Abstract
This study compared the efficacies of two N-methylglucomine antimoniate (MA) dose regimens for treating macaques with Leishmania braziliensis-induced chronic skin disease. Whereas all animals treated with the full dose (20 mg MA/kg/day) were cured, 50% of the monkeys receiving a low-dose regimen (5 mg MA/kg/day) relapsed. The antimony concentrations in macaque plasma and tissue samples were greater in the full-dose group than in that receiving a subtherapeutic MA regimen. Our data also suggest the presence of drug-induced hepatic pathology.
Leishmaniasis is a cause of significant morbidity and mortality throughout the world, with two million new cases of human infection worldwide each year. However, an approved vaccine to prevent leishmaniasis does not exist (7). Treatment for leishmaniasis relies largely on pentavalent antimony (Sbv) compounds (meglumine antimoniate and sodium stibogluconate). A number of other therapeutic agents may be employed, but high costs have limited the large-scale use of the most potent drugs (4). Factors limiting the usefulness of SbV therapy include their adverse toxic effects (arthralgias, myalgias, cardiac arrhythmia, pancreatitis, and hepatic or renal function impairment) and the increasing occurrence of parasite resistance (2, 4). Nevertheless, responses to SbV vary considerably, depending on both the parasite's intrinsic drug sensitivity and the host's immune status (1, 4, 15). Poor clinical responses can also be attributed to inadequately dosed antimony regimens (1, 2) and problems concerning drug pharmacokinetics (PK) or biodisposal (5, 13).
The Leishmania-macaque model has proven to be a valuable in vivo system for anti-infectious disease drug and vaccine development studies (7, 14). The aim of the present study was to compare the pharmacological parameters (PK, toxicity, and efficacy) of a low dose of MA with those of a standard dose of MA in macaques with L. braziliensis infection. All animal studies were performed under the guidance and with the approval of the Institutional Animal Care and Use Committee. Groups of six outbred adult rhesus macaques (Macaca mulatta) were used in this study. A high dose (107 promastigotes) of virulent L. braziliensis (MHOM/BR/2000/CP13396 strain) was injected intradermally above the left upper eyelid of each monkey. The infection was allowed to proceed until the macaques reached skin disease progression. At 9 weeks postinfection, macaques received a 21-day course of a low dose (5 mg/kg/day) or a full dose (20 mg/kg/day) of MA administered through an intramuscular route. A vehicle-only-treated control group was included to assess the development of a local skin lesion caused by the infection. Animals were euthanized on days 55 (140, 142, O6, L30, M2, and O34) and 95 (S62, T32, U48, U12, U46, and X53) after the completion of treatment, and selected necropsy tissue specimens (liver, spleen, and kidneys) were removed from drug-cured and control macaques to determine residual tissue antimony concentrations and for histological examination to assess antimony-induced histopathological changes. Antimony concentrations in macaque plasma and tissues were determined by inductively coupled plasma mass spectrometry (ICP-MS) under the optimized conditions previously described (11).
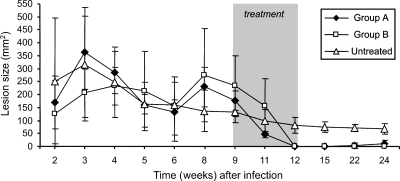
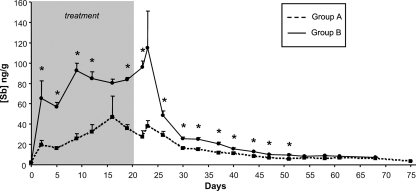
Although L. braziliensis-infected macaques showed a high degree of variability in lesion size (area range, 20 to 540 mm2) before antimonial therapy, the ulcerative cutaneous lesion persisted in untreated animals until the end of the observation period (Fig. 1). Treatment with both therapeutic schemes rapidly reduced the lesion size (in comparison with that of untreated lesions) after treatment. However, while complete healing was achieved in all animals receiving a regular MA schedule, three out of six monkeys treated with a low-dose regimen relapsed with the presentation of macroscopic wound inflammation after a clinical cure and wound reopening 3 to 4 months after the cessation of therapy. The concentration-time profiles of Sb in macaque plasma (Fig. 2) confirmed that drug exposure was much lower in that group (range, 11.3 to 149.3 ng Sb/g) than in macaques treated with a full-dose regimen (range, 36.4 to 150.5 ng Sb/g). Except for days 16, 23, 61, and 68, the differences in plasma Sb concentrations between the two groups were statistically significant (P < 0.001 to 0.05).
FIG. 1.
Response of L. braziliensis cutaneous infections in rhesus macaques to either low-dose (5 mg/kg/day; group A) or standard-dose (20 mg/kg/day; group B) MA treatment. To assess therapy, lesion size development was scored weekly following infection and treatment. All values represent the mean ± standard deviation. Before treatment, there was no statistically significant difference (P > 0.05) in mean lesion size over time between the macaque groups.
FIG. 2.
Time course of plasma Sb concentrations after intramuscular injection of either a low dose (group A) or a full dose (group B) of MA into L. braziliensis-infected macaques. For detection of total Sb, heparin-anticoagulated blood samples were analyzed by ICP-MS as described previously (11). *, Significant differences (P < 0.001 to 0.05) in plasma Sb concentrations between the two groups.
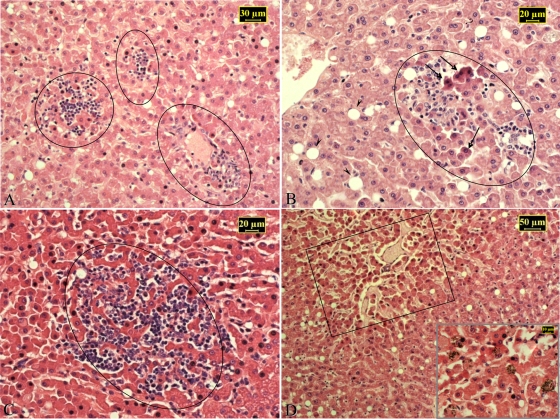
Sustained nadir blood levels of Sb gave rise to residual drug accumulation in different soft tissues. Approximately 2 and 3 months after the last dose of MA, Sb was detected in the livers, spleens, and kidneys of treated macaques, with concentrations in renal tissue significantly lower than those found in hepatic tissue (Table 1). No overt clinical signs of toxicity were observed in any macaque, regardless of the administration scheme. Nevertheless, histological evidence of MA-induced hepatic injury (Fig. 3) was observed in all treated macaques. There was a clear correlation (r = 0.94; P = 0.001) between tissue Sb levels and the extent to which hepatocytes were affected. In contrast, no histopathological alterations were found in any other tissue examined.
TABLE 1.
Residual concentrations of antimony found in selected necropsy tissue specimens from drug-cured L. braziliensis-infected macaquesa
| Posttreatment time,b group, and monkey or parameter | Residual Sb concn (ng/g) in: |
||
|---|---|---|---|
| Liver | Spleen | Kidneys | |
| Group A | |||
| 55 days | |||
| 140 | 2,490 | 1,800 | 340 |
| 142 | 2,890 | 1,050 | 330 |
| 06 | 3,360 | 1,450 | 830 |
| Mean ± SD | 2,913 ± 1,435.5 | 1,433 ± 375.3 | 500 ± 285.8 |
| 95 days | |||
| S62 | 831 | 370 | 169 |
| T32 | 430 | 360 | 125 |
| U48 | 747 | 700 | 162 |
| Mean ± SD | 669.3 ± 211.5 | 476.7 ± 193.5 | 152 ± 23.6 |
| Group B | |||
| 55 days | |||
| L30 | 4,180 | 2,630 | 580 |
| M2 | NDc | 1,100 | 1,000 |
| 034 | 5,420 | 1,050 | 515 |
| Mean ± SD | 4,800 | 1,593 ± 898.1 | 698.3 ± 263.3 |
| 95 days | |||
| U12 | 1,800 | 2,150 | 320 |
| U46 | 2,370 | 1,190 | 374 |
| X53 | 1,230 | 3,320 | 245 |
| Mean ± SD | 1,800 ± 570 | 2,220 ± 1,067 | 313 ± 64.8 |
Primates were treated with either a low dose (5 mg/kg/day; group A) or a full dose (20 mg/kg/day; group B) of MA administered intramuscularly.
Necropsies were performed at different time points (as described in Materials and Methods) after the completion of treatment. For this analysis, specimens from infected but untreated (n = 2) monkeys were also included as negative controls. Residual levels of Sb were compared among organs (matched per monkey) for each dose group by the Friedman test, followed by Dunn's multiple-comparison test: kidneys versus livers in groups A and B, P < 0.05.
ND, not done.
FIG. 3.
Histopathology of liver samples from representative drug-cured L. braziliensis-infected macaques (A, L30; B, 140; C, U46; D, O34). Micrographs show focal hepatocellular acidophilic necrosis (arrows) with surrounding inflammatory infiltrates (circled). These obliterate the sinusoids and protrude into the parenchyma and are associated with fatty changes in stellate cells (arrowheads). Hypotrophy of the hepatic parenchyma at the center of the lobules (boxed) and numerous hemosiderin deposits within liver cells and Kupffer cells (inset in panel D) are also illustrated. Hematoxylin-and-eosin staining was used.
Our main findings in macaques receiving a low SbV dosage regimen are consistent with those obtained in our previous study (14) but differ from the observations of Oliveira-Neto et al. (12), who recommended subtherapeutic MA concentrations for treating L. braziliensis-infected patients. This is likely linked to variabilities in parasite drug susceptibility (1, 4, 15). In fact, taxonomic studies have shown a high degree of genetic variation in natural populations of L. braziliensis from different geographic areas in Brazil (6). It should be noted that sublethal doses contribute to the selection of drug-resistant parasites (9), with the parasites that are inherently most drug resistant being favored (10). Moreover, drug-resistant clones of Leishmania spp. may exhibit cross-resistance (8).
The plasma PK profile of Sb in L. braziliensis-infected macaques treated with MA was similar to that reported for human cases receiving SbV drugs (3, 11, 13). Accordingly, most of the Sb absorbed from the injection site was eliminated rapidly, but long-lived nadir plasma Sb concentrations caused a gradual accumulation of the drug in tissues after repeated daily dosing. With the exception of the spleen in groups treated with the standard dose, tissue Sb concentrations in monkeys necropsied 95 days after the end of treatment were lower than the levels found in monkeys examined 40 days earlier (Table 1). It remains to be determined if the observed variability in tissue Sb levels in macaques in the same dose group results from the outbred genetics of these primates. The species of Sb in the organism and the mechanisms by which it is transported, distributed to tissues, and eliminated from the body remain unclear (4). We also provided evidence for an association between tissue Sb levels and the extent of hepatocyte damage. The drug-induced hepatic injury could be related to the conversion of SbV to SbIII, which has been demonstrated to be considerably more toxic than SbV in different test systems (3).
Acknowledgments
This work was supported by the Fiocruz and the Millennium Institute for Vaccine Development and Technology (CNPq-420067/20005-1), Brazil.
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Azeredo-Coutinho, R. B. G., S. C. F. Mendonça, H. Callahan, A. C. Portal, and M. Grögl. 2007. Sensitivity of Leishmania braziliensis promastigotes to meglumine antimoniate (Glucantime) is higher than that of other Leishmania species and correlates with response to therapy in American tegumentary leishmaniasis. J. Parasitol. 93:688-693. [DOI] [PubMed] [Google Scholar]
- 2.Berman, J. D. 1988. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 10:560-586. [DOI] [PubMed] [Google Scholar]
- 3.Chulay, J. D., L. Fleckenstein, and D. H. Smith. 1988. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans. R. Soc. Trop. Med. Hyg. 82:69-72. [PubMed] [Google Scholar]
- 4.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 5.Cruz, A., P. M. Rainey, B. L. Herwaldt, G. Stagni, R. Palacios, R. Trujillo, and N. G. Saravia. 2007. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J. Infect. Dis. 195:602-608. [DOI] [PubMed] [Google Scholar]
- 6.Cupolillo, E., L. R. Brahim, C. B. Toaldo, M. P. Oliveira-Neto, M. E. F. de Brito, A. Falqueto, M. F. Naiff, and G. Grimaldi, Jr. 2003. Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brazil. J. Clin. Microbiol. 41:3126-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimaldi, G., Jr. 2008. The utility of rhesus monkey (Macaca mulatta) and other non-human primate models for preclinical testing of Leishmania candidate vaccines—a review. Mem. Inst. Oswaldo Cruz 103:629-644. [DOI] [PubMed] [Google Scholar]
- 8.Grogl, M., R. K. Martin, A. M. J. Odula, L. W. K. Milhous, and D. E. Kyle. 1991. Characteristics of multidrug resistance in Plasmodium and Leishmania: detection of P-glycoprotein-like components. Am. J. Trop. Med. Hyg. 45:98-111. [DOI] [PubMed] [Google Scholar]
- 9.Grögl, M., A. M. J. Odula, L. D. C. Cordero, and D. E. Kyle. 1989. Leishmania spp.: development of pentostam-resistant clones in vitro by discontinuous drug exposure. Exp. Parasitol. 69:78-90. [DOI] [PubMed] [Google Scholar]
- 10.Grogl, M. A., T. N. Thomason, and E. D. Franke. 1992. Drug resistance in leishmaniasis: implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am. J. Trop. Med. Hyg. 47:117-126. [DOI] [PubMed] [Google Scholar]
- 11.Miekeley, N., S. R. Mortari, and A. O. Schubach. 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal. Bioanal. Chem. 372:495-502. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira-Neto, M. P., A. Schubach, M. Mattos, S. C. Gonçalves-Costa, and C. Pirmez. 1997. A low-dose antimony treatment in 159 patients with American cutaneous leishmaniasis: extensive follow-up studies (up to 10 years). Am. J. Trop. Med. Hyg. 57:651-655. [DOI] [PubMed] [Google Scholar]
- 13.Rees, P. H., M. I. Keating, P. A. Kager, and W. T. Hockmeyer. 1980. Renal clearance of pentavalent antimony (sodium stibogluconate). Lancet 2:226-229. [DOI] [PubMed] [Google Scholar]
- 14.Teva, A., R. Porrozzi, E. Cupolillo, M. P. Oliveira-Neto, and G. Grimaldi, Jr. 2005. Responses of Leishmania (Viannia) braziliensis cutaneous infection to N-methylglucamine antimoniate in the rhesus monkey (Macaca mulatta) model. J. Parasitol. 91:976-978. [DOI] [PubMed] [Google Scholar]
- 15.Yardley, V., N. Ortuno, A. Llanos-Cuentas, F. Chappuis, S. D. Doncker, L. Ramirez, S. Croft. J. Arevalo, V. Adaui, H. Bermudez, S. Decuypere, and J. C. Dujardin. 2006. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J. Infect. Dis. 194:1168-1175. [DOI] [PubMed] [Google Scholar]