Abstract
The World Health Organization recommends the use of artemisinin-based combination therapies (ACTs) for the treatment of uncomplicated malaria. The two most widely adopted ACT regimens are artemether (AR)-lumefantrine (LR) (the combination is abbreviated AL) and amodiaquine (AQ)-artesunate (AS). Pharmacokinetic (PK) data informing the optimum dosing of these drug regimens is limited, especially in children. We evaluated PK parameters in Ugandan children aged 5 to 13 years with uncomplicated malaria treated with AL (n = 20) or AQ-AS (n = 21), with intensive venous sampling occurring at 0, 2, 4, 8, 24, and 120 h following administration of the last dose of 3-day regimens of AL (twice daily) or AQ-AS (once daily). AS achieved an estimated maximum concentration in plasma (Cmax) of 51 ng/ml and an area under the concentration-time curve from time zero to infinity (AUC0-∞) of 113 ng·h/ml; and its active metabolite, dihydroartemisinin (DHA), achieved a geometric mean Cmax of 473 ng/ml and an AUC0-∞ of 1,404 ng·h/ml. AR-DHA exhibited a Cmax of 34/119 ng/ml and an AUC0-∞ of 168/382 ng·h/ml, respectively. For LR, Cmax and AUC0-∞ were 6,757 ng/ml and 210 μg·h/ml, respectively. For AQ and its active metabolite, desethylamodiaquine (DEAQ), the Cmaxs were 5.2 ng/ml and 235 ng/ml, respectively, and the AUC0-∞s were 39.3 ng·h/ml and 148 μg·h/ml, respectively. Comparison of the findings of the present study to previously published data for adults suggests that the level of exposure to LR is lower in children than in adults and that the level of AQ-DEAQ exposure is similar in children and adults. For the artemisinin derivatives, differences between children and adults were variable and drug specific. The PK results generated for children must be considered to optimize the dosing strategies for these widely utilized ACT regimens.
Malaria is among Africa's leading causes of morbidity and mortality, leading to an estimated 300 million to 660 million cases of Plasmodium falciparum malaria and approximately 1 million deaths per year (15, 30). In the recent past, the management of malaria primarily relied upon monotherapy with chloroquine (CQ) or sulfadoxine-pyrimethamine (SP). However, the widespread and excessive use of these agents led to drug resistance, and as a result, CQ and SP have limited roles in the treatment of malaria. The World Health Organization (WHO) currently recommends new artemisinin-based combination therapy (ACT) for the treatment of uncomplicated malaria in sub-Saharan Africa (38). ACT consists of a short-acting artemisinin derivative that rapidly reduces the parasite burden combined with a longer-acting partner drug that affords adequate treatment efficacy with 3 days of dosing (38). The most widely adopted ACT regimens in Africa are artemether (AR)-lumefantrine (LR) (the combination is abbreviated AL) and amodiaquine (AQ)-artesunate (AS), each of which is a first-line drug for the treatment of uncomplicated malaria in multiple African countries (37). As the availability of ACT increases, the use of hundreds of millions of doses is anticipated in Africa alone, especially by children, the group at the greatest risk for malaria (18).
The artemisinins kill malaria parasites rapidly, and their excellent tolerability and safety provide an additional benefit (23). Resistance is also not a significant problem, although recent reports reveal the emergence of resistance in Southeast Asia (10). Both AS and AR are rapidly converted to the active metabolite dihydroartemisinin (DHA) by cytochrome P450 (CYP) enzymes, with DHA contributing the majority of the antimalarial activity (22, 33). Of the two drugs, AR is more lipid soluble and may exhibit erratic absorption (22). Artemisinins fail to reliably eliminate malaria infections after short courses of treatment if they are used alone. AQ, which is combined with AS, is a 4-aminoquinoline that is converted via CYP enzymes to the active metabolite desethylamodiaquine (DEAQ), which contributes the majority of the antimalarial activity (27). LR, which is combined with AL (Coartem), is an aryl aminoalcohol that is well tolerated (19, 35). The oral bioavailability of LR is highly variable and is dependent on administration with fatty foods; the level of exposure decreases 16-fold when LR is administered in a fasting state compared to the level of exposure achieved when it is administered with a fatty meal (12, 35).
Pharmacokinetic (PK) studies of ACTs informing dosing guidelines have been limited to adults, with less information being available for children. Pediatric dosing of AL and AQ-AS are deduced from adult-based regimens adjusted for body weight, with little consideration of maturational effects on drug absorption and metabolism (6). Indeed, CYP-UDP-glucuronosyltransferase activity and clearance vary with age. Several clinical PK studies have reported that clearance and metabolism in prepubescent children are altered compared to clearance and metabolism in adults (17, 31). For example, for the antimalarial combination SP, PK data generated for children receiving standard weight-based dosing revealed lower levels in children than in adults (4). Importantly, a correlation between low SP levels and the risk of treatment failure has also been noted (32). Likewise, for LR, the levels measured at day 7 were lower in children than in adults (8), which may also compromise outcomes (26). As children are at high risk of severe morbidity with inadequate treatment of malaria, it is important to ensure appropriate dosing of ACTs in this group. We therefore determined the PK parameters for all components and major metabolites of the two most widely adopted ACT regimens in Africa in Ugandan children with acute uncomplicated malaria.
MATERIALS AND METHODS
Subject recruitment and management.
This study was part of a longitudinal drug efficacy trial of 690 children conducted between November 2004 and December 2008 in Kampala, Uganda, where malaria is mesoendemic (11). Details of the study have been published previously (11). Prior to study onset, a census project was conducted to generate a sampling frame of households for recruitment, and all children from randomly selected households were screened for enrollment in the study. Children ages 5 to 13 years presenting to the study clinic with uncomplicated malaria between March 2007 and January 2008 were screened for enrollment in this PK substudy. Study physicians recruited children fulfilling the following eligibility criteria: uncomplicated malaria confirmed by a positive blood smear and fever (tympanic temperature of >38°C or a history of fever within 24 h); age 5 to 13 years; weight of ≥20 kg; no use of AS, AQ, or AL for the prior 2 weeks; hemoglobin concentration of >10 g/dl; no known adverse reactions to the study medications; the absence of severe malnutrition; no known serious chronic disease; no usage of concomitant medications known to alter the metabolism of the study medications (i.e., CYP2C8 and CYP3A4 inducers and inhibitors); and the willingness of the parent or guardian to provide written informed consent (children over 7 years of age were asked to give informed assent). The study received ethical approval from the Uganda National Council of Science and Technology; the Makerere University Research and Ethics Committee; and the University of California, San Francisco, Committee on Human Research.
On the basis of the protocol used for the parent study (20), the study participants were randomly assigned to receive AQ-AS (Camoquin and Arsumax) or AR-LR (Coartem) at the time of their first episode of uncomplicated malaria following enrollment in the study, and they then received the same regimen for subsequent episodes of malaria. The study drugs were acquired from the same source and were dispensed at the single study site. Strict adherence to storage temperatures and shelf life was followed. The treatment doses were as follows: AQ (200-mg tablets) at 10 mg/kg of body weight once a day on the first 2 days and then 5 mg/kg on the third day, with the doses being rounded to the nearest quarter tablet; AS (50-mg tablets) at 4 mg/kg twice a day on all 3 days, with the doses being rounded to the nearest quarter tablet; and AL (20 mg/120-mg tablets) given twice a day for 3 days according to weight, i.e., 20 through 24 kg, 2 tablets per dose; 25 through 34 kg, 3 tablets per dose; and >34 kg, 4 tablets per dose. Dosing was administered as summarized in Fig. 1. AL dosing was scheduled to allow the administration of the last dose in the morning. This scheduling meant that samples obtained 24 and 120 h after administration of the last dose of AL technically occurred on days 4 and 8 (Fig. 1). This dosing strategy should not affect the comparison of our AL results for day 8 samples to the prior day 7 results, as the samples used to determine AL levels were collected at the same times relative to the times of administration of the last two doses of AL, the doses with the most significant impact on the area under the concentration-time curve (AUC) and day 7 concentration estimates. All AL doses were taken with 250 ml of fresh milk containing 3.3 g of fat, provided by the study. The dose was readministered if vomiting occurred within 30 min. The study personnel, the study participants, and the participants' caregivers were informed of the treatment regimen to which the participant was assigned. Treatment outcomes were classified according to 2006 WHO guidelines (38).
FIG. 1.
PK dosing and sampling scheme for ACT regimens. The dosing times and PK sampling schedules are depicted for the AQ-AS and AL regimens. All doses were administered with food and fat. The drug levels in venous plasma were measured.
PK study procedures.
An intensive PK sampling design was employed. For each ACT regimen, venous blood samples were collected just prior to the administration of the last dose and at 2, 4, 8, 24, and 120 h after the administration of the last dose of a standard 3-day regimen (Fig. 1). Three milliliters of whole blood was collected at each time point. For the AQ-AS group, 1.5 ml drawn into potassium oxalate-sodium fluoride tubes was used for the quantification of AS-DHA, and 1.5 ml drawn into sodium heparin tubes was used for the quantification of AQ and DEAQ (AQ-AS group). For the AL group, 3 ml was drawn into sodium heparin tubes for AR-DHA and LR quantification. All samples were immediately placed on ice. For those participants receiving AQ-AS, the samples were cold centrifuged within 30 min at 1,500 × g (or at 3,000 × g for 15 min for AQ-DEAQ) for 5 min, and the plasma was transferred to a cryotube. For those participants receiving AL, the tubes were centrifuged at 3,000 × g for 15 min at room temperature. All samples were stored at −80°C until shipment on dry ice to the Clinical Pharmacology Laboratory at the Mahidol-Oxford Tropical Medicine Research Unit in Thailand for analysis. The maximum duration of sample storage was approximately 1 year at −80°C.
Drug assays.
The plasma concentrations of AS, AR, and DHA were determined by solid-phase extraction and liquid chromatography-tandem mass spectrometry on an API 5000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS SCIEX, Foster City, CA) with a TurboV ionization source operated in the positive ion mode (16). Stable isotope-labeled AS (SIL-AS) and stable isotope-labeled DHA (SIL-DHA) were used as internal standards. Total assay coefficients of variation for AS, AR, and DHA were <5% for inter- and intraday precisions. The lower limits of quantification (LLOQs) for AS and DHA were 1.2 and 2.0 ng/ml, respectively, and the LLOQs for AR and DHA were 1.43 ng/ml for each analyte.
The plasma concentrations of LR were determined by liquid chromatography with UV detection. A hexyl analogue of desbutyl-lumefantrine (no. TA 213/435/516; Novartis) was used as the internal standard (25). The total assay coefficients of variation were <6% for inter- and intraday precisions. The LLOQ for the assay was 25 ng/ml.
AQ and DEAQ concentrations were determined by high-performance liquid chromatography with UV detection (7) The LLOQ for both AQ and DEAQ was 5 ng/ml, and the inter- and intraday precisions were <7%.
Data analysis.
The values of the PK parameters for the antimalarial agents were estimated by noncompartmental PK analysis (Winnonlin, version 5.0.1). The AUC from time zero to infinity (AUC0-∞) was determined beginning just prior to administration of the last ACT dose of a standard 3-day regimen and was estimated by using the linear up-log down trapezoidal rule. The AUC extrapolated to infinity was determined by dividing the last measured concentration for each analyte by the terminal elimination rate constant (λz). λz was estimated by using the regression of the concentration time points that described the terminal linear portion of the concentration-time curve, usually the last three data points. The maximum concentration in plasma (Cmax) was taken directly from the observed data. Samples with concentrations below the LLOQ were handled as missing data, unless the sample was obtained prior to the time of administration of the last dose of drug, in which case it was treated as zero. Geometric means and their associated 90% confidence intervals (CIs) were calculated. Pooling of the data across subjects was carried out for the compounds when limited data for individual subjects were available (e.g., when only three of six time points yielded measurable drug concentrations). In these cases, the data are reported as estimates. Pooling of the data was necessary only for AS and AQ. No pooling of the data was performed for AR, DHA, LR, or DEAQ. The correlation between the AUC and the levels on day 7 was calculated by using nonparametric Spearman's rank correlation coefficient (rs; a P value of <0.05 was considered significant). Graphical and statistical analyses were conducted with the STATA program (version 10.0; StataCorp, College Station, TX) and Prism software (version 3.0; GraphPad Software, San Diego, CA).
RESULTS
A total of 155 children were screened for enrollment in the study, with 110 children being excluded on the basis of the enrollment criteria. The predominant reasons for exclusion were based on either the age or the weight criterion. Forty-five children meeting enrollment criteria for the PK substudy were enrolled: 23 in the AL arm and 22 in the AQ-AS arm. Four patients (three receiving AL and one receiving AQ-AS) failed to complete the study due to the withdrawal of consent (n = 2), the medication was taken incorrectly (n = 1), and the dose failed to be repeated within 30 min of vomiting (n = 1). Thus, a total of 20 children in the AL arm and 21 children in the AQ-AS arm completed the study. One patient in the AQ-AS study arm received a slightly higher total AQ dose (650 mg instead of 600 mg of AQ was administered) than appropriate on the basis of the dosing algorithm; the data for this patient were included. One child vomited a dose of AL within 30 min on the first day of treatment, and this dose was repeated. The baseline mean ± standard deviation age and weight of the participants were 8.9 ± 2.1 years and 26.1 ± 6.8 kg, respectively, for the AQ-AS arm and 8.9 ± 2.3 years and 26.5 ± 5.8 kg, respectively, for the AL arm. The baseline hemoglobin concentrations, heights, and gender proportions were similar for the two groups (data not shown). There were no serious adverse events reported with any of the PK substudy treatments. All patients responded to treatment, with only a single episode of recurrent parasitemia occurring within 28 days in the AL arm. The participant with the recurrence received AL and developed infection with a new parasite on day 25, as classified by parasite genotyping.
PKs of AS-DHA and AR-DHA.
The values of the PK parameters for AS and DHA for children in the group treated with AQ-AS and for AR and DHA in the group treated with AL are summarized in Table 1. Pooling of the data across subjects was necessary for AS but not for its active metabolite, DHA, nor was it necessary for AR-DHA in the AL regimen. For AS, the estimated Cmax and AUC0-∞ were 51 ng/ml and 113 ng·h/ml, respectively; and for DHA, the geometric mean Cmax and AUC0-∞ were 473 ng/ml (90% CI, 42 to 624 ng/ml) and 1,404 ng·h/ml (90% CI, 1,296 to 1,721 ng·h/ml), respectively. For the AL group, the mean estimates of Cmax and AUC0-∞ were 34 ng/ml (90% CI, 31 to 48 ng/ml) and 168 ng·h/ml (90% CI, 153 to 220 ng·h/ml), respectively, for AR and 119 ng/ml (90% CI, 110 to 153 ng/ml) and 382 ng·h/ml (90% CI, 348 to 460 ng·h/ml), respectively, for DHA.
TABLE 1.
PK data for artemisinin derivatives in children
| PK parameter | ASa | DHAb | ARb | DHAb |
|---|---|---|---|---|
| Cmax (ng/ml) | 51.3 | 473 (427-624) | 34 (31-48) | 119 (110-153) |
| AUC0-∞ (ng·h/ml)c | 113.0 | 1,404 (1,296-1,721) | 168 (153-220) | 382 (348-460) |
| t1/2 (h)d | NAe | 1.3 (1.08-1.70) | 3.9 (3.4-5.7) | 1.9 (1.3-3.3) |
Data were estimated by using pooled data from all subjects.
On the basis of estimates obtained with data from individual subjects. Data represent the geometric means (90% CIs).
AUC estimated surrounding the last dose administered.
t1/2, elimination half-life.
NA, not available due to insufficient data points for computation of the half-life.
PKs of AQ-DEAQ.
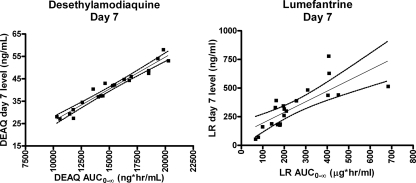
The values of the PK parameters for the longer-acting partner drug AQ and its active metabolite, DEAQ, are summarized in Table 2. Pooling of data for all subjects was necessary for AQ but not for DEAQ. The estimated mean elimination half-life of AQ was 3.3 h, with the estimated Cmax and AUC0-∞ values being 5.2 ng/ml and 39.3 ng·h/ml, respectively. DEAQ exhibited an elimination half-life nearly 20-fold longer than that of AQ, achieving a higher level of exposure, specifically, a geometric mean Cmax of 235 ng/ml (90% CI, 219 to 266 ng/ml) and an AUC0-∞ of 148 μg·h/ml (90% CI, 139 to 162 μg·h/ml) (Fig. 2). The AUC0-∞s for DEAQ showed wide intersubject variability. The median levels of DEAQ on day 3 and day 7 were 124.2 ng/ml (range, 76.2 to 200.1 ng/ml) and 42.0 ng/ml (range, 27.1 to 58 ng/ml), respectively. The correlations between AUC0-∞ and the levels of DEAQ on both day 3 and day 7 were strong (rs = 0.83 and 0.97, respectively; P < 0.0001) (Fig. 3).
TABLE 2.
PK data for longer-acting partner drugs, AQ, DEAQ, and LR, in children
| PK parameter | AQa | DEAQb | LRb |
|---|---|---|---|
| Cmax (ng/ml) | 5.2 | 235 (219-266) | 6,757 (6,118-9,291) |
| AUC0-120 (ng·h/ml)c | NAd | 11,242 (10,580-12,367) | 194,740 (173,935-288,471) |
| AUC0-8 (ng·h/ml)c | 28.9 | NA | NA |
| AUC0-∞ (ng·h/ml)c | 39.3 | 14,770 (13,914-16,226) | 210,040 (188,859-307,217) |
| t1/2 (h)e | 3.3 | 60.0 (56.9-64.6) | 32.7 |
Data were estimated by using pooled data from all subjects.
Data were estimated by using pooled data from individual subjects. Data represent the geometric means (90% CIs).
AUC estimated surrounding the last dose administered.
NA, not applicable.
t1/2, elimination half-life.
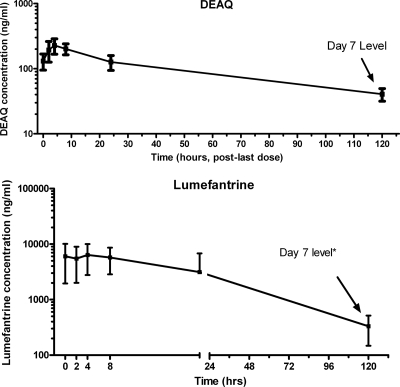
FIG. 2.
Mean ± standard deviation plasma concentration-versus-time profile after administration of the last dose for the longer-acting partner drugs. Profiles are depicted for the principle metabolite, DEAQ, and LR. The concentrations are displayed on logarithmic scales. *, for AL, due to the scheduling of the dosing to allow for administration of the last dose in the morning, the sample collection designed for 120 h after administration of the last dose occurred on day 8.
FIG. 3.
Scatterplot showing the correlation of the levels on day 7 with the AUC0-∞s for both DEAQ and LR (for DEAQ on day 7, rs = 0.97; for LR on day 7, rs = 0.85; P < 0.0001 for all correlations). Dotted lines represent 95% CIs.
PKs of LR in children.
The values of the PK parameters for LR are also summarized in Table 2. LR revealed a long elimination half-life of 33.6 h. The geometric mean Cmax and AUC0-∞ were 6,757 ng/ml (90% CI, 6,118 to 9,291 ng/ml) and 210 μg·h/ml (90% CI 189 to 307 μg·h/ml), respectively (Fig. 2). The LR levels on day 3 and 7 exhibited wide intersubject variability. The median levels on day 3 and day 7 were 1,953.7 ng/ml (range, 650.6 to 16,964.1 ng/ml) and 323.3 ng/ml (range, 53.9 to 778.9 ng/ml), respectively. As for DEAQ, the correlations between AUC0-∞ and the LR levels on both day 3 and day 7 were strong (rs = 0.94 and 0.85, respectively; P < 0.0001) (Fig. 3).
Weight-based dose adjustment and exposure in children.
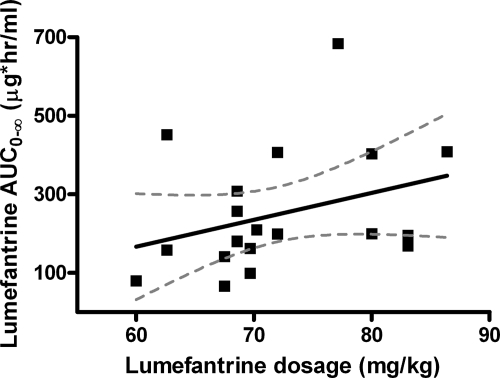
The weight-based dose adjustment scheme for AQ led to a median dosage of 24.2 mg/kg (range, 21.4 to 25.8 mk/kg). There was no correlation between the level of DEAQ exposure, as measured by the AUC0-∞, and the dosage (in mg/kg) of AQ within this narrow range of weight-based dosages (rs = −0.05; P = 0.85)) (data not shown). The weight-based scheme of AL dosing led to a wide range of dosages: the median was 69.67 mg/kg, and the range was 60 to 86.4 mg/kg. There was a linear correlation between the LR dosage (mg/kg) and the subsequent level of LR exposure (AUC0-∞) (rs = 0.44; P = 0.05)) (Fig. 4).
FIG. 4.
Relationship between exposure and weight-based dose adjustment for AL in children. The graph depicts the correlation of the AUC0-∞ and the LR dosage (in mg/kg) in children.
DISCUSSION
Treatment of falciparum malaria has shifted to the use of ACTs, with the majority of countries in Africa where malaria is endemic adopting either AQ-AS or AL as the first-line treatment. In multiple trials, these two regimens have proven to be highly efficacious for the treatment of uncomplicated falciparum malaria. However, little is known regarding the PKs of these regimens in children and whether optimum drug exposure is achieved with the current dosing strategies. Optimum dosing will minimize the risks of treatment failure, drug toxicity, and the selection of drug resistance. In this study, we used an intensive sampling design to study exposure to the components and principal metabolites of AQ-AS and AL in Ugandan children with uncomplicated malaria.
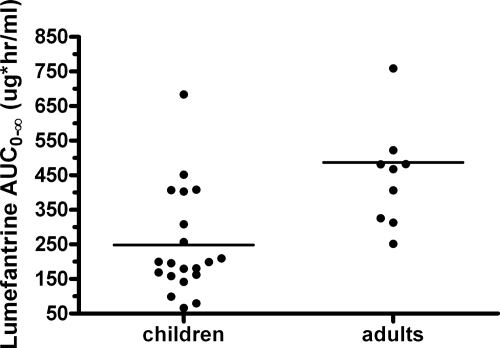
Our most important findings were for LR. These findings suggest that current weight-based dosing for AL in children results in a level of LR drug exposure considerably lower than the level of drug exposure in healthy adults. Current findings were compared to historical data generated in our recent study with healthy uninfected adults who participated in a PK study with a design nearly identical to that of the present study (14). The levels of LR exposure were lower in children than in healthy adults, with the geometric mean AUC0-∞ and Cmax being 46% and 38% lower, respectively (Fig. 5). These differences may be due to higher clearance in prepubescent children, as has been reported for other drugs (13). However, we cannot rule out the possibility that acute malaria alters the level of exposure to LR and thus contributes to this apparent difference. Ezzet et al. reported that the oral bioavailability of LR is lower and displays a higher degree of interindividual variability in the setting of the acute phase of malaria than in the convalescent phase (13), a finding that may be due to the variable food intake that occurs during the acute phase of malaria (8). To control for the effects of food, the children in our study received food with each dose of LR. The ability to compare the findings of the present study with those of other studies with adults is limited since the older studies dosed AL differently and employed different PK sampling schemes that estimated exposure (as measured by the AUC) over the full treatment period, but those studies also supported the differences in LR exposure between children and adults (2, 13).
FIG. 5.
The level of LR exposure is lower in African children with uncomplicated malaria than in healthy American adults. The graph depicts the distribution of AUC0-∞ values for LR in children (n = 20) obtained in the current study and data for healthy adults (n = 10) obtained in a previous study (14). The dosing and sampling strategies were identical between the two studies.
Significant interest has been placed on the utility of PK parameters in predicting the treatment response. Most attention has been placed on the correlates of the AUC, as AUC represents both the duration and the degree of exposure. The accurate measurement of AUC in field studies is difficult, so recent efforts for studying the PKs of artemisinin partner drugs have focused on single day 7 drug levels (34). The rationale for this approach is that by day 7 the remaining parasites will be exposed only to the partner drug, as the rapidly eliminated artemisinin derivatives are no longer present. The level of partner drug in the days following dosing may be critical for determining both the clearance of the infection and the potential selection of drug-resistant parasites. Importantly, for the longer-acting partner drugs, the day 7 levels appear to correlate with the AUC (5), as seen for both DEAQ and LR in our study. Several studies from Thailand have examined the relationship between the day 7 levels and the therapeutic response. Ezzet et al. reported that the median day 7 LR level of 280 ng/ml in adults best predicted an individual's risk of treatment failure (13). Most recently, in a study by Price et al., the day 7 levels of LR were found to be the main determinant of the efficacy of AL (26). The median day 7 level in patients aged 2 to 70 years (median age, 23 years) was 528 ng/ml. In addition, they determined that a level of <175 ng/ml best predicted an individual's risk of recurrent infection. Lastly, Denis et al. assessed the day 7 levels in adults and found a median level of 860 ng/ml in those treated successfully and a median level of 510 ng/ml in those with late treatment failures (9). In our study, there were no true treatment failures, and thus, a correlation of the day 7 levels and outcomes was not possible. It is also worth noting that the day 3 levels of LR correlated well with the AUC in our study, similar to findings reported by Checchi et al. (8). If the day 3 levels correlate well with the AUC and the AUC is associated with the outcome, it may be useful to examine the utility of day 3 levels in predicting the treatment response.
We compared the day 7 levels in our children with those in prior studies with adults. In our study, the median day 7 level in children was 323.3 ng/ml, with the range being 53.9 to 778.9 ng/ml. These values are similar to the median day 7 levels (402 ng/ml) reported in children aged 5 to 14 years in Thailand (8). Comparison of these findings to those of several studies with older individuals suggests that the median levels are similar to (8, 26, 28) or somewhat lower than (2, 12) the levels seen in adults. Several therapeutic day 7 levels, levels below which higher risks of treatment failure are predicted, have also been reported in the literature. In our study, 8/20 (40%) of children had a day 7 level below 280 ng/ml, which was a therapeutic level as reported by Ezzet et al. (12). In addition, Price et al. reported that a level of <175 ng/ml was predictive of treatment failure, and 3/20 of our children had levels below 175 ng/ml (26). Since the degree of LR exposure (as estimated by the day 7 levels or AUC) may influence outcomes, we also evaluated the impact that various doses (in mg/kg) of AL has on the LR AUC in children. The AL dose ranged from 60 to 86 mg/kg. Not surprisingly, as the dosage (mg/kg) of AL increased, there was a trend toward a linear increase in exposure (AUC). Thus, one may speculate that children who receive a lower weight-based dosage of AL may be at risk for a lower level of exposure and may have a higher risk of treatment failure or reinfection, possibilities supported by the findings of Checchi et al. (8).
For AQ, potential differences between children and adults were also noted for both the parent drug and its metabolite. For AQ, the estimated AUC0-∞ was 39 ng·h/ml. This is lower than comparative PK data obtained from two 600-mg single-dose studies of AQ with healthy adults (geometric mean AQ AUC0-∞ values, 154 and 162 ng·h/ml, respectively) (24, 36). For the active metabolite, DEAQ, the mean AUC0-∞ was 15,070 ng·h/ml for our study children, a value actually higher than the AUC reported in the single-dose studies with adults (mean DEAQ AUC0-∞ values, 8,437 ng·h/ml and 8,037 ng·h/ml, respectively) (24, 36). A potential explanation for the lower levels of AQ includes the more rapid conversion to DEAQ in children than in adults or an underestimation of the AQ AUC due to the less intensive sampling employed in our pediatric study. Furthermore, owing to the long half-life of DEAQ and its accumulation with multiple dosing, higher levels of DEAQ exposure, as we observed, are expected following 3 days of treatment than after the administration of the single dose used in the studies with adults. Notably, our study employed the AQ regimen recommended earlier (total dose of AQ, 25 mg/kg over 3 days), whereas the dosage of the currently coformulated AQ-AS regimen is 30 mg/kg of AQ over 3 days.
In contrast to LR, data that may be used to evaluate the relationship between single point measurements and outcomes for AQ are limited. Two studies have explored the utility of day 3 DEAQ levels in children. In a study in Gabon, isolated day 3 levels were obtained for 118 children, aged 6 months to 10 years, treated with AQ (total dose, 30 mg/kg) (3). In this study, the mean DEAQ level on day 3 was 149 ng/ml, with a level of 135 ng/ml reported as being the most predictive of treatment success. In a second population PK study conducted with Ghanaian children treated with AQ-AS (total dose, 30 mg/kg), the day 3 DEAQ level was similar, 156 ng/ml (1). The day 3 levels of DEAQ were similar to those in our study (mean, 127 ng/ml), despite the lower total dose used (25 mg/kg). Although the artemisinins may still be affecting treatment on day 3, the day 3 levels of the partner drug correlated strongly with the AUC in our study and may thus be useful as a correlate of exposure and outcomes, similar to the day 7 levels. Thus, as with AQ, the further study of day 3 levels and outcomes is warranted. We did compare, however, the more commonly utilized day 7 levels of DEAQ in children to those reported in adults. In a small group of Nigerian adult patients (n = 4) given a total AQ dose of 25 mg/kg, the day 7 level of DEAQ was 62 ± 17 ng/ml (36), a value slightly higher than that measured in our children (41 ng/ml). Other studies have reported day 7 levels, although comparisons are limited by the higher total AQ dose (30 mg/kg) given in those studies. Thus, taken together, it appears that metabolism of the parent drug, AQ, into DEAQ or the bioavailability of AQ may be altered in children compared with the metabolism and bioavailability of AQ in adults, but by day 7, the levels of the active metabolite may not be significantly different between children and adults. One additional explanation may be the enhanced elimination of DEAQ in children compared with that in adults. Further comparative data are needed, as lower DEAQ levels may predispose patients to treatment failure, whereas higher levels may lead to higher risks of toxicity. We also assessed the data for a correlation between the AQ dosage (mg/kg) and the subsequent level of DEAQ exposure. Unlike AL, the current weight-based dosing regimen for AQ results in less variability in the actual dosage (mg/kg) administered to children, precluding the ability to make associations between various dosages (mg/kg) and levels of exposure.
The PK results for the artemisinins are also notable, as they are among the first reported for children and indicate distinct disposition characteristics. The results for AR and DHA are also best compared to those from our earlier work with healthy adults, in which a sample collection protocol identical to that used in the present study was employed (14). The geometric mean Cmax and AUC0-∞ values for AR were approximately two- to threefold higher in children than in adults (Cmaxs, 34 and 14 ng/ml, respectively; AUC0-∞s, 168 and 62 ng·h/ml, respectively). Similarly, the values of the PK parameters for the current study were higher than the values reported in a separate study with healthy adults, in which the mean Cmax and the mean AUC from time zero to 8 h (AUC0-8) averaged 27 ng/ml and 64 ng·h/ml, respectively (19). It should be noted that AUC0-∞ and AUC0-8 are comparable, in that both AR and DHA are rapidly eliminated from the plasma. The level of exposure to DHA was similarly higher in our children than in the healthy adults in our previous study (AUC0-∞s, 382 and 198 ng·h/ml, respectively). Comparison of the PK data generated with patients to the data generated with healthy adults is a limitation, in that acute malaria alone may affect the disposition of artemisinin drugs (29). A strength of our comparison, however, is that the pediatric and adult studies used identical study designs.
In contrast to AR, for AS, when it used as part of the AQ-AS regimen, children appeared to have lower levels of exposure than adults. Although good comparative studies with adults are lacking, the best comparison of the results of the present may be made with the results of a study by Orrell et al., completed with uninfected adults receiving a single 4-mg/mg AS dose followed by serial sampling for PK analysis (24). The mean AS Cmax and AUC0-∞ were 142 ng/ml and 183 ng·h/ml, respectively, values higher than those estimated for our children (estimated Cmax, 51 ng/ml; estimated AUC0-∞, 113 ng·h/ml). Importantly, AS is rapidly and extensively converted to DHA (20). In our study, DHA levels were roughly equivalent between children and adults (geometric mean Cmaxs, 526 and 446 ng/ml, respectively; geometric mean AUC0-∞s, 1,509 and 1,411 ng·h/ml, respectively). Thus, perhaps not surprisingly, generalizations regarding the differences in the PKs of artemisinin derivatives between children and adults are difficult to make. Metabolism of the parent drugs is mediated by different CYP isozymes. AS is converted to DHA principally via CYP2A6 (21), and AR is likely converted to DHA via CYP3A4 (22); these isozymes may have different activities at different ages. These enzymes may also display pharmacogenetic differences among individuals of different ethnicities that may affect PK comparisons, similar to what has been seen with in vitro studies of AQ and CYP2C8 (25). In addition, it is possible that other age-dependent effects on drug disposition, such as drug absorption and distribution, vary between the artemisinin derivatives. Although artemisinins are extremely potent and rapidly acting, suboptimal dosing may enhance the selective pressure for resistance to these drugs, especially in settings where resistance to the longer-acting partner drugs has emerged. Of interest is the difference in exposure to the bioactive metabolite DHA when the results for AS and AR are compared. On the basis of current dosing recommendations, a nearly fourfold higher level of DHA exposure was measured following the administration of AS than following the administration of AR. This higher level of exposure to DHA may be important for the early eradication of parasites following ACT and warrants further study.
With over 45 countries having adopted either AL or AQ-AS as the first-line therapy for malaria, it is expected that millions of doses of these drugs will be administered in the coming years (39). Despite this massive deployment, relatively limited data regarding the PKs of these drugs in the most vulnerable population, young children, are available. While the levels of AQ, DEAQ, AR, and DHA seen in children appear to be similar to those seen in adults, this study provides evidence that children have lower levels of exposure to LR. The PK characteristics of artemisinins in children are distinct and must be considered for optimum dosing to preserve the activity of this valuable class of antimalarial drugs.
Acknowledgments
We thank all the parents and guardians for kindly giving their consent and the study participants for their cooperation. We thank all the members of the UO1 study team in Uganda, especially Denise Njama-Meya, Bridget Nzarubara, Catherine Maiteki-Sebuguzi, Leatitia Kampiire, Immaculate Ampeire, Pascal Kwitonda, and Joanitter Nankabirwa.
We have no conflicting interests to declare.
Our related research is supported by the National Institutes of Health (grant U01AI052142 to P.J.R.), the Doris Duke Charitable Foundation (to S.P.), and the UCSF-GIVI Center for AIDS Research (grant 5P30AI022763).
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Adjei, G. O., K. Kristensen, B. Q. Goka, L. C. Hoegberg, M. Alifrangis, O. P. Rodrigues, and J. A. Kurtzhals. 2008. Effect of concomitant artesunate administration and cytochrome P4502C8 polymorphisms on the pharmacokinetics of amodiaquine in Ghanaian children with uncomplicated malaria. Antimicrob. Agents Chemother. 52:4400-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley, E. A., K. Stepniewska, N. Lindegardh, R. McGready, A. Annerberg, R. Hutagalung, T. Singtoroj, G. Hla, A. Brockman, S. Proux, J. Wilahphaingern, P. Singhasivanon, N. J. White, and F. Nosten. 2007. Pharmacokinetic study of artemether-lumefantrine given once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop. Med. Int. Health 12:201-208. [DOI] [PubMed] [Google Scholar]
- 3.Aubouy, A., M. Bakary, A. Keundjian, B. Mbomat, J. R. Makita, F. Migot-Nabias, M. Cot, J. Le Bras, and P. Deloron. 2003. Combination of drug level measurement and parasite genotyping data for improved assessment of amodiaquine and sulfadoxine-pyrimethamine efficacies in treating Plasmodium falciparum malaria in Gabonese children. Antimicrob. Agents Chemother. 47:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes, K. I., F. Little, P. J. Smith, A. Evans, W. M. Watkins, and N. J. White. 2006. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin. Pharmacol. Ther. 80:582-596. [DOI] [PubMed] [Google Scholar]
- 5.Barnes, K. I., W. M. Watkins, and N. J. White. 2008. Antimalarial dosing regimens and drug resistance. Trends Parasitol. 24:127-134. [DOI] [PubMed] [Google Scholar]
- 6.Bartelink, I. H., C. M. Rademaker, A. F. Schobben, and J. N. van den Anker. 2006. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin. Pharmacokinet. 45:1077-1097. [DOI] [PubMed] [Google Scholar]
- 7.Blessborn, D., G. Neamin, Y. Bergqvist, and N. Lindegardh. 2006. A new approach to evaluate stability of amodiaquine and its metabolite in blood and plasma. J. Pharm. Biomed. Anal. 41:207-212. [DOI] [PubMed] [Google Scholar]
- 8.Checchi, F., P. Piola, C. Fogg, F. Bajunirwe, S. Biraro, F. Grandesso, E. Ruzagira, J. Babigumira, I. Kigozi, J. Kiguli, J. Kyomuhendo, L. Ferradini, W. R. Taylor, and J. P. Guthmann. 2006. Supervised versus unsupervised antimalarial treatment with six-dose artemether-lumefantrine: pharmacokinetic and dosage-related findings from a clinical trial in Uganda. Malar. J. 5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis, M. B., R. Tsuyuoka, P. Lim, N. Lindegardh, P. Yi, S. N. Top, D. Socheat, T. Fandeur, A. Annerberg, E. M. Christophel, and P. Ringwald. 2006. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop. Med. Int. Health 11:1800-1807. [DOI] [PubMed] [Google Scholar]
- 10.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsey, G., S. Staedke, T. D. Clark, D. Njama-Meya, B. Nzarubara, C. Maiteki-Sebuguzi, C. Dokomajilar, M. R. Kamya, and P. J. Rosenthal. 2007. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 297:2210-2219. [DOI] [PubMed] [Google Scholar]
- 12.Ezzet, F., R. Mull, and J. Karbwang. 1998. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br. J. Clin. Pharmacol. 46:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzet, F., M. van Vugt, F. Nosten, S. Looareesuwan, and N. J. White. 2000. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob. Agents Chemother. 44:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.German, P., S. Parikh, J. Lawrence, G. Dorsey, P. J. Rosenthal, D. Havlir, E. Charlebois, W. Hanpithakpong, N. Lindegardh, and F. T. Aweeka. 2009. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J. Acquir. Immune Defic. Syndr. 51:424-429. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood, B. M., D. A. Fidock, D. E. Kyle, S. H. Kappe, P. L. Alonso, F. H. Collins, and P. E. Duffy. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanpithakpong, W., B. Kamanikom, A. M. Dondorp, P. Singhasivanon, N. J. White, N. P. Day, and N. Lindegardh. 2008. A liquid chromatographic-tandem mass spectrometric method for determination of artesunate and its metabolite dihydroartemisinin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 876:61-68. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, R. F., G. L. Kearns, A. L. Brown, J. M. Trang, and R. B. Kluza. 1984. Renal clearance of imipenem in children. Eur. J. Clin. Microbiol. 3:471-474. [DOI] [PubMed] [Google Scholar]
- 18.Kindermans, J. M., J. Pilloy, P. Olliaro, and M. Gomes. 2007. Ensuring sustained ACT production and reliable artemisinin supply. Malar. J. 6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefevre, G., M. Bindschedler, F. Ezzet, N. Schaeffer, I. Meyer, and M. S. Thomsen. 2000. Pharmacokinetic interaction trial between co-artemether and mefloquine. Eur. J. Pharm. Sci. 10:141-151. [DOI] [PubMed] [Google Scholar]
- 20.Li, Q. G., J. O. Peggins, L. L. Fleckenstein, K. Masonic, M. H. Heiffer, and T. G. Brewer. 1998. The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J. Pharm. Pharmacol. 50:173-182. [DOI] [PubMed] [Google Scholar]
- 21.Li, X. Q., A. Bjorkman, T. B. Andersson, L. L. Gustafsson, and C. M. Masimirembwa. 2003. Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur. J. Clin. Pharmacol. 59:429-442. [DOI] [PubMed] [Google Scholar]
- 22.Navaratnam, V., S. M. Mansor, N. W. Sit, J. Grace, Q. Li, and P. Olliaro. 2000. Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 39:255-270. [DOI] [PubMed] [Google Scholar]
- 23.Nosten, F., and N. J. White. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181-192. [PubMed] [Google Scholar]
- 24.Orrell, C., F. Little, P. Smith, P. Folb, W. Taylor, P. Olliaro, and K. I. Barnes. 2008. Pharmacokinetics and tolerability of artesunate and amodiaquine alone and in combination in healthy volunteers. Eur. J. Clin. Pharmacol. 64:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh, S., J. B. Ouedraogo, J. A. Goldstein, P. J. Rosenthal, and D. L. Kroetz. 2007. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin. Pharmacol. Ther. 82:197-203. [DOI] [PubMed] [Google Scholar]
- 26.Price, R. N., A. C. Uhlemann, M. van Vugt, A. Brockman, R. Hutagalung, S. Nair, D. Nash, P. Singhasivanon, T. J. Anderson, S. Krishna, N. J. White, and F. Nosten. 2006. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin. Infect. Dis. 42:1570-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pussard, E., F. Verdier, F. Faurisson, J. M. Scherrmann, J. Le Bras, and M. C. Blayo. 1987. Disposition of monodesethylamodiaquine after a single oral dose of amodiaquine and three regimens for prophylaxis against Plasmodium falciparum malaria. Eur. J. Clin. Pharmacol. 33:409-414. [DOI] [PubMed] [Google Scholar]
- 28.Rahman, M. M., A. M. Dondorp, N. P. Day, N. Lindegardh, M. Imwong, M. A. Faiz, A. M. Bangali, A. T. Kamal, J. Karim, J. Kaewkungwal, and P. Singhasivanon. 2008. Adherence and efficacy of supervised versus non-supervised treatment with artemether/lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Bangladesh: a randomised controlled trial. Trans. R. Soc. Trop. Med. Hyg. 102:861-867. [DOI] [PubMed] [Google Scholar]
- 29.Simpson, J. A., T. Agbenyega, K. I. Barnes, G. Di Perri, P. Folb, M. Gomes, S. Krishna, S. Krudsood, S. Looareesuwan, S. Mansor, H. McIlleron, R. Miller, M. Molyneux, J. Mwenechanya, V. Navaratnam, F. Nosten, P. Olliaro, L. Pang, I. Ribeiro, M. Tembo, M. van Vugt, S. Ward, K. Weerasuriya, K. Win, and N. J. White. 2006. Population pharmacokinetics of artesunate and dihydroartemisinin following intra-rectal dosing of artesunate in malaria patients. PLoS Med. 3:e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strolin Benedetti, M., and E. L. Baltes. 2003. Drug metabolism and disposition in children. Fundam. Clin. Pharmacol. 17:281-299. [DOI] [PubMed] [Google Scholar]
- 32.Terlouw, D. J., J. M. Courval, M. S. Kolczak, O. S. Rosenberg, A. J. Oloo, P. A. Kager, A. A. Lal, B. L. Nahlen, and F. O. ter Kuile. 2003. Treatment history and treatment dose are important determinants of sulfadoxine-pyrimethamine efficacy in children with uncomplicated malaria in western Kenya. J. Infect. Dis. 187:467-476. [DOI] [PubMed] [Google Scholar]
- 33.White, N. J. 1994. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):S41-S43. [DOI] [PubMed] [Google Scholar]
- 34.White, N. J., K. Stepniewska, K. Barnes, R. N. Price, and J. Simpson. 2008. Simplified antimalarial therapeutic monitoring: using the day-7 drug level? Trends Parasitol. 24:159-163. [DOI] [PubMed] [Google Scholar]
- 35.White, N. J., M. van Vugt, and F. Ezzet. 1999. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin. Pharmacokinet. 37:105-125. [DOI] [PubMed] [Google Scholar]
- 36.Winstanley, P. A., O. Simooya, J. M. Kofi-Ekue, O. Walker, L. A. Salako, G. Edwards, M. L. Orme, and A. M. Breckenridge. 1990. The disposition of amodiaquine in Zambians and Nigerians with malaria. Br. J. Clin. Pharmacol. 29:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. 2006, posting date. Facts on ACTs. January 2006 update. World Health Organization, Geneva, Switzerland. http://www.searo.who.int/LinkFiles/Drug_Policy_RBMInfosheet_9.pdf.
- 38.World Health Organization. 2006, posting date. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland. http://apps.who.int/malaria/docs/TreatmentGuidelines2006.pdf.
- 39.World Health Organization. 2008, posting date. World malaria report. World Health Organization, Geneva, Switzerland. http://apps.who.int/malaria/wmr2008/malaria2008.pdf.