 to 36, 36 to 48, 48 to 72, and 72 to 96 h after dosing on day 10. Urine was also collected from the 500-mg fed cohort over the same time intervals as in the fasting study until 48 h after dosing. Blood and urine samples were stored at −70°C until subsequent analysis.
Sample analysis.
Bioanalyses of plasma and urine samples were performed at Charles River Laboratories (Worcester, MA) using a process of nemonoxacin extraction followed by solid-phase extraction, evaporation, reconstitution, and final analysis by high-performance liquid chromatography with a tandem mass spectrometer. The method was validated over a nemonoxacin concentration range of 5 to 5,000 ng/ml in urine. The limit of quantification was 5 ng/ml. The between- and within-run coefficients of variation over this range were less than 6% and 14%, respectively. For the plasma samples, the analytical method was validated for a concentration range of 5 to 5,000 ng/ml in plasma. The coefficient of variation over this range was no greater than 16%.
Pharmacokinetic analysis.
Concentration-versus-time data for nemonoxacin in plasma were derived by standard noncompartmental methods (12) with WinNonlin (version 4.1; Scientific Consulting Inc., KY). The maximum plasma concentration (Cmax) and the time at which this concentration was achieved (Tmax) were estimated by visual inspection of the plasma concentration-versus-time data. The minimum plasma concentration (Cmin) was also determined by direct measurement. The apparent t1/2 was calculated as ln(2)/λz, where λz is the elimination rate constant derived from the slope of the linear regression line of the apparent terminal linear portion of the log concentration-time curve at a minimum of the last three data points. The area under the plasma concentration-time curve from 0 to 24 h (AUC0-24) and the area under the plasma concentration-time curve from 0 to the last observation after dosing (AUC0-last) were calculated using the linear trapezoidal method. The AUC from time zero to infinity (AUC0-∞) was calculated as the sum of AUC0-last and Clast/λz, where Clast is the last measurable drug concentration. Oral clearance (CLo) was calculated as the dose divided by AUC0-∞. The degree of accumulation was calculated as the ratio of Cmax and AUC0-24 at day 10 to day 1.
The amount of nemonoxacin excreted into urine was calculated based on the drug concentration in each urine sample multiplied by the volume of urine obtained during the respective collection period. The amounts of unchanged nemonoxacin in the urine from 0 to 24 h (U0-24) were calculated using the cumulative amount excreted in the urine on days 1 and 10, and on day 16 for the 500-mg fed cohort. Total urinary recovery (Ae) of nemonoxacin was expressed as a percentage of the administered dose. Renal clearance (CLr) was calculated as U0-24/AUC0-24.
Statistical analysis.
Dose proportionality of Cmax, AUC0-24, and AUC0-last by each cohort and study day was assessed using the power model (13) of the form y = α(x)β, where y denotes the PK parameter and x the dose. Applying the natural log on both sides gives a linear equation in x and y with the slope represented by β, which was estimated for each PK parameter. The criterion for dose proportionality, which was the slope (β) close-in value to 1 based on the 95% confidence interval (CI) for the slope, was computed (28). All data were expressed as the mean ± standard deviation (SD).
RESULTS
Subject demographics and baseline characteristics.
In total, 46 healthy subjects—2 females and 44 males—were enrolled in the study. Of these, 43 were evaluable for pharmacokinetics and safety on all study days. The mean age was 31 years (range, 19 to 45 years); the mean body weight was 80.04 kg (range, 60.2 to 106.6 kg). Twenty-six subjects (56.6%) were African-American, 13 Caucasian (28.3%), 6 Hispanic (13%), and one Asian (2.2%). Two subjects, one in the placebo group and the other in the 250-mg cohort, withdrew on day 1 and day 10, respectively, because of personal reasons. One subject in the 750-mg cohort withdrew because of mild skin rash on day 8.
Safety and tolerability.
Nemonoxacin was generally safe and well tolerated across all the doses. There were no significant clinical abnormalities in vital signs or physical examinations.
No serious or severe AEs were reported in the study. Table 1 summarizes all AEs in the treatment groups by causality. In total, 64 AEs were reported by 23 of the 46 (50%) subjects, including 11 AEs in 5 of the 10 (50%) subjects who received the placebo and 53 AEs in 18 of the 36 (50%) subjects who received nemonoxacin. No apparent difference was observed on comparing the numbers of drug-related AEs among the treatment groups. Although more AEs were reported in the 75- and 1,000-mg cohorts (20 and 15 AEs, respectively), multiple AEs were reported by the same subject in these two dosing groups. One subject from the 750-mg group was withdrawn from the study because of a mild rash. The most common AE was headache (13.9% in the nemonoxacin group and 10% in the placebo group). The other frequently reported AEs (by two or more subjects) were contact dermatitis due to ECG electrode application (8.3% in the nemonoxacin group and 10% in the placebo group), rash (8.3% in the nemonoxacin group), abdominal pain (12.8% in the nemonoxacin group and 10% in the placebo group), ECG electrode application site pruritus (2.8% in the nemonoxacin group and 10% in the placebo group), diarrhea (5.6% in the nemonoxacin group), pollakiuria (5.6% in the nemonoxacin group), pruritus (5.6% in the nemonoxacin group), and somnolence (5.6% in the nemonoxacin group). The most common drug-related AE determined by the investigator was headache. All AEs were mild in intensity. In conclusion, a dose-related increase in the incidence of AEs was not observed.
TABLE 1.
Number and frequency of subjects with adverse events by causality for all treatment groups
| AE | No. (%) of subjects |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nemonoxacin |
Placeboa (n = 10) | Total (n = 46) |
||||||||
| 75 mg (n = 6) | 250 mg (n = 6) | 500 mga (n = 8) | 750 mg (n = 8) | 1,000 mg (n = 8) | Total (n = 36) |
|||||
| Related | Not related | Related | Not related | |||||||
| Headache | 2 | 1 | 1 | 1 | 0 | 3 (8.3) | 2 (5.6) | 1 (10) | 4 (8.7) | 2 (4.3) |
| ECG electrode application site dermatitis | 2 | 0 | 0 | 0 | 1 | 0 (0.0) | 3 (8.3) | 1 (10) | 0 (0.0) | 4 (8.7) |
| Rash | 0 | 0 | 1 | 2 | 0 | 1 (2.8) | 2 (5.6) | 0 (0.0) | 1 (2.2) | 2 (4.3) |
| Abdominal pain | 1 | 0 | 0 | 0 | 0 | 1 (2.8) | 0 (0.0) | 1 (10) | 1 (2.2) | 1 (2.2) |
| ECG electrode application site pruritus | 0 | 0 | 0 | 0 | 1 | 0 (0.0) | 1 (2.8) | 1 (10) | 0 (0.0) | 2 (4.3) |
| Diarrhea | 2 | 0 | 0 | 0 | 0 | 2 (5.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) |
| Pollakiuria | 0 | 0 | 0 | 1 | 1 | 2 (5.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) |
| Pruritus | 0 | 0 | 0 | 1 | 1 | 2 (5.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) |
| Somnolence | 2 | 0 | 0 | 0 | 0 | 2 (5.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) |
| Otherb | 3 | 5 | 2 | 2 | 5 | 12 (33) | 5 (13.9) | 4 (40) | 2 (4.3) | 0 (0.0) |
| No. (%) of subjects reporting an AE | 4 | 3 | 3 | 4 | 4 | 18 (50.0) | 5 (50) | 23 (50.0) | ||
| Total no. of AEs reportedc | 20 | 6 | 5 | 7 | 15 | 53 | 11 | 64 | ||
| No. (%) of relatedd AEsc | 16 | 4 | 1 | 4 | 10 | 35 (66.0) | 4 (36.4) | 39 (60.9) | ||
Includes AEs reported following dosing in both the fed and fasting states.
Other AEs, each reported once, included ECG electrode application site irritation, burning sensation, altered bowel habits, dizziness, dysgeusia, dysmenorrhoea, dyspepsia, pancreatitis, dysuria, erythema, flatulence, flushing, injury, muscle tightness, nausea, noncardiac chest pain, abnormal urine odor, abdominal discomfort, constipation, ear pain, and ocular hyperemia.
Some subjects reported the same AE more than once.
Related: possible, probable, and definite combined causalities.
There were sporadic increases in creatine phosphokinase values in some subjects. One subject in the 1,000-mg group had an elevated serum amylase concentration. The investigator did not consider the elevated levels of creatine phosphokinase and serum amylase to be clinically significant. An increased lipase concentration was also observed in one subject in the 750-mg group, but the level was found to have returned to normal when followed up 1 month later.
None of the subjects in any of the dose cohorts had a QTc (QTcB or QTcF) greater than 500 ms or a QTc change from baseline greater than 60 ms. A blinded review of ECG data from cohort 5 (1,000-mg nemonoxacin or placebo) was performed by an independent cardiologist, who concluded that the ECG findings were not clinically alarming or suggestive of any kind of drug-related effect. The ECG changes detected in this study are similar to those frequently reported in healthy subjects. There were no apparent differences in the ECG intervals in subjects taking nemonoxacin with food or in the fasting state.
Pharmacokinetics.
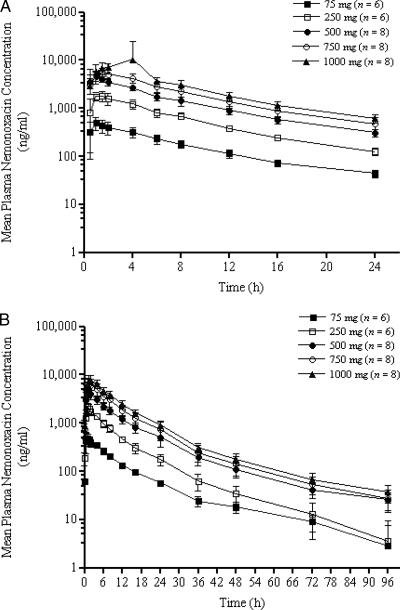
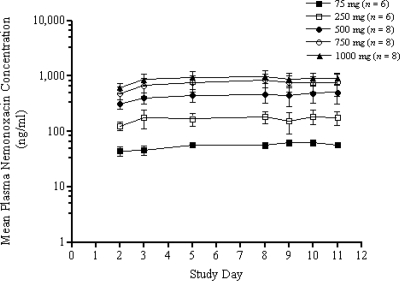
The pharmacokinetics of nemonoxacin determined in single and repeat doses are summarized in Table 2. The plasma concentrations of nemonoxacin on days 1 and 10 are shown in Fig. 2A and B, respectively. Nemonoxacin was absorbed rapidly following oral administration in the fasting state, with the Cmax attained in 1 to 2 h after dosing and the mean Tmax values attained between 0.92 and 2.07 h after dosing. The Tmax values appeared to increase slightly with increasing doses but remained relatively constant within individual dose groups. The mean t1/2 of nemonoxacin was calculated as 19.65 h at multiple doses in the steady state. Plasma concentrations were similar on days 1 and 10 within each cohort, suggesting little accumulation over the 10-day dosing period (Table 2 and Fig. 3). The theoretical accumulation ratios (mean ± SD) of the Cmax and AUC0-24 on day 10 compared to those on day 1 were 1.11 ± 0.08 and 1.21 ± 0.06, respectively. Mean trough concentrations of nemonoxacin in plasma were compared across days 2, 3, 5, 8, 9, 10, and 11 for all the dose groups. As shown in Fig. 3, a dose-dependent increase in the trough concentrations was observed 3 to 5 days before a steady state was reached.
TABLE 2.
PK parameters of nemonoxacin following single oral administration and once-daily oral administration for 10 daysa
| Dose group and time | Cmax (ng/ml) | Tmax (h) | AUC0-24 (h·ng/ml) | AUC0-last (h·ng/ml) | t1/2 (h) | CLo (ml/h) | Ae24 (%) | CLr (ml/h) |
|---|---|---|---|---|---|---|---|---|
| 75 mg | ||||||||
| Day 1 (n = 6) | 502.4 (156.4) | 1.09 (1.0-1.5) | 3,782 (822) | 3,779 (822) | NA | 18,170 (3,848) | 56.4 (7.6) | 11,869 (4,200) |
| Day 10 (n = 6) | 514.2 (149.8) | 0.92 (0.5-1.0) | 4,268 (461) | 5,452 (515) | 22.96 (7.96) | 13,453 (15,187) | 56.7 (6.5) | 10,102 (1,871) |
| 250 mg | ||||||||
| Day 1 (n = 6) | 2,001.7 (373.6) | 1.25 (1.0-1.5) | 13,663 (1,298) | 13,653 (1,639) | NA | 16,733 (1,495) | 36.8 (7.6) | 6,887 (1,994) |
| Day 10 (n = 6) | 2,391.7 (258.4) | 1.09 (1.0-1.5) | 16,096 (1,298) | 18,676 (2,020) | 17.58 (7.71) | 13,373 (1,422) | 49.9 (10.7) | 7,763 (1,731) |
| 500 mg | ||||||||
| Day 1 (n = 8) | 5,121.3 (1,035.2) | 1.00 (0.5-1.5) | 31,601 (4,333) | 31,578 (4,330) | NA | 14,545 (1,993) | 42.2 (13.1) | 6,920 (3,020) |
| Day 10 (n = 8) | 5,557.5 (1,389.0) | 1.31 (1.0-2.0) | 38,599 (7,368) | 46,730 (9,778) | 18.56 (4.67) | 11,018 (2,723) | 57.8 (9.6) | 7,846 (2,810) |
| Day 16 (n = 8)b | 2,747.5 (1,123.3) | 3.07 (0.5-4.0) | 22,747 (2,531) | 26,043 (3,061) | NA | 18,781 (2,547) | 30.5 (7.9) | 6,806 (1,905) |
| 750 mg | ||||||||
| Day 1 (n = 8) | 5,752.5 (1,177.4) | 1.50 (1.0-2.0) | 46,064 (9,276) | 46,029 (9,269) | NA | 15,094 (3,178) | 47.1 (10.9) | 7,955 (2,323) |
| Day 10 (n = 7) | 6,817.1 (1,812.2) | 1.51 (1.0-2.0) | 58,433 (14,323) | 69,254 (16,910) | 19.73 (4.32) | 11,335 (3,082) | 41.8 (10.3) | 5,631 (1,741) |
| 1,000 mg | ||||||||
| Day 1 (n = 8) | 7,753.8 (2,147.3) | 2.00 (1.0-4.0) | 59,650 (12,459) | 59,626 (12,460) | NA | 15,727 (3,503) | 47.9 (8.7) | 7,484 (1,799) |
| Day 10 (n = 8) | 8,195.0 (2,032.3) | 2.07 (1.5-4.0) | 74,837 (14,270) | 88,320 (16,423) | 19.42 (2.74) | 11,558 (2,327) | 48.6 (13.7) | 6,871 (2,630) |
Values for Tmax are means (ranges); all others are arithmetic means (standard deviations). NA, not applicable.
After a high-fat meal.
FIG. 2.
Mean ± SD concentration-versus-time plasma concentrations for nemonoxacin following oral administration of 75, 250, 500, 750, and 1,000 mg on day 1 (A) and day 10 (B). The error bars indicate SDs.
FIG. 3.
Mean ± SD trough plasma concentration of nemonoxacin prior to the subsequent oral administration of 75, 250, 500, 750, and 1,000 mg. The error bars indicate SDs.
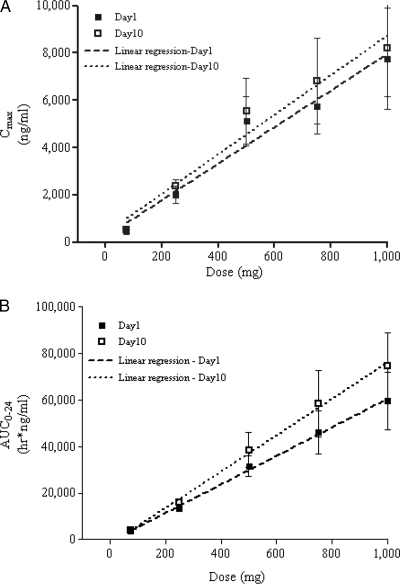
The concentrations of nemonoxacin in plasma increased with increasing doses following once-daily administration of the study doses for 10 days (Fig. 2A and B). The dose-normalized Cmax ratios were 6.7:8.0:10.2:7.7:7.7 on day 1 and 6.8:9.6:11.1:9.1:8.2 on day 10, showing a slight increase in the dose-normalized Cmax from the 75-mg to the 500-mg dosing group. The dose-normalized AUC0-24 ratios were 50.4:54.7:63.2:61.4:59.7 on day 1 and 56.9:64.4:77.2:77.9:74.8 on day 10, showing a slight increase from the 75-mg to the 500-mg dosing group and then reaching a plateau for the 500-mg group. The mean Cmax and AUC0-24 values, with a 95% CI, were plotted against the dose (Fig. 4) and showed dose linearity over the dose range studied. Dose proportionality was evaluated using the power model. The slope parameters of Cmax, AUC0-24, and AUC0-∞ were 1.07 (95% CI, 0.96, 1.17), 1.08 (95% CI, 0.96, 1.17), and 1.08 (95% CI, 1.00, 1.15), respectively, using single-dose (i.e., day 1) data. When multiple-dose (i.e., day 10) data were used, the slope parameters of Cmax (1.08; 95% CI, 0.97, 1.19), AUC0-24 (1.12; 95% CI, 1.04, 1.19), and AUC0-∞ (1.09; 95% CI, 1.02, 1.16) also showed dose proportionality. The t1/2, CLo, and Ae24 (cumulative amount of nemonoxacin recovered in urine from 0 to 24 h) values, which did not change with increasing doses, indicate the linearity of nemonoxacin pharmacokinetics at single and multiple doses (Table 2).
FIG. 4.
Mean ± SD Cmax (A) and AUC0-24 (B) versus dose following a single dose (day 1) and multiple doses (day 10) of nemonoxacin. The error bars indicate the 95% CI.
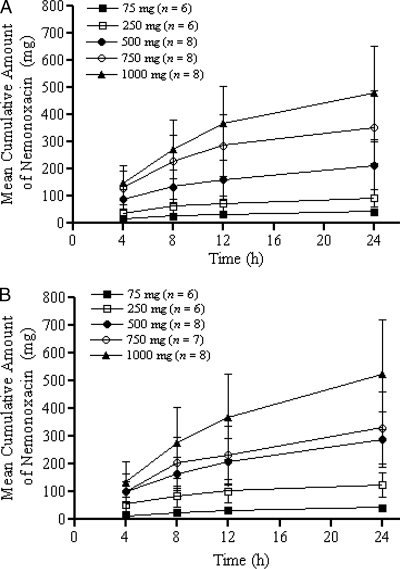
The cumulative amount of nemonoxacin in urine over 24 h was approximately the same for subjects receiving the single- and multiple-dose treatments. Approximately 37 to 58% of the administered nemonoxacin was excreted unchanged in the urine in the fasting state (Table 2 and Fig. 5). A slight accumulation of the drug was found within each cohort on day 10 compared with day 1, based on the mean Ae24.
FIG. 5.
Cumulative amounts of nemonoxacin (mean ± SD) in urine following oral administration of 75, 250, 500, 750, and 1,000 mg on day 1 (A) and day 10 (B). The error bars indicate SDs.
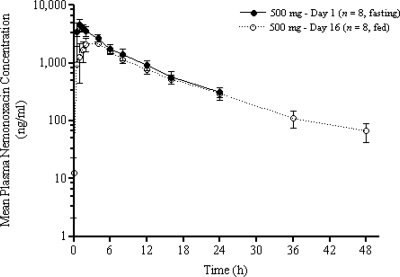
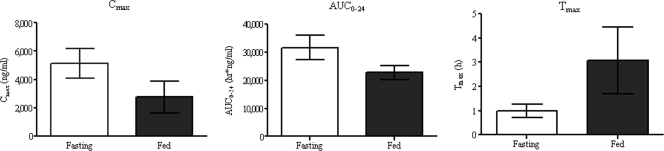
The extents of nemonoxacin absorption were different under the fasting and fed conditions. The mean Cmax and AUC0-24 decreased by approximately 46% and 27%, respectively, in the fed state, and the Tmax increased twofold from 1.31 to 3.07 h (Table 2 and Fig. 6 and 7). Dosing in the fed state also led to a decrease in the Ae24 (Table 2). The AUC0-last decreased by about 17%, and the mean trough plasma concentration at 24 h postdosing was similar to that in the fasting state.
FIG. 6.
Mean ± SD plasma concentrations versus time following oral administration of 500 mg of nemonoxacin on day 1 (fasting state) and day 16 (fed state). The error bars indicate SDs.
FIG. 7.
Effect of food on the mean ± SD Cmax, AUC0-24, and Tmax of nemonoxacin. The error bars indicate SDs.
DISCUSSION
This is the first report on the safety and pharmacokinetics of nemonoxacin following multiple once-daily oral administration to healthy subjects. Orally administered nemonoxacin was generally safe and well tolerated by the volunteers at doses of 75 to 1,000 mg per day for 10 days, showing favorable results without the common concerns associated with quinolone class antibiotics (29).
In this study, all AEs were generally mild and resolved spontaneously without medication. There was no drug- or dose-related increase in the incidence of reported AEs. With the exception of a transient elevation in serum amylase and lipase in one subject, no clinical laboratory findings of potential concern were found. Elevation of liver function parameters has been reported with many fluoroquinolones (9, 34); however, there were no clinically significant changes in the liver function test results in this study. Further, no clinically significant abnormalities in vital signs, physical examination, ECG intervals, or morphology findings were observed.
Nemonoxacin has been carefully evaluated in a battery of preclinical safety studies due to reported safety concerns about FQs. There has been no evidence of phototoxicity (6), significant hepatotoxicity, or severe central nervous system toxicity (unpublished data). The nonclinical studies completed to date indicate that nemonoxacin has a safety profile comparable with, if not better than, those of many of the currently marketed quinolones.
The PK profile of nemonoxacin with multiple-dose oral administration in the fasting state is similar to the profile at a single dose. The PK findings of this study showed that the Cmax and AUC0-24 were dose proportional and linear over the range of 75 to 1,000 mg, and the Cmax was reached within 2 h after a single oral administration. The mean Cmax and AUC0-24 increased slightly from day 1 to day 10 in each cohort. This slight accumulation following once-daily dosing regimens is in agreement with the theoretical calculation based on the single-dose data. The values of the t1/2, CLo, and Ae24 remained unchanged over the escalating doses, indicating that nemonoxacin exhibits linear PK over the range of 75 to 1,000 mg. The t1/2 was determined to be 17.6 to 19.8 h. The Ae24 accounted for 37 to 58% of the drug. The range of renal elimination for nemonoxacin falls into the range of data reported for other quinolones (20 to 30% for gemifloxacin, 80% for levofloxacin, 20% for moxifloxacin, and 30 to 50% for garenoxacin) (1, 2, 5, 11, 21, 30). Metabolism studies completed to date indicate that no metabolite or a minor metabolite (less than 5%) of nemonoxacin was formed either in vitro or ex vivo (reference 7 and unpublished data).
Comparison of the PK data on day 1 (fasting) and day 16 (fed) indicated that food affected the pharmacokinetics of nemonoxacin. The presence of food led to a decrease in both the Cmax and AUC and a delay in the Tmax, suggesting that the rate and extent of nemonoxacin absorption are significantly reduced by food. Even so, the mean trough plasma concentration of nemonoxacin (0.297 μg/ml) was well above the MIC90 of the antibiotic-resistant pathogens in our studies (e.g., MRSA and PRSP clinical isolates) (17, 24, 33).
The overall PK parameters demonstrated that, over the dose range studied, sufficient concentrations of nemonoxacin could be maintained in the plasma over 24 h postdosing. In the early phase Ia study, plasma binding of nemonoxacin was shown to be about 16% (18). When administered at a dose of 500 mg or higher, the free plasma concentration of nemonoxacin was well above the MIC90s for a wide spectrum of Gram-positive, Gram-negative, and atypical pathogens for the full 24-h dosing interval (1, 4). Data from a variety of in vitro, animal, and human studies have suggested that the bactericidal activity of FQs is rapid and concentration dependent (10, 20, 26, 27). The free AUC0-24 (fAUC0-24) over the MIC90 ratio of FQs has been correlated with bacterial eradication. fAUC0-24/MIC90 ratios of 30 and 40 were used to assess bacterial eradication, and ratios of 100 to 120 were used to assess resistance development (22, 32). Nemonoxacin at 500 mg showed favorable fAUC0-24/MIC90 ratios for Gram-positive pathogens of community-acquired pneumonia and skin infections tested in our in vitro studies, including MRSA, QR-MRSA, and PRSP (33). If the food effect is considered, fAUC0-24/MIC90 ratios of more than 100 for nemonoxacin above a 500-mg dose are still attainable for most pathogens tested.
In conclusion, oral administration of nemonoxacin is generally safe and well tolerated at doses of 75 to 1,000 mg per day for 10 consecutive days. The PK parameters of single and repeat administrations are dose proportional and show little accumulation. Further investigation of the efficacy of once-daily oral administration of nemonoxacin against clinical bacterial infections is warranted.
Acknowledgments
We thank the investigator, Ronald Goldwater, and the healthy volunteers who participated in the study.
The study was supported by an MOEA grant from the Government of Taiwan, Republic of China.
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Allen, A., E. Bygate, M. Vousden, S. Oliver, M. Johnson, C. Ward, A.-J. Cheon, Y. S. Choo, and I.-C. Kim. 2001. Multiple-dose pharmacokinetics and tolerability of gemifloxacin administered orally to healthy volunteers. Antimicrob. Agents Chemother. 45:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, A., E. Bygate, S. Oliver, M. Johnson, C. Ward, A. J. Cheon, Y. S. Choo, and I. C. Kim. 2000. Pharmacokinetics and tolerability of gemifloxacin (SB-265805) after administration of single oral doses to healthy volunteers. Antimicrob. Agents Chemother. 44:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andriole, V. T. 1999. The future of the quinolones. Drugs 58(Suppl. 2):1-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S. J., L. Lin, and L. W. Chang. 2007. Analysis of antibacterial response of nemonoxacin (TG-873870) against major pathogens from respiratory tract and skin infections, abstr. 441. Abstr. 45th Infect. Dis. Soc. Am.
- 5.Chow, A. T., C. Flowler, R. R. Williams, N. Morgan, S. Kaminski, and J. Nataranjan. 2001. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 45:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow, C. P., C. P. Sambuco, and A. M. Hoberman. 2007. Phototoxicity of nemonoxacin (TG-873870) in hairless mice, abstr. A-28. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 7.Chow, C. P., C. Y. Tsai, C. F. Yeh, and S. J. Chen. 2007. In vitro metabolism and interaction of nemonoxacin (TG-873870) on human hepatic CYP3A4, abstr. A-27. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 8.Chow, C. P., C. Y. Tsai, and Y. M. Chen. 2007. Cardiovascular safety evaluation of nemonoxacin (TG-873870) in dogs and monkeys, abstr. B-823. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 9.Coleman, C. I., J. V. Spencer, J. O. Chung, and P. Reddy. 2002. Possible gatifloxacin-induced fulminant hepatic failure. Ann. Pharmacother. 36:1162-1167. [DOI] [PubMed] [Google Scholar]
- 10.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 11.Gajjar, D. A., A. Bello, Z. Ge, L. Christopher, and D. M. Grasela. 2003. Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects. Antimicrob. Agents Chemother. 47:2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, NY.
- 13.Gough, K., M. Hutchinson, O. Keene, B. Byron, S. Ellis, L. Lacey, and J. McKellar. 1995. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmacokinetic UK joint working party. Drug Inform. J. 29:1039-1048. [Google Scholar]
- 14.Hsu, C. H., Y. M. Chen, and C. P. Chow. 2008. Fertility and early embryonic developmental toxicity of nemonoxacin after oral administration to rats, abstr. F1-2056. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 15.Hsu, C. H., Y. M. Chen, and C. P. Chow. 2008. Systemic hypersensitivity test of nemonoxacin, a novel potent broad-spectrum non-fluorinated quinolone in guinea pigs, abstr. F1-2055. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 16.King, C. H. R., L. Lin, and R. Leunk. 2008. In vitro resistance development to nemonoxacin for Streptococcus pneumoniae, abstr. C1-1971. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 17.Lauderdale, T. L., Y. R. Shiau, J. H. Lai, H. C. Chen, and C. H. King. 2007. In vitro antibacterial activity of nemonoxacin (TG-873870), a new non-fluorinated quinolone, against clinical isolates, abstr. E-1635. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 18.Lin, L., K. M. Chiu, and T. K. Chung. 2007. Single-dose safety, tolerability, and pharmacokinetics of nemonoxacin (TG-873870), a novel potent broad-spectrum nonfluorinated quinolone, in healthy volunteers, abstr. 439. Abstr. 45th Infect. Dis. Soc. Am. [DOI] [PMC free article] [PubMed]
- 19.Lipsky, B. A., and C. A. Baker. 1999. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin. Infect. Dis. 28:352-364. [DOI] [PubMed] [Google Scholar]
- 20.Lode, H., K. Borner, and P. Koeppe. 1998. Pharmacodynamics of fluoroquinolones. Clin. Infect. Dis. 27:33-39. [DOI] [PubMed] [Google Scholar]
- 21.Lubasch. A., I. Keller, K. Borner, P. Koeppe, and H. Lode. 2000. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob. Agents Chemother. 47:2600-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noreddin, A. M., A. A. Reese, M. Ostroski, D. J. Hoban, and G. G. Zhanel. 2007. Comparative pharmacodynamics of garenoxacin, gemifloxacin, and moxifloxacin in community-acquired pneumonia caused by Streptococcus pneumoniae: a Monte Carlo simulation analysis. Clin. Ther. 29:2685-2689. [DOI] [PubMed] [Google Scholar]
- 23.Owens, R. C., Jr., and P. G. Ambrose. 2005. Antimicrobial safety: focus on fluoroquinolones. Clin. Infect. Dis. 41(Suppl. 2):S144-S157. [DOI] [PubMed] [Google Scholar]
- 24.Pankuch, G. A., K. Kosowska-Shick, P. McGhee, C. H. R. King, and P. C. Appelbaum. 2008. Comparative antistaphylococcal activity of nemonoxacin, a novel broad-spectrum quinolone, abstr. C1-189. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 25.Roychoudhury, S., and B. Ledoussal. 2002. Non-fluorinated quinolones (NFQs): new antibacterials with unique properties against quinolone-resistant gram-positive pathogens. Curr. Drug Targets Infect. Disord. 2:51-65. [DOI] [PubMed] [Google Scholar]
- 26.Schentag, J. J., A. K. Meagher, and A. Forrest. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 1: in vitro and animal models. Ann. Pharmacother. 37:1287-1298. [DOI] [PubMed] [Google Scholar]
- 27.Schentag, J. J., A. K. Meagher, and A. Forrest. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 2: human trials. Ann. Pharmacother. 37:1478-1488. [DOI] [PubMed] [Google Scholar]
- 28.Smith, B. P., F. R. Vandenhende, K. A. DeSante, N. A. Farid, P. A. Welch, J. T. Callaghan, and S. T. Forgue. 2000. Confidence interval criteria for assessment of dose proportionality. Pharm. Res. 17:1278-1283. [DOI] [PubMed] [Google Scholar]
- 29.Stahlmann, R. 2002. Clinical toxicological aspects of fluoroquinolones. Toxicol. Lett. 127:269-277. [DOI] [PubMed] [Google Scholar]
- 30.Stass, H., A. Dalhoff, D. Kubitza, and U. Schühly. 1998. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 42:2060-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 2006. Proposed INN list 96. World Health Organ. Drug Inf. 20:292. [Google Scholar]
- 32.Zhanel, G. G., and A. M. Noreddin. 2001. Pharmacokinetics and pharmacodynamics of the new fluoroquinolones: focus on respiratory infections. Curr. Opin. Pharmacol. 1:459-463. [DOI] [PubMed] [Google Scholar]
- 33.Zhanel, G. G., N. Laing, M. DeCorby, K. Nichol, C. H. R. King, H. Adam, and D. J. Hoban. 2008. Activity of nemonoxacin, an investigational C8-methoxy non-fluorinated quinolone, against Gram-positive cocci obtained from Canadian hospitals: CANWARD 2007, abstr. F1-2057. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 34.Zimpfer, A., A. Propst, G. Mikuz, W. Vogel, L. Terracciano, and S. Stadlmann. 2004. Ciprofloxacin-induced acute liver injury: case report and review of literature. Virchows Arch. 444:87-89. [DOI] [PubMed] [Google Scholar]