Abstract
Nemonoxacin (TG-873870) is a novel nonfluorinated quinolone with potent broad-spectrum activity against Gram-positive and Gram-negative pathogens, including methicillin-resistant Staphylococcus aureus, penicillin- and quinolone-resistant Streptococcus pneumoniae, and vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. The safety, tolerability, and pharmacokinetics of nemonoxacin were investigated in a double-blind, ascending-single-dose study involving 56 healthy subjects (48 males and 8 females) who were randomly assigned to 1 of 7 dose cohorts. In each successive cohort, two subjects received a placebo and six received single oral doses of 25, 50, 125, 250, 500, 1,000, or 1,500 mg nemonoxacin. Nemonoxacin was well tolerated up to the maximum dose of 1,500 mg. No severe or serious adverse events were observed. The most frequent adverse events were contact dermatitis, pruritus, and erythema. No clinically significant abnormalities were noted in the electrocardiograms, vital signs, or laboratory tests. The plasma concentrations increased over the dose range, and at 500 mg, the free area under the plasma concentration-time curve/MIC90 ratios and free maximum nemonoxacin concentration/MIC90 ratios against drug-sensitive/drug-resistant S. pneumoniae and S. aureus were greater than 227 and 24, respectively. The peak time and elimination half-life of nemonoxacin were 1 to 2 h and 9 to 16 h, respectively. The oral clearance was approximately 0.22 liter/h/kg. The plasma protein binding was approximately 16%. The results of this study support further evaluation of the multiple-dose safety, tolerability, and pharmacokinetics of nemonoxacin.
Newer-generation quinolones are largely fluoroquinolones (FQs); they are characterized by the presence of a fluorine at the R6 position of the quinolone nucleus (2, 9, 19). Owing to their improved activity against Gram-positive and atypical pathogens, they have been widely used for the treatment and prophylaxis of bacterial infections. However, the use of FQs has been compromised by the emergence of bacterial resistance (3, 8, 14, 18, 22, 30) and the potential for adverse side effects (15, 21), including ECG QTc interval prolongation, hepatotoxicity, and phototoxicity. Thus, there is an unmet need for newer compounds that are broad-spectrum agents effective against existing bacterial resistance and that have the potential to minimize the risk of use-limiting adverse effects.
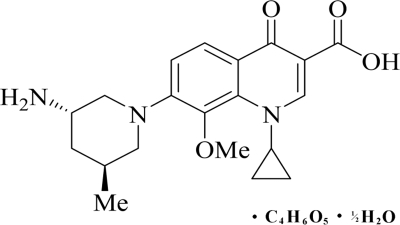
Nemonoxacin (TG-873870) (36), a novel nonfluorinated quinolone, has recently been developed from a series of 8-methoxy nonfluorinated quinolones (29) as a malate salt. It differs from FQs in that it lacks the fluorine at the R6 position (Fig. 1); this was formerly believed to be important for the antibacterial potency of FQs. Nemonoxacin has demonstrated broad-spectrum activity (4, 20, 28) against Gram-positive, Gram-negative, and atypical pathogens. Furthermore, nemonoxacin appears to be more potent than several FQs, including ciprofloxacin, levofloxacin, and moxifloxacin (20). In preclinical studies, it has been found that nemonoxacin neither induces nor inhibits human hepatic CYP3A4 activity (7). Metabolism studies completed to date indicate that no metabolite or a minor metabolite (less than 5%) of nemonoxacin was formed either in vitro or ex vivo (reference 7 and unpublished data). Nemonoxacin has a minimal effect on cardiac conduction as measured by ECG QTc interval prolongation (6) and a low potential to produce phototoxicity (5).
FIG. 1.
Chemical structure of nemonoxacin as a malate salt (C20H25N3O4·C4H6O5·H2O). Nemonoxacin is the free base, and its molecular mass is 371.44 g/mol. The molecular mass of the salt, nemonoxacin malate, is 514.53 g/mol.
Owing to promising in vitro activity and a favorable safety profile of nemonoxacin in animals, we investigated its safety, tolerability, and pharmacokinetics (PK) in an ascending single-dose phase 1 study of healthy male and female volunteers.
MATERIALS AND METHODS
Subjects and study design.
The study was a randomized, double-blind, placebo-controlled, parallel-design, single-ascending-dose study involving seven sequential doses of nemonoxacin (25, 50, 125, 250, 500, 1,000, and 1,500 mg). The initial dose selected for this study was 25 mg, or approximately 0.42 mg/kg of body weight for a 60-kg person. This dose is lower than 10% of the human equivalent derived from the lowest no-observed-adverse-effect level (NOAEL) found in preclinical 1-month repeat-dose toxicity studies. This starting dose of 25 mg was deemed to allow judicious and efficient dose escalation to levels well beyond the anticipated therapeutic range. Cohorts of healthy male and female subjects were randomly assigned to orally receive capsules of nemonoxacin malate salt (six subjects per dose group) or placebo (two subjects per dose group). A prestudy screening, including medical history, a physical examination, and clinical laboratory tests, was performed within 22 days prior to admission to the study center. Eligible subjects were admitted the day prior to dosing (day 1) and were confined at the study center for 5 nights. On day 1 of the study, after fasting for 10 h, the subjects were orally administered nemonoxacin or the placebo with 240 ml water. The subjects continued to fast for 4 h after dosing. They remained at the study center until 96 h after administration. During this period, blood and urine samples were collected for PK and safety evaluation before proceeding to the next cohort for the administration of the higher dose. The study was conducted at a single center in the United States in accordance with the principles of the Declaration of Helsinki and all of its amendments. All volunteers provided written informed consent.
Safety evaluation.
Safety and tolerability were evaluated by physical examination, measurement of vital signs (blood pressure, heart rate, and temperature), electrocardiography (ECG), cardiac telemetry, laboratory tests (clinical chemistry, hematology, urinalysis, serology, coagulation, thyroid panel, and drug abuse), and clinical adverse events (AEs). Blood pressure and pulse rates were recorded before dosing and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 18, 36, 48, 72, and 96 h after dosing. The temperature and respiratory rate were recorded at screening and admission and at 18, 36, 48, 72, and 96 h after dosing. Predose 12-lead ECGs were obtained at time points corresponding to 0, 1, 2, 3, 4, 6, 8, 10, 12, 15, 18, and 21 h after dosing. Cardiac telemetry was initiated within 12 h prior to dosing and was continued up to 12 h after dosing. Clinical chemistry and hematology testing and urinalysis were conducted before dosing and at 24, 48, and 96 h after dosing. AEs were recorded throughout the dosing period and at follow-up.
Sample collection.
Blood samples for pharmacokinetic analysis were collected within 1 h before dosing and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 15, 18, 21, 24, 36, 48, 72, and 96 h after dosing. The blood samples (5 ml) were drawn into heparinized, evacuated specimen tubes and were processed to obtain plasma. For each sample, the plasma was aliquoted and stored at approximately −70°C until further analysis. Urine samples were collected before dosing (−1 to 0 h) and during the following collection periods: 0 to 2, 2 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 36, 36 to 48, 48 to 72, and 72 to 96 h after dosing. The urine samples were pooled; for each sample, the urine was aliquoted and stored at approximately −70°C until further analysis.
Sample analysis.
Nemonoxacin was extracted using the solid-phase extraction method and was quantified by a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay with positive multiple reaction monitoring detection. Separation was achieved using a Waters Symmetry Shield RP-18 column (2.1 by 50 mm; 3.5 μm) with gradient elution. Both human plasma and urine calibration standards were used to quantify nemonoxacin in the human quality control samples and unknown specimens. Sample concentrations were determined by back calculation using weighted linear (1/x2) regression of a calibration curve generated from spiked matrix standards. The limit of quantification was defined as the lowest concentration of the analyte in plasma or urine measured with interrun accuracy and precision. A 25-μl aliquot was used for plasma analysis. The nominal range of quantitation of the analyte was 5 to 5,000 ng/ml. The inter- and intrarun relative percent error and percent coefficient of variation for nemonoxacin were −7.9% to 5.1% and 4.9% to 8.2%, respectively, over the linear range. A 20-μl aliquot was used for urinalysis. The nominal range of quantitation of the analyte was 200 to 200,000 ng/ml. The inter- and intrarun relative percent error and percent coefficient of variation for nemonoxacin were −5.9% to 2.1% and 8.0% to 10.7%, respectively, over the linear range.
Plasma protein binding.
The level of plasma protein binding of nemonoxacin was evaluated ex vivo using an ultrafiltration membrane (molecular weight cutoff, 30,000) method (P and G Pharmaceuticals method no. BA920_proteinBinding_01_03_04). Plasma samples were obtained from volunteers at two points (0.5 and 6 h postdosing) over the range of 25- to 1,000-mg doses in the study. A 0.4-ml aliquot was used for ultrafiltrate sample analysis and quantitated using the LC-MS/MS method. The nominal range of quantitation of the analyte was 50 to 10,000 ng/ml. Nonspecific protein binding was also measured (nonspecific protein binding, 0.0415) and used as a correction factor to determine the final percentage of protein binding. The percentage of the drug bound to the plasma protein was calculated by comparing the nemonoxacin concentrations measured in plasma ultrafiltrate solutions with those reported in plasma.
Pharmacokinetic analysis.
Pharmacokinetic parameters were calculated according to standard noncompartmental analysis (13) using SAS version 9.0 (SAS Institute Inc., Cary, NC). Plasma concentrations below the lower limit of quantification (5 ng/ml) were set to zero if they occurred prior to the peak; they were omitted from the PK analysis. Urine concentrations below the lower limit of quantification (200 ng/ml) were set to zero. The peak or maximum nemonoxacin concentration (Cmax) and the time to reach Cmax (Tmax) were determined from visual inspection of individual plasma concentration-time profiles. The terminal-phase elimination rate constant was determined using linear least-squares regression of the terminal phase of the log plasma concentration-time profile. The terminal-phase elimination half-life (t1/2) was obtained as 0.693/terminal-phase elimination rate constant. The area under the plasma concentration-time curve from 0 to the last observation after dosing (AUC0-last) was calculated using the linear trapezoidal method. The AUC from time zero to infinity (AUC0-∞) was calculated as the sum of the (AUC0-last) and Clast/λz, where Clast is the last measurable drug concentration and λz is the elimination rate constant derived from the slope of the linear regression line of the apparent terminal linear portion of the log concentration-time curve at a minimum of the last three data points. Oral clearance (CLo) and the terminal volume of distribution (Vz/F), uncorrected for bioavailability, were determined using standard equations (31). Renal clearance (CLr) was determined based on the cumulative amount of nemonoxacin excreted in urine divided by its corresponding AUC over the same collection interval. The percentage of the administered dose recovered in urine (Ae) and the percentage of nemonoxacin bound to plasma proteins were also determined.
Statistical analysis.
Data were analyzed using SAS version 8.2 (SAS Institute Inc., Cary, NC). All tests were two sided, and a significance level of 0.05 was used. The dose proportionality was assessed by evaluating the PK parameters, i.e., Cmax, AUC0-∞, AUCtlast (area under the plasma concentration-time curve from zero to the last quantifiable concentration), Ae, Tmax, t1/2, CLo, CLr, and Vz/F, using the power model. The linear relationships between the log-transformed PK parameters and the natural log of the dose were tested using a linear lack-of-fit test. Estimation of the slope parameter, β, and the 95% confidence interval and the two-sided H0: β = 1 or H0: β = 0 test were performed as appropriate. Dose proportionality was said to exist if β was equal to 1 for the dose-dependent parameters (AUC0-∞, AUCtlast, and Cmax) and if β was equal to 0 for the dose-independent parameters (Tmax, t1/2, CLo, CLr, Ae, and Vz/F). Residual analysis was used to assess the assumptions of normality and variance homogeneity for the statistical model. The parameters that were not dose proportional over the original range of 25 to 1,500 mg were investigated over two smaller ranges, i.e., 25 to 125 mg and 250 to 1,500 mg, in order to coincide with the two dose levels (25 mg and 250 mg). Supplemental analysis of dose proportionality involving the stepwise elimination of dose levels (highest or lowest) for the reduced ranges listed above was performed when the lack-of-fit test was significant or dose proportionality was not achieved. For parameters that did not have normally distributed Studentized residuals, least absolute deviation regression was used to estimate the model for the purpose of comparison. ECG and clinical laboratory data were listed.
RESULTS
Study population.
A total of 56 subjects (48 males and 8 females) completed all the assessments. The mean age was 31.1 years (range, 18 to 45 years), and the mean body weight was 73.8 kg (range, 54 to 94 kg). A majority of the subjects (40 subjects; 71%) were Hispanic, 7 were black (13%), 5 were Caucasian (9%), and 4 were multiracial (7%). Fifty-four subjects completed the study. Two subjects voluntarily withdrew after dosing owing to personal reasons. One subject who withdrew from the 50-mg group after the collection of the 72-h-postdosing samples was included in the PK analysis; the other, who withdrew from the 500-mg group after the collection of the 8-h-postdosing samples, was not included in the PK analysis.
Safety and tolerability.
There were no serious AEs or withdrawals due to AEs. Twelve subjects who were administered nemonoxacin experienced a total of 20 AEs (Table 1), including 5 cases of mild ECG electrode contact dermatitis (12%), 5 cases of moderate pruritus (12%), 4 cases of mild erythema (10%), 2 cases of mild headache (5%), and 1 case each of mild oral hypoesthesia, abdominal pain, rash, and nausea (2%). Eight of the AEs were considered doubtful drug-related AEs, while 4 were thought to be possible drug-related AEs (2 headache, 1 oral hypoesthesia, and 1 abdominal pain) and 8 were considered probable drug-related AEs (4 pruritus and 4 erythema). Further dose escalation was stopped at 1,500 mg owing to pruritus and erythema, i.e., probable drug-related AEs. Most subjects recovered from the reported AEs during the observation period. Moderate pruritus resolved after treatment with intramuscular diphenhydramine or topical calamine lotion. No significant abnormalities were reported in the vital signs, physical examinations, ECGs, or laboratory tests.
TABLE 1.
Number of subjects reporting adverse events
| Adverse event | No. [n (%)] of subjects reporting adverse eventsa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nemonoxacin |
Placebo (pooled) (N = 14) | ||||||||
| 25 mg (N = 6) | 50 mg (N = 6) | 125 mg (N = 6) | 250 mg (N = 6) | 500 mg (N = 6) | 1,000 mg (N = 6) | 1,500 mg (N = 6) | Overall (N = 42) | ||
| Contact dermatitis | 3 (50) | 0 (0) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (12) | 1 (7) |
| Pruritus | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 3 (50) | 5 (12) | 1 (7) |
| Erythema | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 3 (50) | 4 (10) | 0 (0) |
| Headache | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (17) | 2 (5) | 0 (0) |
| Oral hypoesthesia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 1 (2) | 0 (0) |
| Abdominal pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (2) | 0 (0) |
| Pustular rash | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (2) | 0 (0) |
| Ear pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) |
N, number of subjects given the specified treatment; n, number of subjects who reported adverse events; %, percentage of subjects who reported adverse events [(n/N) × 100].
Pharmacokinetics.
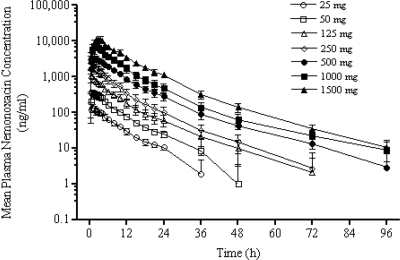
Figure 2 shows the mean plasma concentration-time profiles of nemonoxacin for each dose. Table 2 presents the values of all the pharmacokinetic parameters determined using a noncompartmental model. In general, nemonoxacin was rapidly absorbed following oral administration and Cmax was attained within 1 to 2 h after dosing.
FIG. 2.
Mean ± standard deviation (SD) plasma concentration-time profiles following single-dose oral administration of nemonoxacin to healthy volunteers.
TABLE 2.
Mean (standard deviation) PK parameters of nemonoxacin after ascending single dosing
| Dose (mg) | AUC0-∞ (h·μg/ml) | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | Vz/F (liters/kg) | CLo (liters/h/kg) | CLr (liters/h/kg) | Ae (%) |
|---|---|---|---|---|---|---|---|---|
| 25 | 1.19 (0.19) | 0.15 (0.05) | 0.92 (0.49) | 9.71 (1.50) | 4.21 (0.62) | 0.30 (0.050) | 0.18 (0.060) | 56.24 (15.84) |
| 50 | 2.99 (0.18) | 0.37 (0.045) | 1.08 (0.20) | 8.49 (1.31) | 2.78 (0.27) | 0.23 (0.035) | 0.12 (0.049) | 51.11 (17.06) |
| 125 | 7.67 (1.46) | 1.22 (0.31) | 0.75 (0.27) | 11.13 (4.09) | 3.38 (0.87) | 0.22 (0.046) | 0.080 (0.029) | 38.28 (15.66) |
| 250 | 15.45 (3.94) | 2.40 (0.66) | 0.92 (0.20) | 10.86 (3.91) | 3.58 (0.89) | 0.24 (0.056) | 0.11 (0.055) | 44.85 (17.48) |
| 500 | 32.36 (3.01) | 3.41 (0.58) | 2.0 (0.87) | 14.75 (3.06) | 4.25 (1.31) | 0.20 (0.024) | 0.068 (0.021) | 34.15 (9.05) |
| 1,000 | 63.31 (10.01) | 7.22 (0.88) | 1.67 (0.26) | 16.41 (2.54) | 5.28 (0.78) | 0.22 (0.025) | 0.084 (0.031) | 36.54 (12.03) |
| 1,500 | 117.84 (10.74) | 12.1 (1.70) | 2.67 (0.68) | 13.04 (1.51) | 3.42 (0.42) | 0.18 (0.025) | 0.069 (0.0077) | 37.58 (1.99) |
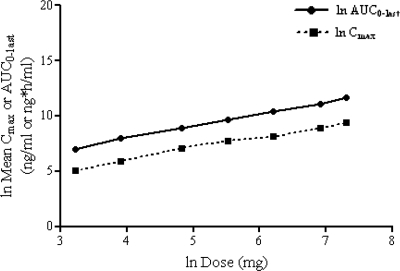
In this study, pharmacokinetic linearity and/or dose proportionality could not be achieved statistically over the entire dose range of 25 to 1,500 mg (a 60-fold increase in the dose) (Fig. 3). The AUC0-∞ and Cmax increased by more than the expected 60-fold range, i.e., by 41% and 14%, respectively. Analysis over a smaller range showed that, over the anticipated clinically relevant dose range of 250 to 1,500 mg (a sixfold increase in the dose), the increases in the AUC0-∞ and Cmax were 23% more and 14% less than the expected range, respectively. There was a small (<10%) but statistically significant lack of linearity for Cmax over the dose range of 250 to 1,500 mg. However, Cmax was dose proportional over the dose range of 500 to 1,500 mg.
FIG. 3.
Mean ± SD ln AUC and ln Cmax of nemonoxacin plotted against the ln dose.
The mean Tmax ranged from approximately 1 to 2 h. Analysis over the smaller range indicated that the Tmax was approximately 1 h over the dose range of 25 to 250 mg and increased to approximately 2 h in the dose range of 500 to 1,500 mg. The mean t1/2 was approximately 10 h over the dose range of 25 to 250 mg and increased to approximately 15 h at higher doses.
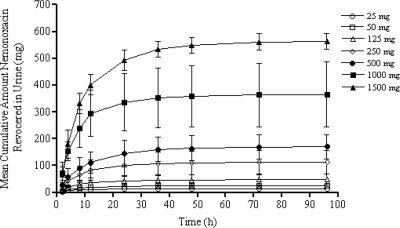
Figure 4 provides an overview of the cumulative amounts of urinary excretion of nemonoxacin. High dose-dependent concentrations ranging from 6.03 to 177.38 mg/liter were observed in the first 24 h postdosing. The mean CLr was approximately 0.09 liter/h/kg (111 ml/min), while the mean CLo was approximately 0.22 liter/h/kg. The mean Vz/F was approximately 3.8 liters/kg (281 liters). The mean Ae was approximately 45% of the administered dose in the dose range of 25 to 1,500 mg, with more than 75% of urinary excretion occurring within the first 24 h after dosing. A mean plasma protein binding of approximately 16% was observed over a concentration range of 141 to 4,270 ng/ml. The CLr of the free (unbound) fraction of nemonoxacin reached 132 ml/min.
FIG. 4.
Mean ± SD urinary recovery-time profiles following single-dose oral administration of nemonoxacin to healthy volunteers. The error bars indicate standard deviations.
DISCUSSION
In this study, single escalating doses of 25, 50, 125, 250, 500, 1,000, and 1,500 mg of nemonoxacin were evaluated in healthy volunteers (age range, 18 to 45 years). Further dose escalation was stopped at 1,500 mg owing to the occurrence of probable drug-related AEs. Thus, the 1,500-mg dose is defined as the maximum tolerated dose for nemonoxacin in humans.
There were no severe or life-threatening AEs related to nemonoxacin. The most common probable drug-related clinical AEs were pruritus and erythema. The safety of single oral doses of nemonoxacin was further demonstrated by the lack of any significant effect on vital signs, physical examination findings, laboratory values, or ECG data. These safety and tolerability findings compare favorably with those of presently available FQs (1, 33).
FQs such as clinafloxacin, grepafloxacin, temafloxacin, sparfloxacin, and trovafloxacin have been withdrawn from the market due to their phototoxicity, inhibition of cytochrome P450, QT interval prolongation, glucose homeostasis dysregulation, and/or hepatotoxicity (11, 27, 32). This study showed that nemonoxacin did not cause QT interval prolongation, glucose homeostasis dysregulation, or hepatotoxicity. These data are consistent with those reported in preclinical studies of nemonoxacin (5, 6, 7).
The pharmacokinetic findings of this study show that following oral administration, nemonoxacin rapidly reached its Cmax (within 1 to 2 h) with a relatively long t1/2 (approximately 9 to 16 h). These observations are similar to the data obtained in single-dose studies of existing FQs (1, 33, 34). Although the Cmax and AUC0-∞ were not linear and dose proportional over the dose range evaluated in this study, deviation from the dose proportionality over the anticipated clinically relevant dose range (250 to 1,500 mg) was within the variability of the pharmacokinetic parameters.
The apparent Vz/F of nemonoxacin was similar to the values reported for the available FQs (1, 33, 37) and exceeded the total body volume in the study population (35); this was consistent with the extensive tissue distribution pattern of quinolones (25, 26). The CLr (132 ml/min) of the free drug was greater than the normal glomerular filtration rate (GFR), i.e., 125 ml/min (24), which is suggestive of active renal secretion of the drug. Plasma protein binding was lower than that of existing FQs (10, 12, 33), indicating a higher percentage of tissue penetration and a lower potential for protein binding-based drug interactions (23).
Quinolone antibiotics are generally considered to have concentration-dependent bactericidal activity; Cmax/MIC and AUC/MIC ratios have been identified as pharmacodynamic predictors of clinical and microbiological outcomes, as well as the development of bacterial resistance (38). In studies that have been carried out over the past 20 years, investigators have suggested that AUC/MIC ratios of 100 to 125 or Cmax/MIC ratios of >10 are required to predict clinical and microbiological success and to limit the development of bacterial resistance (38). Table 3 summarizes the pharmacodynamic parameters of 500 mg nemonoxacin against Streptococcus pneumoniae and Staphylococcus aureus, the main causative pathogens of community-acquired pneumonia and skin and skin structure infections, respectively. The MIC at which 90% of S. pneumoniae and S. aureus isolates were inhibited (MIC90) by nemonoxacin was ≤0.125 μg/ml. Following administration of 500 mg nemonoxacin, the free AUC/MIC90 ratios and free Cmax/MIC ratios were greater than 227 and 24, respectively. These results also include drug-resistant isolates of S. pneumoniae and S. aureus. Therefore, nemonoxacin could be considered for the treatment of patients with community-acquired pneumonia and skin and skin structure infections.
TABLE 3.
Pharmacodynamic parameters of nemonoxacin
| Pathogen (no. of isolates)a | MIC90b (μg/ml) | 500 mg nemonoxacinc |
|
|---|---|---|---|
| fAUC/MIC90 | fCmax/MIC90 | ||
| S. pneumoniae (655) | 0.015 | 1812 | 191 |
| Penicillin-S S. pneumoniae (519) | 0.015 | 1812 | 191 |
| Penicillin-I S. pneumoniae (103) | 0.015 | 1812 | 191 |
| Penicillin-R S. pneumoniae (33) | 0.03 | 906 | 95 |
| Ciprofloxacin-R S. pneumoniae (29) | 0.12 | 227 | 24 |
| Methicillin-S S. aureus (375) | 0.12 | 227 | 24 |
| Methicillin-R S. aureus (26) | 0.06 | 453 | 48 |
Based on the good safety and tolerability of nemonoxacin, as observed in this study, and its sensitivity against Gram-positive microorganisms (4, 16, 17, 20), a once-daily dosing regimen with an anticipated clinically relevant dose (e.g., 500 mg once daily) may be possible.
In summary, all doses of nemonoxacin were well tolerated, no serious AEs or laboratory abnormalities were observed, and the pharmacokinetic profile as demonstrated in this study supports the further clinical development of nemonoxacin.
Acknowledgments
We are grateful to Chi-Hsin R. King, Judy Yuan, and Yu-Mai Chen for discussion and critical review of the manuscript and to Stephen Hoi Chuen Ip and Kit-Mui Chiu for their assistance in data transfer. Yi-Chien Lee provided valuable assistance in the preparation of the manuscript.
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Allen, A., E. Bygate, S. Oliver, M. Johnson, C. Ward, A. J. Cheon, Y. S. Choo, and I. C. Kim. 2000. Pharmacokinetics and tolerability of gemifloxacin (SB-265805) after administration of single oral doses to healthy volunteers. Antimicrob. Agents Chemother. 44:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriole, V. T. 1999. The future of the quinolones. Drugs 58(Suppl. 2):1-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen, D. K., A. McGeer, J. C. De Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S. J., L. Lin, and L. W. Chang. 2007. Analysis of antibacterial response of nemonoxacin (TG-873870) against major pathogens from respiratory tract and skin infections, abstr. 441. Abstr. 45th Infect. Dis. Soc. Am.
- 5.Chow, C. P., C. P. Sambuco, and A. M. Hoberman. 2007. Phototoxicity of nemonoxacin (TG-873870) in hairless mice, abstr. A-28. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 6.Chow, C. P., C. Y. Tsai, and Y. M. Chen. 2007. Cardiovascular safety evaluation of nemonoxacin (TG-873870) in dogs and monkeys, abstr. B-823. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 7.Chow, C. P., C. Y. Tsai, C. F. Yeh, and S. J. Chen. 2007. In vitro metabolism and interaction of nemonoxacin (TG-873870) on human hepatic CYP3A4, abstr. A-27. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 8.Deshpande, L. M., T. R. Fritsche, G. J. Moet, D. J. Biedenbach, and R. N. Jones. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 58:163-170. [DOI] [PubMed] [Google Scholar]
- 9.Fass, R. J. 1990. Ciprofloxacin. Best use of this new broad-spectrum antibiotic. Postgrad. Med. 87:117-122, 124, 127-131. [DOI] [PubMed] [Google Scholar]
- 10.Fish, D. N., and A. T. Chow. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32:101-119. [DOI] [PubMed] [Google Scholar]
- 11.Frothingham, R. 2004. Quinolone safety and efficacy more important than potency. Emerg. Infect. Dis. 10:156-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee, T., J. M. Andrews, J. P. Ashby, G. Marshall, and R. Wise. 2001. Pharmacokinetics and tissue penetration of gemifloxacin following a single oral dose. J. Antimicrob. Chemother. 47:431-434. [DOI] [PubMed] [Google Scholar]
- 13.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, NY.
- 14.Henwood, C. J., D. M. Livermore, A. P. Johnson, D. James, M. Warner, and A. Gardiner. 2000. Susceptibility of Gram-positive cocci from 25 UK hospitals to antimicrobial agents including linezolid. The Linezolid Study Group. J. Antimicrob. Chemother. 46:931-940. [DOI] [PubMed] [Google Scholar]
- 15.Hooper, D. C. 2000. New uses for new and old quinolones and the challenge of resistance. Clin. Infect. Dis. 30:243-254. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, C. H., L. Lin, R. Leunk, and D. Reichart. 2008. In vivo efficacy of nemonoxacin in a mouse pulmonary infection model, abstr. B-056. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 17.Hsu, C. H., L. Lin, R. Leunk, and D. Reichart. 2008. In vivo efficacy of nemonoxacin in a mouse protection model, abstr. B-1005. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 18.Kahlmeter, G. 2000. The ECO.SENS Project: a prospective, multinational, multicentre epidemiological survey of the prevalence and antimicrobial susceptibility of urinary tract pathogens—interim report. J. Antimicrob. Chemother. 46(Suppl. 1):15-22. [PubMed] [Google Scholar]
- 19.Koga, H., A. Itoh, S. Murayama, S. Suzue, and T. Irikura. 1980. Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J. Med. Chem. 23:1358-1363. [DOI] [PubMed] [Google Scholar]
- 20.Lauderdale, T. L., Y. R. Shiau, J. F. Lai, H. C. Chen, and C. H. King. 2007. In vitro antibacterial activity of nemonoxacin (TG-873870), a new nonfluorinated quinolone, against clinical isolates, abstr. E-1635. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 21.Lipsky, B. A., and C. A. Baker. 1999. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin. Infect. Dis. 28:352-364. [DOI] [PubMed] [Google Scholar]
- 22.Lister, P. D. 2000. Emerging resistance problems among respiratory tract pathogens. Am. J. Manag. Care 6:S409-S418. [PubMed] [Google Scholar]
- 23.Liu, S., L. W. Zhang, and X. X. Zhang. 2006. Interaction between fluoroquinolones and bovine serum albumin studied by affinity capillary electrophoresis. Anal. Sci. 22:1515-1518. [DOI] [PubMed] [Google Scholar]
- 24.Manjunath, G., M. J. Sarnak, and A. S. Levey. 2001. Prediction equations to estimate glomerular filtration rate: an update. Curr. Opin. Nephrol. Hypertens. 10:785-792. [DOI] [PubMed] [Google Scholar]
- 25.Nix, D. E., S. D. Goodwin, C. A. Peloquin, D. L. Rotella, and J. J. Schentag. 1991. Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning. Antimicrob. Agents Chemother. 35:1947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nix, D. E., S. D. Goodwin, C. A. Peloquin, D. L. Rotella, and J. J. Schentag. 1991. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob. Agents Chemother. 35:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens, R. C., Jr., and P. G. Ambrose. 2005. Antimicrobial safety: focus on fluoroquinolones. Clin. Infect. Dis. 41:S144-S157. [DOI] [PubMed] [Google Scholar]
- 28.Pankuch, G. A., K. Kosowska-Shick, P. McGhee, C. H. R. King, and P. C. Appelbaum. 2008. Comparative antistaphylococcal activity of nemonoxacin, a novel broad-spectrum quinolone, abstr. C1-189. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 29.Roychoudhury, S., and B. Ledoussal. 2002. Non-fluorinated quinolones (NFQs): new antibacterials with unique properties against quinolone-resistant Gram-positive pathogens. Curr. Drug Targets Infect. Disord. 2:51-65. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz, F. J., A. C. Fluit, S. Brisse, J. Verhoef, K. Kohrer, and D. Milatovic. 1999. Molecular epidemiology of quinolone resistance and comparative in vitro activities of new quinolones against European Staphylococcus aureus isolates. FEMS Immunol. Med. Microbiol. 26:281-287. [DOI] [PubMed] [Google Scholar]
- 31.Spokane, W. A. 1986. Guidelines for the collection and analysis of pharmacokinetic data, p. 9-54. In W. J. Jusko, W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics: principles of therapeutic drug monitoring, 2nd ed. Applied Therapeutics Inc., Lippincott Williams & Wilkins, Baltimore, MD.
- 32.Stahlmann, R. 2002. Clinical toxicological aspects of fluoroquinolones. Toxicol. Lett. 127:269-277. [DOI] [PubMed] [Google Scholar]
- 33.Stass, H., A. Dalhoff, D. Kubitza, and U. Schuhly. 1998. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 42:2060-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagenlehner, F. M., M. Kinzig-Schippers, U. Tischmeyer, C. Wagenlehner, F. Sorgel, A. Dalhoff, and K. G. Naber. 2006. Pharmacokinetics of ciprofloxacin XR (1000 mg) versus levofloxacin (500 mg) in plasma and urine of male and female healthy volunteers receiving a single oral dose. Int. J. Antimicrob. Agents 27:7-14. [DOI] [PubMed] [Google Scholar]
- 35.Watson, P. E., I. D. Watson, and R. D. Batt. 1980. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am. J. Clin. Nutr. 33:27-39. [DOI] [PubMed] [Google Scholar]
- 36.W. H. O. 2006. Proposed INN list 96. World Health Organ. Drug Inf. 20:292. [Google Scholar]
- 37.Wingender, W., K. H. Graefe, W. Gau, D. Forster, D. Beermann, and P. Schacht. 1984. Pharmacokinetics of ciprofloxacin after oral and intravenous administration in healthy volunteers. Eur. J. Clin. Microbiol. 3:355-359. [DOI] [PubMed] [Google Scholar]
- 38.Wright, D. H., G. H. Brown, M. L. Peterson, and J. C. Rotschafer. 2000. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 46:669-683. [DOI] [PubMed] [Google Scholar]
- 39.Zhanel, G. G., N. Laing, M. DeCorby, K. Nichol, C. H. R. King, H. Adam, and D. J. Hoban. 2008. Activity of nemonoxacin, an investigational C8-methoxy non-fluorinated quinolone, against Gram-positive cocci obtained from Canadian hospitals: CANWARD 2007, abstr. F1-2057. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.