Abstract
Enfuvirtide (also known as Fuzeon, T-20, or DP-178) is an antiretroviral fusion inhibitor which prevents human immunodeficiency virus type 1 (HIV-1) from entering host cells. This linear 36-mer synthetic peptide is indicated, in combination with other antiretroviral agents, for the treatment of HIV-1-infected individuals and AIDS patients with multidrug-resistant HIV infections. Although enfuvirtide is an efficient anti-HIV-1 drug, its clinical use is limited by a short plasma half-life, i.e., approximately 2 h, which requires twice-daily subcutaneous injections, often resulting in skin sensitivity reaction side effects at the injection sites. Ultimately, 80% of patients stop enfuvirtide treatment within 6 months because of these side effects. We report on the development of long-lasting enfuvirtide conjugates by the use of the site-specific conjugation of enfuvirtide to an antithrombin-binding carrier pentasaccharide (CP) through polyethylene glycol (PEG) linkers of various lengths. These conjugates showed consistent and broad anti-HIV-1 activity in the nanomolar range. The coupling of the CP to enfuvirtide only moderately affected the in vitro anti-HIV-1 activity in the presence of antithrombin. Most importantly, one of these conjugates, enfuvirtide-PEG12-CP (EP40111), exhibited a prolonged elimination half-life of more than 10 h in rat plasma compared to the half-life of native enfuvirtide, which was 2.8 h. On the basis of the pharmacokinetic properties of antithrombin-binding pentasaccharides, the anticipated half-life of EP40111 in humans would putatively be about 120 h, which would allow subcutaneous injection once a week instead of twice daily. In conclusion, EP40111 is a promising compound with strong potency as a novel long-lasting anti-HIV-1 drug.
Enfuvirtide (also known as Fuzeon, T-20, or DP-178) is a well-described antiviral fusion inhibitor which prevents human immunodeficiency virus type 1 (HIV-1) from entering host cells (28, 38, 57). This linear 36-mer synthetic peptide is indicated, in combination with other antiretroviral agents, for the treatment of HIV-1-infected individuals and AIDS patients with multidrug-resistant HIV infections who no longer respond to current highly active antiretroviral therapies (i.e., HIV reverse transcriptase and protease inhibitors). Because enfuvirtide interferes with an earlier stage of infection than highly active antiretroviral therapies, no development of cross-resistance to existing antiretroviral drugs is expected, given their distinct mechanisms of action (9, 31, 32, 35, 43). Recently, the small molecule maraviroc (Selzentry/Celsentry; Pfizer), which acts as a chemokine receptor 5 (CCR5) antagonist, was the second HIV entry inhibitor to receive approval from the U.S. FDA (16, 19).
The entry of HIV-1 into target cells is mediated by the viral envelope, which is a trimer consisting of three surface gp120 glycoproteins noncovalently associated with three transmembrane gp41 subunits (17, 20, 26). The extracellular domain (ectodomain) of gp41 is the key structure responsible for fusion and consists of a hydrophobic fusion peptide at the N terminus, an N-terminal heptad repeat (NHR or HR1), a C-terminal heptad repeat (CHR or HR2), and a tryptophan-rich region. The precise mechanism of the viral entry process remains unclear; but in the current model, the first step of infection is initiated by the binding of gp120 to CD4, the primary receptor of HIV on the target cell (11). The binding of gp120 to CD4 causes conformational changes of the V1, V2, and V3 variable loop regions of gp120, resulting in the subsequent interaction with the chemokine receptors CCR5 and/or CXCR4. The viral gp120-gp41 complex then undergoes further conformational changes, exposing the N-terminal domain of gp41 and allowing the fusion peptide sequence to insert into the membrane of the host cell. At this stage of fusion, gp41 adopts an extended prehairpin intermediate conformation that bridges both the viral and the cellular membranes. This transient intermediate can last for several minutes, but ultimately, gp41 folds into a hairpin-like structure in which the two hydrophobic heptad repeats (NHR and CHR) lie antiparallel and form a six-helix bundle, thus bringing the viral and host cell membranes into close proximity. This allows the fusion of the two membranes, and the viral content is expelled into the host cell.
The prehairpin intermediate state of gp41 is the target for the peptide enfuvirtide, which derives from the gp41 CHR region (corresponding to HIV-1 IIIB/LAI gp41 residues 127 to 162) and which binds to the viral gp41 NHR region, preventing the formation of the six-helix bundle required for membrane fusion (15, 28).
Despite its therapeutic efficacy, the clinical use of enfuvirtide is limited by a short plasma half-life, i.e., approximately 2 h (28, 29, 31, 48, 58), which requires twice-daily subcutaneous injections of 90 mg drug product, which often result in skin sensitivity side effects at the injection sites (3, 37, 39, 56). Thus, improvement of the pharmacokinetic (PK) properties of this type of molecule is highly desired to facilitate its therapeutic use and to bring medical benefit to patients.
Several technologies have been developed to improve the PK and pharmacodynamics (PD) of polypeptides and proteins (7). For instance, (bio)chemical modification (e.g., pegylation [52], acylation [22], or glycosylation [47]) and fusion with or binding to plasma proteins (e.g., albumin [13], transferrin [2], or immunoglobulin Fc fragments [34]) have yielded numerous clinical protein candidates with improved PK-PD profiles. Pegylation, the covalent attachment of polyethylene glycol (PEG) polymers to compounds, is some 20 years old and has become one of the best-validated methods for the development of long-acting peptide/protein analogs (33, 44, 55). The PEG polymer is added to therapeutic peptides/proteins in order to prolong their absorption, decrease their renal clearance, retard their enzymatic degradation, increase their elimination half-life, and reduce their immunogenicity (25). A pegylated version of enfuvirtide was patented (2a), but up to now, there are still no long-acting enfuvirtide derivatives on the market or even in late-stage clinical development. Recently, Stoddart et al. (49) reported on the conjugation of the C34 anti-HIV fusion peptide with human serum albumin that resulted in an albumin-conjugated C34 peptide with improved PK properties. Therefore, the goal of the present study was to develop an enfuvirtide-based molecule with sustained in vitro and in vivo anti-HIV efficacy and a prolonged in vivo half-life, which would eventually allow reduction of the injection frequency and, thus, increase patient convenience and compliance.
Our strategy exploits the properties of a synthetic pentasaccharide (42) that reversibly binds with a high affinity to antithrombin, a 60-kDa plasma protein with a half-life of approximately 3 days that circulates at a relatively high concentration (2.5 μM) in blood and which regulates the activity of blood-clotting proteinases (8, 23). The plasma half-life of synthetic pentasaccharides that bind to antithrombin is governed by their affinity for the protein (50). For instance, the anticoagulant pentasaccharidic drugs idraparinux and fondaparinux are synthetic factor Xa inhibitors that reversibly bind with a high affinity to antithrombin. Due to its increased sulfation, idraparinux exhibits a 30-fold higher binding affinity to antithrombin than fondaparinux does (53). As a result, idraparinux exhibits a significantly longer half-life in humans of about 120 h compared to that of fondaparinux, which is 17 h (27, 36, 54). Therefore, this novel approach takes advantage of the long plasma half-life of such a high-affinity pentasaccharide to enhance the PK-PD properties of therapeutic peptides and proteins, as was previously described for α-NAPAP, a peptidomimetic anticoagulant drug (10), and insulin (14). The purpose of this study was to investigate whether this approach could be applied to the peptide enfuvirtide. The acquired half-lives of these novel enfuvirtide derivatives should be close to that of the pentasaccharide (which has a chemical structure similar to but different from that of idraparinux), i.e., up to 120 h in humans, which is about 60 times the half-life of the original peptide (i.e., 2 h).
We report here on the development of long-lasting enfuvirtide conjugates by the use of the site-specific conjugation of enfuvirtide to an antithrombin-binding carrier pentasaccharide (CP) through PEG linkers of various lengths. These conjugates showed consistent and broad anti-HIV-1 activities in the nanomolar range, and the length of the PEG linker did not significantly influence their antiviral activities. Coupling of the CP to enfuvirtide through a PEG linker only moderately affected its in vitro anti-HIV-1 activity in the presence of antithrombin. The most potent compound, enfuvirtide-PEG12-CP (EP40111), exhibited in vivo a prolonged elimination half-life compared to that of native enfuvirtide.
While the present work was in progress, a report on the proof of principle of this technology applied to insulin appeared in the literature (14).
MATERIALS AND METHODS
Materials.
Unless stated otherwise, all chemicals were purchased from Sigma-Aldrich. The heterobifunctional PEG linkers, succinimidyl-[(N-maleimidopropionamido)-polyethyleneglycol] ester, were purchased from Pierce (NHS-PEG4-Mal and NHS-PEG12-Mal), Nektar (NHS-PEG-Mal-3,400, which corresponds to NHS-PEG70-Mal), and Polypure (NHS-PEG28-Mal). The chromatographic Q-Sepharose resin, Superdex Peptide 10/300, and Superdex 200 10/300 GL columns were supplied by GE Healthcare. The N-terminal cysteine analogue of enfuvirtide, Ac-CYTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF-NH2 (where Ac is acetyl), and the 13C,15N-labeled enfuvirtide peptide Ac-YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF-NH2 containing one [13C, 15N]asparagine-labeled residue (highlighted in boldface) were prepared by 9-fluorenylmethoxycarbonyl (Fmoc)/tert-butyl (tBu) solid-phase chemistry and were subsequently purified by reversed-phase high-pressure liquid chromatography (HPLC) by the Almac Group Ltd., United Kingdom. Optical rotations were measured on a 243 automatic polarimeter (Perkin-Elmer, Brussels, Belgium) by using a sodium lamp (λ = 365 nm) with a standard cell (10 cm/1 ml). Native enfuvirtide used in this study was the commercial drug (Fuzeon; Roche, Switzerland). Human antithrombin was purchased from Hyphen BioMed, France.
Viruses.
The HIV-1 IIIB strain was provided by R. C. Gallo (at that time at the National Institutes of Health, Bethesda, MD, and currently at the Institute of Human Virology, University of Maryland, Baltimore, MD), the HIV-1 LAI strain was provided by F. Barré-Sinoussi (Institut Pasteur, Paris, France), and the HIV-2 ROD strain was provided by L. Montagnier (at that time at Institut Pasteur, Paris, France). The CXCR4-using (X4) HIV-1 NL-4.3 strain was obtained from the AIDS Reagent Program, NIAID, National Institutes of Health. The CCR5-using (R5) HIV-1 Ba-L strain was obtained from the Medical Research Council AIDS Reagent Project (Herts, United Kingdom). It should be noted that strains HIV-1 IIIB (21) and LAI (formerly named LAV and then BRU) (6) derived from the same original strain and are therefore considered to be the same strain in this study. Hence, for the clarity of the work described herein, these viruses are named HIV-1 IIIB/LAI. HIV-1 NL4.3 is actually a chimeric recombinant virus engineered in laboratory (1). The 3′ half of the proviral DNA coding for the viral envelope was cloned from HIV-1 LAI (the 5′ half, which encodes the gag and pol genes, was cloned from HIV-1 NY5). Infectious HIV-1 NL4.3 particles were obtained upon transfection in mammalian cells. These three HIV-1 laboratory strains, IIIB, LAI, and NL4.3, are therefore identical at the level of the viral envelope glycoproteins gp120 and gp41, with gp41 being the target of the enfuvirtide conjugates.
The primary clinical isolates included in this study were I-2496 (clade A, R5), UG273 (clade A, R5), US2 (clade B, R5), BZ167 (clade B, X4), ETH2220 (clade C, R5), and DJ259 (clade C, R5). All these viruses were kindly provided by J. L. Lathey, who was then at Biotech Research Laboratories Inc. (Gaithersburg, MD). The coreceptor specificity (R5 or X4) of these isolates was determined on the astroglioma U87.CD4 cell line expressing either CCR5 or CXCR4 (5).
Synthesis of enfuvirtide-PEGn-CP.
For the chemoselective ligation of enfuvirtide to the maleimide (Mal)-functionalized Mal-PEGn-CP (i.e., a maleimide-functionalized PEG linker of n ethylene glycol units chemically attached to the antithrombin-binding pentasaccharide), a cysteine residue was introduced at the N terminus of enfuvirtide by using standard Fmoc/tBu solid-phase peptide chemistry to give cysteine-containing enfuvirtide.
(i) General procedure.
An aqueous solution of the maleimide-functionalized Mal-PEGn-CP (1.5 equivalents) was added dropwise to 1 equivalent of the Cys-enfuvirtide peptide (molecular mass, 4,595 Da) dissolved in 4 ml of a mixture (1/1, vol/vol) of phosphate buffer (100 mM, pH 7.2) and acetonitrile. The reaction mixture was stirred under an argon atmosphere at room temperature overnight and was then lyophilized. The freeze-dried product was then dissolved in water and subjected to gel filtration chromatography (Superdex Peptide or Superdex 200 10/300 GL chromatography) to remove the Mal-PEGn-CP and unmodified enfuvirtide. The column was eluted with H2O and the elution was followed by measurement of the absorbance at 280 nm. The product-containing fraction was subjected to freeze-drying to obtain enfuvirtide-PEGn-CP. Electrospray ionization (ESI)-quadrupole time-of-flight (Q-TOF) mass spectrometry (MS) analysis confirmed the predicted molecular masses of the maleimide-functionalized Mal-PEGn-CP moieties as well as those of conjugates EP40111 and EP40112. Matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) MS analysis confirmed the predicted molecular masses of the EP40113 and EP40114 conjugates, which could not be determined by ESI-Q-TOF MS. The purities of the final products were estimated to be >80%, as judged by the use of silver-stained sodium dodecyl sulfate-polyacrylamide gels and high-field 1H-nuclear magnetic resonance spectroscopy.
(ii) Enfuvirtide-PEG4-CP (EP40114).
Mal-PEG4-CP (741 μl from a 2.2 mM stock solution in H2O, 1.6 μmol) was conjugated to Cys-enfuvirtide (5 mg, 1.1 μmol) and purified according to the general procedure to give enfuvirtide-PEG4-CP as a white powder (3.4 mg, 46%). MALDI-TOF MS, [M] calculated for C264H390N55Na9O121S8: 6,733. Found: 6,421 [M-3SO3Na].
(iii) Enfuvirtide-PEG12-CP (EP40111).
Mal-PEG12-CP (2.5 ml from a 2.9 mM stock solution in H2O, 7.2 μmol) was conjugated to Cys-enfuvirtide (22 mg, 4.8 μmol) and purified according to the general procedure to give enfuvirtide-PEG12-CP as a white powder (32.6 mg, 96%). ESI-Q-TOF MS, positive mode, Q-Star, [M] calculated for C280H422N55Na9O129S8: 7,086. Found: 7,084.
(iv) Enfuvirtide-PEG28-CP (EP40112).
Mal-PEG28-CP (1.0 ml from a 5.2 mM stock solution in H2O, 5.5 μmol) was conjugated to Cys-enfuvirtide (17 mg, 3.7 μmol) and purified according to the general procedure to give enfuvirtide-PEG28-CP as a white powder (21.7 mg, 76%). ESI-Q-TOF MS, positive mode, Q-Star, [M] calculated for C312H486N55Na9O145S8: 7,791. Found: 7,592 [M-9Na].
(v) Enfuvirtide-PEG70-CP (EP40113).
Mal-PEG70-CP (950 μl from a 4.5 mM stock solution in H2O, 4.3 μmol) was conjugated to Cys-enfuvirtide (13.2 mg, 2.9 μmol) and purified according to the general procedure to give enfuvirtide-PEG70-CP as a white powder (22 mg, 80%). MALDI-TOF MS, [M] calculated for C393H648N53Na9O187S8: 9,571. Found: 9,583.
Factor Xa inhibition-based bioassay.
Anti-factor Xa activity was determined with a Stachrom HP kit and an STA compact automated device (Diagnostica Stago, France). The test compound was mixed with bovine antithrombin (2.5 μM) in Tris-EDTA buffer (50 mM, pH 8.4), and the mixture was incubated with bovine factor Xa (3.5 nkat/ml) for 1 min at 37°C. During this incubation, factor Xa was neutralized by the complex formed between antithrombin and the pentasaccharide part of the test compound. A factor Xa chromogenic substrate (CBS 31.39, CH3SO2-d-Leu-Gly-Arg-para-nitroaniline) was then added (final concentration,1 mM), and the release of para-nitroaniline was immediately measured by spectrophotometry detection at 405 nm. The 50% inhibitory concentration (IC50) was obtained by plotting the percentage of the remaining anti-factor Xa activity as a function of the test compound concentrations.
Anti-HIV activity in CEM cells.
CEM cells (4.5 × 105 cells per ml) were suspended in fresh culture medium and were infected either with HIV-1 IIIB/LAI or HIV-2 ROD at 100 50% cell culture infective doses per ml of cell suspension. Then, 100-μl aliquots of the infected cell suspension were transferred to microplate wells, 100 μl of the appropriate dilutions of the test compounds was added to the wells, and the plates were further incubated at 37°C. After 4 to 5 days, the level of giant cell formation in the CEM cell cultures was scored microscopically. The 50% effective concentration (EC50) corresponds to the compound concentration required to prevent giant cell formation by 50% in the HIV-1- or HIV-2-infected CEM cell cultures.
Cell-cell fusion (syncytium formation) assay.
The inhibition of envelope-mediated cell-cell fusion by enfuvirtide and the conjugates was evaluated in persistently HIV-1-infected HUT-78 cells (designated HUT-78/HIV-1 IIIB/LAI cells) cocultured with the SupT1 CD4+ T-cell line. The first syncytia arose after about 6 h of cocultivation at 37°C in a humidified 5% CO2 atmosphere. After 16 to 20 h of coculture, marked syncytium formation was noted, and the number of syncytia was determined by optical microscopy, as previously described in detail (4). The EC50 corresponds to the compound concentration required to prevent syncytium formation by 50%.
Anti-HIV activity in PBMCs.
Testing of the activities of the conjugates against various HIV-1 isolates in peripheral blood mononuclear cells (PBMCs) was performed as follows: PBMCs from healthy donors were isolated by density gradient centrifugation and were stimulated with phytohemagglutinin (PHA) at 2 μg/ml (Sigma, Bornem, Belgium) for 3 days at 37°C. The PHA-stimulated blasts were washed twice with phosphate-buffered saline and counted after they were stained with trypan blue. The cells were then seeded at 0.5 × 106 cells per well into a 48-well plate in duplicate with various concentrations of compound in cell culture medium (RPMI 1640) containing 10% fetal calf serum and interleukin-2 (25 U/ml; R&D Systems Europe, Abingdon, United Kingdom). The virus stocks were diluted in medium and added at a final dose of 250 pg/ml p24, as determined by use of a specific p24 core antigen enzyme-linked immunosorbent assay (ELISA) kit (Perkin-Elmer). The cell supernatant was collected on day 12, and the level of HIV-1 p24 core antigen was quantified by use of the p24 ELISA kit. The EC50 corresponds to the compound concentration required to inhibit virus replication by 50%, as assessed by the level of p24 antigen in the culture medium.
Anti-HIV activity in the presence of antithrombin.
The influence of antithrombin on the anti-HIV-1 activities of the enfuvirtide conjugates was tested by using an assay similar to the one mentioned above and as described previously (45), except that human PBMCs were infected with HIV-1 IIIB/LAI in the presence (or the absence, as a control) of 2.5 μM antithrombin.
Cell viability assay.
The potential cytotoxicity induced by the conjugates was assessed by the in situ reduction of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) by a CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison, WI). The absorbance at 490 nm was then measured spectrophotometrically with a 96-well plate reader (Molecular Devices, Sunnyvale, CA) and was compared to the absorbance of four cell control replicates (cells without drugs).
Half-life determination.
Female Wistar rats (weight, ∼200 g; Elevage Janvier, France) cannulated in the jugular vein were anesthetized, and then EP40111 or enfuvirtide (dose, 4 mg/kg of body weight) was injected intravenously via the cannula (two rats were used for each compound). After administration, blood samples were collected from the cannula at several time points (5, 15, and 30 min and 1, 2, 4, 8, 24, and 48 h after drug injection) and placed in citrate-containing tubes.
The concentrations of EP40111 and enfuvirtide in rat plasma (μg compound/ml plasma) was determined by liquid chromatography (LC)-tandem MS (LC-MS/MS) after trypsin digestion of the plasma samples, as described below.
The plasma compound concentrations were determined as a function of time, and the half-life was then calculated by regression of the semilogarithmic concentration-versus-time data by using the PK Functions for Microsoft Excel program (J. L. Usansky, A. Desai, and D. Tang-Liu, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA [http://www.boomer.org/pkin/soft.html]).
Animal studies were conducted in full compliance with the good practice principles set forth by the European Ethical Guidelines of Animal Experimentation and according to European Directive 86/609/EEC and Additional Protocol ETS 123.
LC-MS/MS quantification of EP40111 and enfuvirtide in rat plasma.
Rat plasma samples (50 μl) were spiked with the isotopic internal standard, [13C, 15N]asparagine-labeled enfuvirtide (250 μg/ml, 5 μl). Protein precipitation was then carried out by addition of 1 M HCl (20 μl) and acetonitrile (200 μl). The samples were vortexed, centrifuged, and vacuum dried; and the resulting pellets were reconstituted in 100 μl of an acetonitrile-water mixture (5/95, vol/vol) and subjected to reduction for 10 min by addition of ammonium bicarbonate (50 mM, 70 μl) and dithiothreitol (50 mM, 10 μl) and then to alkylation for 45 min by adding iodoacetamide (75 mM, 20 μl). The samples were then treated with trypsin (2.5 mg/ml, 20 μl) overnight at 37°C and analyzed by LC-MS/MS.
LC-MS/MS analysis was performed with a system consisting of an HP 1100 series HPLC device (Agilent Technologies, Waldbronn, Germany) coupled to an API 2000 triple quadrupole (Applied Biosystems/MDS Analytical Technologies, Foster City, CA) equipped with an ESI. The LC separation was carried out on a Symmetry C18 column (Waters, Milford, MA); and the mobile phase consisted of water (A) and acetonitrile (B), each of which contained 0.1% (vol/vol) formic acid. The solvent flow rate was 300 μl/min; and the following linear gradient (A/B, vol/vol) was used: at 0 to 10 min, from 95/5 to 50/50; at 10 to 11 min, from 50/50 to 0/100; and at 11 to 14 min, 0/100.
Instrument control, data acquisition, and processing were performed with the associated Analyst 1.4.2 software. Selected reaction monitoring experiments were performed in the positive ionization mode to detect ion transitions at m/z 615.8/244.1 and m/z 618.8/250.1, which correspond to the fragment NEQELLELDK of enfuvirtide (dwell time, 300 ms) and the internal standard, [13C, 15N]asparagine-labeled enfuvirtide (dwell time, 100 ms), respectively.
RESULTS
Drug design and synthesis.
The enfuvirtide-CP conjugates were prepared by using the well-established thiomaleimide coupling technology. A cysteine-containing enfuvirtide peptide, in which a cysteine motif was added at the N terminus of enfuvirtide, was first synthesized. This modification does not alter the biological activity of the peptide and is well suited for covalent conjugation (40). The enfuvirtide-CP conjugates were synthesized by the chemoselective ligation of the cysteine-containing enfuvirtide peptide to maleimide-functionalized Mal-PEGn-CP. Under specific conditions (CH3CN/phosphate buffer, pH 7.2), the maleimide group of Mal-PEGn-CP reacts with the sulfhydryl group of the peptide to form a stable thioether bond. The resulting compounds had a much better aqueous solubility than native enfuvirtide.
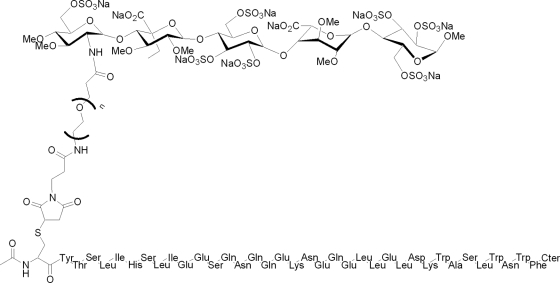
It was anticipated that the molecular distance between enfuvirtide and the pentasaccharide part of the conjugates would be a parameter critical for the retention of both the antifusion activity mediated by enfuvirtide and the affinity to antithrombin mediated by the pentasaccharide, with the latter governing the half-life of the conjugates. Thus, four enfuvirtide-PEGn-CP conjugates with PEG linkers of various lengths (n = 4, 12, 28, and 70) were synthesized (Fig. 1).
FIG. 1.
Chemical structures of the enfuvirtide-CP conjugates. Compounds EP40114, EP40111, EP40112, and EP40113 correspond to enfuvirtide-PEGn-CP with 4, 12, 28, and 70 (labeled n) ethylene glycol units (i.e., PEG), respectively. Enfuvirtide is connected to the PEG linker through its N terminus. The C terminus (Cter) of enfuvirtide is a carboxamide, CONH2.
Anti-factor Xa activity.
The affinities of the enfuvirtide-CP conjugates for antithrombin were assessed by using an anti-factor Xa bioassay. In this bioassay, the pentasaccharide-containing compound binds to antithrombin and induces a conformational change of the protein, which results in the accelerated and selective inhibition of factor Xa. Although this is not a method that allows the direct measurement of affinity (Kd), the anti-factor Xa activity is a reliable marker for evaluation of the interaction between poly- and oligosaccharidic anticoagulants (heparin derivatives, fondaparinux, idraparinux) and antithrombin (42, 50). The enfuvirtide-CP conjugates inhibited the activity of factor Xa in a dose-dependent manner, with the IC50s being 93, 56, 53, and 32 nM for EP40114 (n = 4), EP40111 (n = 12), EP40112 (n = 28), and EP40113 (n = 70), respectively. These activities were lower than the activity of the pentasaccharide alone (IC50, 16 nM); but except maybe for EP40114, which has the shortest PEG linker, they were still comparable to the activity of the pentasaccharide alone.
Anti-HIV activity profile.
The first series of experiments was designed to evaluate the in vitro anti-HIV activity profiles of the four conjugates (EP40111, EP40112, EP40113, and EP40114) in viral infection assays with a CD4+ T-cell line (CEM) infected with reference HIV laboratory strains HIV-1 IIIB/LAI and HIV-2 ROD. All four enfuvirtide-CP conjugates displayed potent anti-HIV-1 activity and inhibited HIV-1 IIIB/LAI infection, with the EC50s being in the low-nanomolar range (6 to 18 nM) and with EP40111 consistently being two- to threefold more active than the other conjugates (Table 1). These values were comparable to the value for native enfuvirtide (EC50, 3 nM). Like native enfuvirtide, enfuvirtide-CP conjugates were much less active against HIV-2 ROD.
TABLE 1.
Anti-HIV activities of enfuvirtide-CP conjugates in virus infection (CEM cells infected with HIV-1 IIIB/LAI or HIV-2 ROD) and cell-cell fusion (HUT-78/HIV-1 IIIB/LAI cocultured with SupT1 cells) assays
| Compound | No. of ethylene glycol units (PEG) | Length (Å) of PEG linker | EC50 (nM)a |
||
|---|---|---|---|---|---|
| CEM cells infected with: |
HUT-78/HIV-1 IIIB/LAI cocultured with SupT1 | ||||
| HIV-1 IIIB/LAI | HIV-2 ROD | ||||
| EP40114 | 4 | 25 | 13 ± 12 | 280 ± 110 | 65 ± 4 |
| EP40111 | 12 | 54 | 6 ± 3 | 670 ± 67 | 72 ± 15 |
| EP40112 | 28 | 106 | 18 ± 7 | 630 ± 64 | 61 ± 0 |
| EP40113 | 70 | 247 | 15 ± 9 | 990 ± 730 | 51 ± 15 |
| Enfuvirtide | 3 ± 1 | 360 ± 46 | 275 ± 150 | ||
The results represent the means ± SDs of three independent experiments, each of which was performed in triplicate.
The anti-HIV-1 activities of enfuvirtide-CP conjugates EP40111, EP40112, EP40113, and EP40114 were also evaluated in a cell-cell fusion assay, in which persistently HIV-1-infected cells (HUT-78/HIV-1 IIIB/LAI cells) were cocultured with uninfected CD4+ T cells (SupT1 cells). It was previously reported that this system is relatively resistant to the antiviral activity of enfuvirtide (5). In this system, the enfuvirtide-CP conjugates were consistently about fivefold more potent than native enfuvirtide in preventing syncytium formation. The enfuvirtide-CP conjugates displayed similar EC50s that ranged from 51 nM to 72 nM, whereas native enfuvirtide showed an EC50 of about 275 nM (Table 1).
The activities of compounds EP40111 and EP40112 against X4 or R5 laboratory strains and also against six different HIV-1 clinical isolates (two clade A, two clade B, and two clade C viruses) were then evaluated with PBMCs from different donors (Table 2). In general, EP40111 and EP40112 were somewhat less active than native enfuvirtide, but broad and consistent anti-HIV-1 activity was observed. When the data obtained with the PBMCs and the various HIV-1 strains and clades used in these experiments are averaged, the mean EC50 of enfuvirtide was about 20 nM, whereas the mean EC50s of EP40111 and EP40112 were about 100 and 130 nM, respectively. The CXCR4 antagonist AMD3100 (which is active only against X4 viruses) (46) and the CCR5 antagonist maraviroc (which is active only against R5 viruses) (16) were included as control compounds and displayed antiviral activities according to the coreceptor specificities of the HIV-1 strains/clades used in this study. Unlike AMD3100 and maraviroc, the enfuvirtide-CP conjugates and native enfuvirtide inhibited HIV-1 independently of the coreceptor specificity.
TABLE 2.
Anti-HIV-1 activities of enfuvirtide-CP conjugates EP40111 and EP40112 against various X4 and R5 HIV-1 strains and clades in PBMCs
| Compound | EC50 (nM)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NL4.3b (X4) | Ba-L (R5) | BZ167 (clade B, X4) | I-2496 (clade A, R5) | UG273 (clade A, R5) | US2 (clade B, R5) | ETH2220 (clade C, R5) | DJ259 (clade C, R5) | Mean for all strains and cladesd | |
| Enfuvirtide | 3.6 | 17.9 | 17.9 | 12.4 | 55.9 | 6.3 | 17.7 | 17.9 | 20 |
| EP40111 | 89.4 | 88.9 | 105.7 | 54.8 | 277.6 | 72.7 | 92.2 | 9.4 | 100 |
| EP40112 | 13.2 | 108.0 | 445.0 | 80.7 | 136.0 | 43.8 | 111.0 | 89.4 | 130 |
| AMD3100c | 3.6 | >1,000 | 18.5 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 | NCe |
| Maravirocc | >1,000 | 3.8 | >1,000 | 0.4 | 2.6 | 0.3 | 3.6 | 3.7 | NCe |
Representative values from several independent set of experiments in PBMCs from different donors.
The HIV-1 NL4.3 envelope sequence is identical to that of HIV-1 IIIB/LAI.
The CXCR4 antagonist (AMD3100) and the CCR5 antagonist (maraviroc) (kindly provided by G. Bridger, then at AnorMED, Langley, BC, Canada) were used as controls.
Mean values based on the EC50s obtained with all virus strains and clinical isolates.
NC, not calculated, since some EC50s were >1,000 nM.
It is worth noticing that none of the enfuvirtide-CP conjugates was cytotoxic at concentrations of up to 100 μM for uninfected CD4+ T-cell cultures (CEM cells and PBMCs), as determined by microscopic evaluation and as measured by the cell viability MTS assay (data not shown).
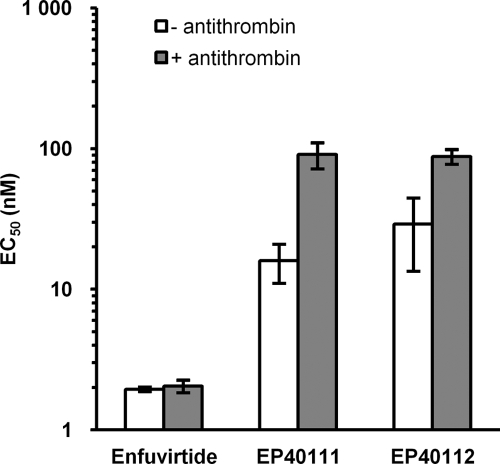
In plasma, the conjugates will bind to antithrombin, which could putatively abolish their antiviral activity by steric hindrance. The effect of antithrombin on the antiviral activity of the enfuvirtide-CP conjugates was thus studied under conditions mimicking the physiological situation in humans, in whom antithrombin is present at a concentration of about 2.5 μM. The activities of enfuvirtide-CP conjugates EP40111 and EP40112 against HIV-1 IIIB/LAI were compared to the activity of enfuvirtide in PBMCs (Fig. 2). In the presence of 2.5 μM antithrombin, both compounds retained their anti-HIV-1 activities, although they were lowered by a factor 3 for EP40112 and a factor 6 for EP40111 compared to their activities under conditions without antithrombin. Nevertheless, the mean EC50s remained below 100 nM. As expected, native enfuvirtide was not sensitive to the presence of antithrombin.
FIG. 2.
Antiviral activities (EC50s) of enfuvirtide-CP conjugates EP40111 and EP40112 against HIV-1 IIIB/LAI infecting PBMCs in the absence or the presence of 2.5 μM antithrombin. These results represent the means and standard deviations of three independent experiments, each of which was performed in triplicate.
Half-life determination.
EP40111 and enfuvirtide were injected into rats in order to measure their respective elimination half-lives. An LC-MS/MS detection assay was specifically developed to detect the enfuvirtide peptide within EP40111. In this assay, isotope-labeled enfuvirtide was used as an internal standard. This method allowed the direct comparison of EP40111 and enfuvirtide in the same study and by the same method.
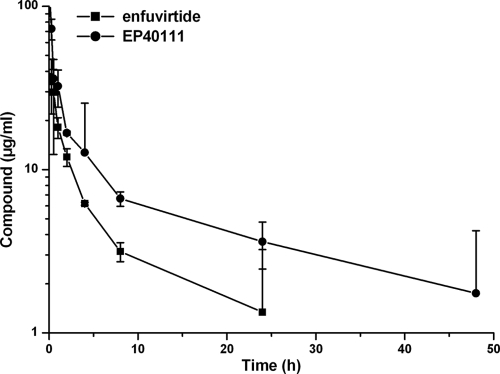
The concentration of the compounds in plasma was quantified by LC-MS/MS after protein precipitation and subsequent enzymatic cleavage with trypsin (12, 51). The time-course profiles of EP40111 and enfuvirtide in rat plasma after the administration of a single intravenous dose of the compounds at 4 mg/kg are presented in Fig. 3. The calculated elimination half-lives of EP40111 and enfuvirtide were 10.4 h and 2.8 h, respectively. These data clearly demonstrate that the attachment of a carrier pentasaccharide to enfuvirtide significantly increased its plasma half-life.
FIG. 3.
PK profiles of EP40111 and enfuvirtide in rats. Both compounds were administered intravenously at 4 mg/kg (i.e., 0.56 μmol/kg of EP40111 and 0.89 μmol/kg of enfuvirtide) to rats, and blood was sampled at the indicated times. The corresponding concentrations of EP40111 and enfuvirtide were evaluated by LC-MS/MS by using the 13C- and 15N-labeled enfuvirtide peptide as an internal standard. These results represent the means and standard deviations of two independent experiments. The enfuvirtide plasma concentration at 48 h was below the detection limit, so the point could not be indicated on this semilogarithmic graph.
DISCUSSION
The design of the long-lasting enfuvirtide-CP conjugates reported herein was based on the expectation that the known long half-life of the pentasaccharide component (similar to but different from that of idraparinux), i.e., 8.3 h in rat plasma, would be transferred to the entire molecule. As this half-life is mainly governed by the strong reversible interaction of the pentasaccharide with the plasma protein antithrombin, it was of paramount importance to design a molecular structure in which the binding to antithrombin would be minimally perturbed by the appendage attached to the pentasaccharide. For this reason we synthesized several conjugates that differed by the lengths of their PEGn linkers, EP40114 (n = 4), EP40111 (n = 12), EP40112 (n = 28), and EP40113 (n = 70).
Rather than performing cumbersome binding assays, the affinities of the enfuvirtide-PEGn-CP conjugates for antithrombin were assessed by determination of their respective anti-factor Xa activities. Although there is no direct correlation between the IC50 determined by the anti-factor Xa bioassay and the affinity usually expressed by the dissociation equilibrium constant (Kd), this bioassay is commonly used for this purpose to evaluate the interaction between drugs and antithrombin (42, 50). The anti-factor Xa activities of the enfuvirtide-CP conjugates were dependent on the size of the PEG linker. The conjugate with the shortest linker (EP40114) displayed an IC50 (93 nM) higher than the IC50s of the other conjugates. This could obviously be explained by the steric hindrance brought by the enfuvirtide peptide. The conjugate that showed the best anti-factor Xa activity (IC50, 32 nM) was EP40113, which has the longest PEG linker. EP40111 and EP40112 displayed intermediate IC50s of about 50 nM. Compared to the anti-factor Xa activity of the pentasaccharide alone (IC50, 16 nM), it could be concluded that the conjugation of the pentasaccharide to enfuvirtide did not significantly alter the capacities of the enfuvirtide-CP conjugates to bind to antithrombin, except maybe for EP40114, which displayed the lowest anti-factor Xa activity.
As was the case for the binding to antithrombin, the anti-HIV activity of the enfuvirtide-CP conjugates can also be affected by the pentasaccharide-PEG linker appendage. Anti-HIV activity was therefore evaluated in several virus infection and fusion assays, in the absence and in the presence of antithrombin.
In a first series of assays with laboratory strains (HIV-1 IIIB/LAI and HIV-2 ROD) and a human T-cell line (CEM), all compounds (EP40111, EP40112, EP40113, and EP40114) were tested in order to evaluate the impact of the length of the PEG linker and, eventually, the structure-activity relationship of the compounds. The enfuvirtide-CP conjugates showed consistent and broad anti-HIV-1 activity in the nanomolar range. Compared to the EC50 of native enfuvirtide (3 nM), the conjugates displayed two- to sixfold higher EC50s in this assay with the HIV-1 IIIB/LAI-CEM cells. EP40111 (EC50, 6 nM) appeared to be more potent than the three other conjugates, albeit the differences are probably not statistically or functionally significant. However, these results were very reproducible in several independent experiments and were confirmed in another cellular assay with the laboratory strain HIV-1 NL-4.3 (which is identical to strain IIIB/LAI at the level of the envelope) and the MT-4 T-cell line (data not shown). As expected, like enfuvirtide, these conjugates were much less potent against HIV-2 ROD.
Interestingly, the enfuvirtide-CP conjugates had consistently better activity than native enfuvirtide in the cell-cell fusion (syncytium formation) assay, in which persistently HIV-1-infected cells (HUT-78/HIV-1 IIIB/LAI cells) were cocultured with uninfected CD4+ T cells (SupT1 cells). These results were somewhat surprising, but they were consistently observed in all experiments. Globally, in this fusion assay all compounds displayed EC50s higher than those obtained in virus infection assays. The very high EC50 of native enfuvirtide obtained in this assay (EC50, 275 nM) was consistent with data already reported in the literature (5). For unobvious reasons, this system appeared to be relatively resistant to the antiviral activity of enfuvirtide. The fact that enfuvirtide-CP conjugates were more potent than native enfuvirtide in this system could not be explained by the current models of virus-cell or cell-cell membrane fusion mechanisms. Laboratories working on the field of HIV-1 entry use either one system or the other to establish models, which could tend toward a paradigm without making a difference between virus-cell or cell-cell fusion (11, 15, 17, 26, 30). More mechanistic studies must be performed to figure out the differences between the fusion process for HIV-1 entry into CD4+ T cells and the cell-cell fusion mechanism mediated by the HIV-1 envelope expressed at the surface of persistently infected cells. Multiple cellular factors (e.g., receptor/coreceptor density, proteoglycans, and the lipid composition of membranes) are clearly involved in these complex fusion processes and could adopt different conformations or could be presented differently at the virus or the cell surface.
In a second series of experiments, enfuvirtide-CP conjugates were evaluated in a system closer to clinical reality by using HIV-1 clinical isolates infecting PBMCs. At this stage of development, only two conjugates (EP40111 and EP40112) were further studied. EP40111 and EP40112 showed consistent and broad activity against six HIV-1 clinical isolates from three different clades (clades A, B, and C) independently of the coreceptor (X4 or R5) used by the virus to enter into host cells. However, these enfuvirtide-CP conjugates appeared to be generally less potent than native enfuvirtide. When all strains and clades are considered together as a virus population, the mean EC50 of enfuvirtide was about 20 nM, whereas the mean EC50s of EP40111 and EP40112 were about 100 and 130 nM, respectively. Thus, overall, the enfuvirtide-CP conjugates lost activity against HIV-1 clinical isolates by approximately five- to sevenfold compared to the activity of native enfuvirtide. Nevertheless, this loss of activity is still acceptable and is incommensurate with the complete lack of activity of AMD3100 (X4 specific) and maraviroc (R5 specific) against viruses that are not using the matching drug-specific coreceptor.
The EC50s for the HIV-1 clinical isolates in PBMCs obtained in these experiments were relatively variable. Since the NHR region of gp41, the target of enfuvirtide, is well conserved among HIV-1 strains and clades, the inconsistent binding capacity of the conjugates or enfuvirtide to NHR alone cannot explain this variability. Other intrinsic viral characteristics (envelope sequences outside the NHR, infection and replication rates, and the envelope density at the virus surface) and methodological biases (interindividual variability in PBMCs, the percentage of CD4+ T lymphocytes, the level of PBMC activation, the accuracy of the infectious doses, and the variability of the assay itself) have consequences on the level of activity of a given drug and could explain the differences observed in these experiments. Nevertheless, this variability does not have any impact on the global antiviral activity of the enfuvirtide-CP conjugates, as assessed in these studies.
Enfuvirtide-CP conjugates were less potent against clinical isolates (in PBMCs) than against laboratory-adapted strains (in T-cell lines). Native enfuvirtide also lost activity, but to a lesser extent. The reason for this decrease in activity is unclear but is commonly observed with entry inhibitors. One could argue that the envelope (gp120/gp41) of clinical isolates forms a more stable structure than that of laboratory-adapted strains and is therefore more resistant to gp120 shedding (41). gp120 is known to be the primary component of the steric block for preventing macromolecules, such as neutralizing monoclonal antibodies, from accessing gp41 (18). Enfuvirtide-CP conjugates EP40111 and EP40112 are obviously larger molecules than native enfuvirtide and may have more difficulties accessing their target (i.e., the NHR region of gp41), due to the steric hindrance of the pentasaccharide moiety.
The steric hindrance of enfuvirtide-CP conjugates would eventually be increased when the pentasaccharide moiety binds to antithrombin. Antithrombin is a 60-kDa plasma glycoprotein that circulates at a relatively high concentration (2.5 μM) in human blood. The binding between the pentasaccharide moiety of the enfuvirtide-CP conjugates and antithrombin is reversible, but it was wise to assess the potential consequence of this interaction on the antiviral activities of the conjugates. The results showed that, in the presence of antithrombin, EP40111 and EP40112 had lower antiviral activities against HIV-1 IIIB/LAI in PBMCs by three- to sixfold compared to the activity achieved under conditions without antithrombin. As anticipated, this decrease in activity could be explained by the additional steric hindrance brought by antithrombin bound to the pentasaccharide moiety of the conjugates. Nevertheless, the mean EC50s remained below 100 nM, which is still acceptable to maintain interest in the further development of these compounds.
Finally, the most important feature of these conjugates, i.e., their half-lives, was assessed in vivo in rats upon intravenous injection. The concentrations of EP40111 and enfuvirtide in rat plasma were determined by LC-MS/MS using isotope-labeled enfuvirtide as an internal standard. The half-life of native enfuvirtide in this animal model was about 2.8 h, which was consistent with its known half-life in rats as well as in humans (24, 28, 29, 48). Unlike native enfuvirtide, EP40111 displayed a much longer half-life in rat plasma, i.e., 10.4 h, in good agreement with the half-life of the pentasaccharide alone (i.e., 8.3 h). Because of the prolonged half-life of EP40111, the level of exposure of the animals to this conjugate was also higher than that to enfuvirtide (Fig. 3).
Allometric extrapolation of the half-life from one animal species to another is well documented for antithrombin-binding pentasaccharides (36, 53). Fondaparinux, which displays a half-life of 1 h in rats, shows a half-life of 17 h in humans. Similarly, the half-life of idraparinux is approximately 10 h in rats and 120 h in humans. On the basis of these preclinical and clinical observations with fondaparinux and idraparinux, a major improvement of the half-life of enfuvirtide could be predicted and expected in clinical practice.
In this first series of in vivo experiments, enfuvirtide and EP40111 were administered intravenously instead of subcutaneously, which is how enfuvirtide is administered to humans, because the primary goal of this study was to determine the absolute half-lives of the compounds without any interference from the absorption phase. All other pharmacokinetics parameters (the time to reach the maximum concentration in plasma, the maximum concentration in plasma, the area under the concentration-time curve, and clearance) will be assessed in a formal PK study that will be performed by the use of both routes of administration, intravenous and subcutaneous, and several doses.
Potential future studies with HIV-susceptible transgenic rats (24) would explore the in vivo anti-HIV-1 activities of these promising compounds and, hence, their potential for use as long-acting anti-HIV-1 drugs. In such an animal model, the intrinsic anticoagulant properties of the carrier pentasaccharide moiety could also be addressed. Indeed, for such a conjugate, it is necessary to evaluate the benefit/risk ratio to balance its antiviral potency with its antithrombotic effect.
All the results described and discussed above suggest that EP40111 may have a half-life of about 120 h in humans. This would open the possibility for the use of subcutaneous injections much less frequently, e.g., once a week instead of twice daily, which is the standard prescribing protocol currently applied for enfuvirtide. In addition, the conjugation of enfuvirtide to a pentasaccharide may overcome the low solubility limits commonly associated with this family of inhibitors, thus rendering this peptide more amenable to subcutaneous delivery. This novel schedule of treatment with enfuvirtide-CP conjugates would eventually increase patient convenience and compliance by decreasing or eliminating skin sensitivity reaction side effects at the injection sites and would definitively bring significant medical benefit to the patients.
Acknowledgments
We are grateful to the following for excellent technical assistance: F. Cantais and C. Villedieu from Endotis Pharma; S. Claes, R. Provinciael, L. Ingels, and E. Fonteyn from the Rega Institute for Medical Research; and N. Dereuddre-Bosquet and R. Yousfi from SPI-Bio.
D.S. and J.B. thank the European Commission EMPRO no. 503558 of the 6th Frame Work Program, the Fonds voor Wetenschappelijk Onderzoek (FWO) Krediet (no. G-485-08), and the Centers of Excellence of the K. U. Leuven (Krediet no. EF/05/015) for financial support. We also thank the MS facility of the Université des Sciences et Technologies de Lille 1 used in this study, which is funded by the European Community (FEDER), the region Nord-Pas de Calais (France), the CNRS, and the Université de Lille 1.
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, Y., and W. C. Shen. 2006. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm. Res. 23:2116-2121. [DOI] [PubMed] [Google Scholar]
- 2a.Bailon, P. S., and C. Y. Won. 23 May 2006. Pegylated T20 polypeptide. U.S. patent 7, 049, 415.
- 3.Ball, R. A., and T. Kinchelow. 2003. Injection site reactions with the HIV-1 fusion inhibitor enfuvirtide. J. Am. Acad. Dermatol. 49:826-831. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., S. Hatse, K. Vermeire, K. Princen, S. Aquaro, C. F. Perno, E. De Clercq, H. Egberink, G. Vanden Mooter, W. Peumans, E. Van Damme, and D. Schols. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 48:3858-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini, J., K. Van Laethem, S. Hatse, K. Vermeire, E. De Clercq, W. Peumans, E. Van Damme, A. M. Vandamme, A. Bölmstedt, and D. Schols. 2004. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J. Virol. 78:10617-10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barré-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vézinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 7.Beals, J. M., and A. B. Shanafelt. 2006. Enhancing exposure of protein therapeutics. Drug Discov. Today Technol. 3:87-94. [DOI] [PubMed] [Google Scholar]
- 8.Björk, I., and S. T. Olson. 1997. Antithrombin. A bloody important serpin, p. 17-33. In F. C. Church, D. D. Cunningham, D. Ginsburg, M. Hoffman, S. R. Stone, and D. M. Tollefsen (ed.), Chemistry and biology of serpins. Plenum Press, New York, NY. [PubMed]
- 9.Briz, V., E. Poveda, and V. Soriano. 2006. HIV entry inhibitors: mechanisms of action and resistance pathways. J. Antimicrob. Chemother. 57:619-627. [DOI] [PubMed] [Google Scholar]
- 10.Buijsman, R. C., J. E. Basten, T. G. Van Dinther, G. A. Van der Marel, C. A. Van Boeckel, and J. H. Van Boom. 1999. Design and synthesis of a novel synthetic NAPAP-penta-saccharide conjugate displaying a dual antithrombotic action. Bioorg. Med. Chem. Lett. 9:2013-2018. [DOI] [PubMed] [Google Scholar]
- 11.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 12.Chang, D., S. J. Kolis, K. H. Linderholm, T. F. Julian, R. Nachi, A. M. Dzerk, P. P. Lin, J. W. Lee, and S. K. Bansal. 2005. Bioanalytical method development and validation for a large peptide HIV fusion inhibitor (enfuvirtide, T-20) and its metabolite in human plasma using LC-MS/MS. J. Pharm. Biomed. Anal. 38:487-496. [DOI] [PubMed] [Google Scholar]
- 13.Chuang, V. T., U. Kragh-Hansen, and M. Otagiri. 2002. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm. Res. 19:569-577. [DOI] [PubMed] [Google Scholar]
- 14.de Kort, M., B. Gianotten, J. A. Wisse, E. S. Bos, M. H. Eppink, E. Mattaar, G. M. Vogel, W. H. Dokter, M. Honing, S. Vonsovic, M. J. Smit, J. C. Wijkmans, and C. A. Van Boeckel. 2008. Conjugation of ATIII-binding pentasaccharides to extend the half-life of proteins: long-acting insulin. Chem. Med. Chem. 3:1189-1193. [DOI] [PubMed] [Google Scholar]
- 15.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 16.Dorr, P., M. Westby, S. Dobbs, P. Griffin, B. Irvine, M. Macartney, J. Mori, G. Rickett, C. Smith-Burchnell, C. Napier, R. Webster, D. Armour, D. Price, B. Stammen, A. Wood, and M. Perros. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 18.Eckert, D. M., Y. Shi, S. Kim, B. D. Welch, E. Kang, E. S. Poff, and M. S. Kay. 2008. Characterization of the steric defense of the HIV-1 gp41 N-trimer region. Protein Sci. 17:2091-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flexner, C. 2007. HIV drug development: the next 25 years. Nat. Rev. Drug Discov. 6:959-966. [DOI] [PubMed] [Google Scholar]
- 20.Freed, E. O., and M. A. Martin. 1995. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J. Biol. Chem. 270:23883-23886. [DOI] [PubMed] [Google Scholar]
- 21.Gallo, R. C., P. S. Sarin, E. P. Gelmann, M. Robert-Guroff, E. Richardson, V. S. Kalyanaraman, D. Mann, G. D. Sidhu, R. E. Stahl, S. Zolla-Pazner, J. Leibowitch, and M. Popovic. 1983. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 220:865-867. [DOI] [PubMed] [Google Scholar]
- 22.Garber, A. J. 2005. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes. Metab. 7:666-674. [DOI] [PubMed] [Google Scholar]
- 23.Gettins, P. G. 2002. Serpin structure, mechanism, and function. Chem. Rev. 102:4751-4804. [DOI] [PubMed] [Google Scholar]
- 24.Goffinet, C., I. Allespach, and O. T. Keppler. 2007. HIV-susceptible transgenic rats allow rapid preclinical testing of antiviral compounds targeting virus entry or reverse transcription. Proc. Natl. Acad. Sci. U. S. A. 104:1015-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris, J. M., and R. B. Chess. 2003. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2:214-221. [DOI] [PubMed] [Google Scholar]
- 26.Harrison, S. C. 2005. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 64:231-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbert, J. M., J. P. Hérault, A. Bernat, R. G. Van Amsterdam, J. C. Lormeau, M. Petitou, C. Van Boeckel, P. Hoffmann, and D. G. Meuleman. 1998. Biochemical and pharmacological properties of SANORG 34006, a potent and long-acting synthetic pentasaccharide. Blood 91:4197-4205. [PubMed] [Google Scholar]
- 28.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 29.Kilby, J. M., J. P. Lalezari, J. J. Eron, M. Carlson, C. Cohen, R. C. Arduino, J. C. Goodgame, J. E. Gallant, P. Volberding, R. L. Murphy, F. Valentine, M. S. Saag, E. L. Nelson, P. R. Sista, and A. Dusek. 2002. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp41-mediated virus fusion, in HIV-infected adults. AIDS Res. Hum. Retrovir. 18:685-693. [DOI] [PubMed] [Google Scholar]
- 30.Krambovitis, E., F. Porichis, and D. A. Spandidos. 2005. HIV entry inhibitors: a new generation of antiretroviral drugs. Acta Pharmacol. Sin. 26:1165-1173. [DOI] [PubMed] [Google Scholar]
- 31.Lalezari, J. P., J. J. Eron, M. Carlson, C. Cohen, E. DeJesus, R. C. Arduino, J. E. Gallant, P. Volberding, R. L. Murphy, F. Valentine, E. L. Nelson, P. R. Sista, A. Dusek, and J. M. Kilby. 2003. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS 17:691-698. [DOI] [PubMed] [Google Scholar]
- 32.Lazzarin, A., B. Clotet, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, H. J. Stellbrink, J. F. Delfraissy, J. Lange, L. Huson, R. DeMasi, C. Wat, J. Delehanty, C. Drobnes, and M. Salgo. 2003. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348:2186-2195. [DOI] [PubMed] [Google Scholar]
- 33.Leader, B., Q. J. Baca, and D. E. Golan. 2008. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 7:21-39. [DOI] [PubMed] [Google Scholar]
- 34.Low, S. C., S. L. Nunes, A. J. Bitonti, and J. A. Dumont. 2005. Oral and pulmonary delivery of FSH-Fc fusion proteins via neonatal Fc receptor-mediated transcytosis. Hum. Reprod. 20:1805-1813. [DOI] [PubMed] [Google Scholar]
- 35.Lu, J., S. G. Deeks, R. Hoh, G. Beatty, B. A. Kuritzkes, J. N. Martin, and D. R. Kuritzkes. 2006. Rapid emergence of enfuvirtide resistance in HIV-1-infected patients: results of a clonal analysis. J. Acquir. Immune Defic. Syndr. 43:60-64. [DOI] [PubMed] [Google Scholar]
- 36.Ma, Q., and J. Fareed. 2004. Idraparinux sodium. Sanofi-Aventis. IDrugs 7:1028-1034. [PubMed] [Google Scholar]
- 37.Maggi, P., N. Ladisa, E. Cinori, A. Altobella, G. Pastore, and R. Filotico. 2004. Cutaneous injection site reactions to long-term therapy with enfuvirtide. J. Antimicrob. Chemother. 53:678-681. [DOI] [PubMed] [Google Scholar]
- 38.Matthews, T., M. Salgo, M. Greenberg, J. Chung, R. DeMasi, and D. Bolognesi. 2004. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat. Rev. Drug Discov. 3:215-225. [DOI] [PubMed] [Google Scholar]
- 39.Myers, S. A., A. A. Selim, M. A. McDaniel, R. Hall, Y. Zhang, J. A. Bartlett, and A. L. True. 2006. A prospective clinical and pathological examination of injection site reactions with the HIV-1 fusion inhibitor enfuvirtide. Antivir. Ther. 11:935-939. [PubMed] [Google Scholar]
- 40.Naicker, K. P., H. Li, A. Heredia, H. Song, and L. X. Wang. 2004. Design and synthesis of alpha Gal-conjugated peptide T20 as novel antiviral agent for HIV-immunotargeting. Org. Biomol. Chem. 2:660-664. [DOI] [PubMed] [Google Scholar]
- 41.Orloff, S. L., M. S. Kennedy, A. A. Belperron, P. J. Maddon, and J. S. McDougal. 1993. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J. Virol. 67:1461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petitou, M., and C. A. Van Boeckel. 2004. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Ed. Engl. 43:3118-3133. [DOI] [PubMed] [Google Scholar]
- 43.Reeves, J. D., F. H. Lee, J. L. Miamidian, C. B. Jabara, M. M. Juntilla, and R. W. Doms. 2005. Enfuvirtide resistance mutations: impact on human immunodeficiency virus envelope function, entry inhibitor sensitivity, and virus neutralization. J. Virol. 79:4991-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts, M. J., M. D. Bentley, and J. M. Harris. 2002. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 54:459-476. [DOI] [PubMed] [Google Scholar]
- 45.Roisin, A., J. P. Robin, N. Dereuddre-Bosquet, A. L. Vitte, D. Dormont, P. Clayette, and P. Jalinot. 2004. Inhibition of HIV-1 replication by cell-penetrating peptides binding. Rev. J. Biol. Chem. 279:9208-9214. [DOI] [PubMed] [Google Scholar]
- 46.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinclair, A. M., and S. Elliott. 2005. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J. Pharm. Sci. 94:1626-1635. [DOI] [PubMed] [Google Scholar]
- 48.Stocker, H., C. Kloft, N. Plock, A. Breske, G. Kruse, C. Herzmann, H. Schulbin, P. Kreckel, C. Weber, F. Goebel, J. Roeling, S. Staszewski, A. Plettenberg, C. Moecklinghoff, K. Arasteh, and M. Kurowski. 2006. Pharmacokinetics of enfuvirtide in patients treated in typical routine clinical settings. Antimicrob. Agents Chemother. 50:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoddart, C. A., G. Nault, S. A. Galkina, K. Thibaudeau, P. Bakis, N. Bousquet-Gagnon, M. Robitaille, M. Bellomo, V. Paradis, P. Liscourt, A. Lobach, M. E. Rivard, R. G. Ptak, M. K. Mankowski, D. Bridon, and O. Quraishi. 2008. Albumin-conjugated C34 peptide HIV-1 fusion inhibitor: equipotent to C34 and T-20 in vitro with sustained activity in SCID-hu Thy/Liv mice. J. Biol. Chem. 283:34045-34052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Amsterdam, R. G., G. M. Vogel, A. Visser, W. J. Kop, M. T. Buiting, and D. G. Meuleman. 1995. Synthetic analogues of the antithrombin III-binding pentasaccharide sequence of heparin. Prediction of in vivo residence times. Arterioscler. Thromb. Vasc. Biol. 15:495-503. [DOI] [PubMed] [Google Scholar]
- 51.Van den Broek, I., R. W. Sparidans, J. H. Schellens, and J. H. Beijnen. 2007. Enzymatic digestion as a tool for the LC-MS/MS quantification of large peptides in biological matrices: measurement of chymotryptic fragments from the HIV-1 fusion inhibitor enfuvirtide and its metabolite M-20 in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 854:245-259. [DOI] [PubMed] [Google Scholar]
- 52.Veronese, F. M., and G. Pasut. 2005. PEGylation, successful approach to drug delivery. Drug Discov. Today 10:1451-1458. [DOI] [PubMed] [Google Scholar]
- 53.Walenga, J. M., W. P. Jeske, and J. Fareed. 2005. Short- and long-acting synthetic pentasaccharides as antithrombotic agents. Expert Opin. Investig. Drugs 14:847-858. [DOI] [PubMed] [Google Scholar]
- 54.Weitz, J. I. 2004. New anticoagulants for treatment of venous thromboembolism. Circulation 110:I19-I26. [DOI] [PubMed] [Google Scholar]
- 55.Werle, M., and A. Bernkop-Schnurch. 2006. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 30:351-367. [DOI] [PubMed] [Google Scholar]
- 56.Wheeler, D. A., J. P. Lalezari, J. M. Kilby, J. Wheat, J. Delehanty, R. DeMasi, I. Patel, and M. Salgo. 2004. Safety, tolerability, and plasma pharmacokinetics of high-strength formulations of enfuvirtide (T-20) in treatment-experienced HIV-1-infected patients. J. Clin. Virol. 30:183-190. [DOI] [PubMed] [Google Scholar]
- 57.Wild, C., T. Greenwell, and T. Matthews. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, X., K. Nieforth, J. M. Lang, R. Rouzier-Panis, J. Reynes, A. Dorr, S. Kolis, M. R. Stiles, T. Kinchelow, and I. H. Patel. 2002. Pharmacokinetics of plasma enfuvirtide after subcutaneous administration to patients with human immunodeficiency virus: inverse Gaussian density absorption and 2-compartment disposition. Clin. Pharmacol. Ther. 72:10-19. [DOI] [PubMed] [Google Scholar]