Abstract
The clinical application of conventional peptide drugs often is limited by their short in vivo half-life and potential immunogenicity. Frequent injection presents challenges to the treatment of chronic diseases, such as HIV infection. We chemically modified a peptide HIV fusion inhibitor with 3-maleimidopropionic acid (MPA), which allows rapid and irreversible conjugation with serum albumin at a 1:1 molar ratio. FB006M, with an MPA modification at the 13th amino acid, rapidly formed conjugate with albumin upon intravenous injection, and it exhibited a remarkably extended in vivo half-life. The albumin conjugate of FB006M displayed potent inhibitory activity against a number of laboratory and clinical isolates of HIV-1 in vitro and in vivo. No immunogenicity or antibody formation was detected after repeated dosing. The clinical application of FB006M may decrease the cost of treatment and improve treatment compliance and patient quality of life.
The current treatment of HIV infection and AIDS faces the challenge of the widespread emergence of drug-resistant HIV-1 variants in both newly infected and drug-experienced patients (17). This emphasizes the need for therapeutics with new mechanisms of action. Enfuvirtide, the first and only FDA-approved HIV-1 fusion inhibitor, showed potent activity against both wild-type and drug-resistant HIV-1 (11, 12). Being a peptide of 36 amino acid residues, however, it suffers from a short in vivo half-life of 3.46 to 4.35 h and requires twice-daily injection (16). To reduce the cost of treatment and improve patient quality of life, we were interested in developing a peptide-based and long-acting HIV fusion inhibitor by covalently linking an anti-HIV fusion peptide to serum albumin at a 1:1 molar ratio.
Albumin is the most abundant protein in plasma. It is well distributed in different tissues and exhibits a half-life of 15 to 19 days in humans. Because of these properties, albumin has been used as a drug carrier (1, 6, 7). This approach has been applied to small-molecule drugs (9), peptides (10), and protein therapeutics (2). These albumin-drug conjugates demonstrated prolonged in vivo half-life, excellent safety profiles, and therapeutic efficacy. To apply this approach to peptide-based HIV-1 fusion inhibitors, we recognized three important questions that had to be addressed. First, albumin is one magnitude greater in molecular weight than the anti-fusion peptides. The linkage of albumin to peptide may prevent the peptide from accessing its target by steric hindrance (8). Therefore, the linkage site in both albumin and peptide has to be selected carefully so that the final peptide-albumin conjugate retains its biological activities. Second, the molar ratio of peptide to albumin in a conjugate can affect both activity and half-life (20). A chemical modification of peptide that allows a 1:1 molar ratio was used to assure the minimum structural alteration of albumin after peptide linkage. Third, an albumin conjugate in circulation with a long half-life may be immunogenic. It could, therefore, compromise the drug activity or cause adverse reactions. A careful investigation of immune responses would be required prior to human application.
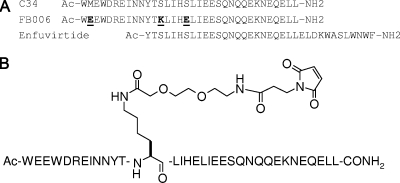
Based on these considerations, we set out the program with the peptide HIV-1 fusion inhibitor C34, a 34-amino-acid peptide from the C-terminal heptad repeat region of HIV-1 gp41 envelope protein (5). At its C terminus, C34 shares an identical 24-amino-acid sequence with the N terminus of enfuvirtide. Nevertheless, these two peptides are believed to utilize nonoverlapping molecular targets in the HIV-1 membrane glycoprotein gp41 (13). Assisted by the crystal structure of C34 in complex with HIV-1 gp41 N peptides, three residues of C34 not involved in target binding were replaced by lysine and glutamic acid to improve the solubility and antiviral activity (Fig. 1A). This peptide, FB006, is to be chemically modified further and conjugated to albumin.
FIG. 1.
(A) Aligned sequences of C34, enfuvirtide, and FB006. The boldface and underlined letters in the FB006 sequence are the residues that differ from those in C34. The amino termini of all peptides were acetylated, and the carboxyl termini were amidated. (B) Structure of FB006M.
Albumins of rodents, rabbits, dogs, monkeys, and humans all possess a conserved cysteine residue (Cys34 in humans) that has the only free thiol group in the protein. To enable binding to this thiol group, FB006 was modified by 3-maleimidopropionic acid (MPA), which allows an irreversible reaction between the maleimide and the free thiol to form a specific 1:1 peptide-albumin conjugate. Guided by the crystal structure of the HIV-1 gp41 ectodomain, we chemically modified FB006 with a single MPA at different residual positions. These peptides were conjugated to human serum albumin (HSA) in vitro and subjected to a human peripheral blood mononuclear cell (PBMC) assay to determine their anti-HIV activities (22). Based on the results, a lead molecule named FB006M (Fig. 1B) was selected for further studies.
MATERIALS AND METHODS
Peptides and proteins.
The peptides shown in Fig. 1 were prepared by standard solid-phase synthesis using Rinkam resin and 9-fluorenylmethoxy carbonyl-protected amino acids. The side chain of the 13th lysine residue was protected by allyloxycarbonyl (Aloc), which allows the specific deprotection and addition of the linker molecule and MPA.
The purities were greater than 90% as determined by reverse-phase high-performance liquid chromatography analysis. The molecular weight was verified by liquid chromatography-mass spectrometry (LC-MS) measurements.
Human serum albumin, 99% pure, was purchased from Sigma Chemical Co., St Louis, MO.
Cells and viruses.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (gag/env subtype, coreceptor tropism, and syncytium-inducing phenotype information are in parentheses as listed in the Reagent Program catalog): HIV-1IIIB and H9 cells, HIV-1Ba-L, HIV-192RW016 (subtype A/A, R5); HIV-192BR025 (subtype C/C, R5, NSI); HIV-1CMU02 (subtype/EA, X4, SI); HIV-1302056 (subtype/B, R5, NSI); HIV-1JV1083 (subtype/G, R5); HeLa CD4 long terminal repeat β-galactosidase cells, GHOST (3) X4/R5 cells, and HL2/3 cells. VK2/E6E7 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The low-passage, lymphotropic clinical isolate HIV-1SLKA was obtained from a pediatric patient attending the AIDS Clinic at the University of Alabama at Birmingham and was isolated in the laboratories of the Southern Research Institute as previously described (3). HIV-1SK1 was obtained from Miles Cloyd (Duke University Medical Center) (4). The H9-SK1 cell line was developed by the long-term culture of H9 cells acutely infected with HIV-1SK1 to allow for the outgrowth of a chronically infected population of cells. All cell lines were maintained in culture as recommended in the data sheets supplied by either the NIH AIDS Reference Reagent Program or the ATCC. Low-passage stocks of each virus were prepared using fresh human PBMCs and stored in liquid nitrogen. Stocks of HIV-1IIIB for use in virus attachment assays were prepared by standard methods using either CEM-SS or H9 cells. Pretitered aliquots of all viruses were removed from the freezer (−80°C) and thawed rapidly to room temperature immediately before use.
In vivo pharmacology and toxicology studies.
The toxicology studies were conducted at WestChina-Frontier Pharmatech Co. (WCFP; Chengdu, China), certified by the Chinese State Food and Drug Administration for GLP compliance, and accredited by AAALAC International (Bethesda, MD). All study procedures complied with the Guide for the Care and Use of Laboratory Animals (NRC) and the standard operating procedures at WCFP. The study protocols were in accordance with the Animal Welfare Act and were approved by the WCFP IACUC.
In vivo serum protein binding of FB006M.
The in vivo protein binding study was performed with two male Sprague-Dawley rats, with body weights of 270 to 300 g. FB006M was intravenously injected at 63.0 mg/kg of body weight. Serial blood samples were collected from each animal at predose (before injection) and at 1, 2, 6, and 10 h after the injection. The samples then were centrifuged at 4°C and 3,000 rpm for 6 min to obtain plasma samples, which were subjected to LC-MS analysis.
An LC-MS method was developed for the analysis of FB006M-albumin in rat plasma. An Agilent 1100 LC/MSD (SL) detector and an Agilent ZORBAX SB300-C3 chromatographic column (5 μm; 4.6 by 150 mm) were used. Briefly, the chromatographic conditions of sample analysis were as follows: the mobile phase A was 0.1% trifluoroacetic acid (TFA)-water, and 0.1% TFA-acetonitrile was used as mobile phase B for gradient elution at a flow rate of 1 ml/min. The mass spectrum was determined under the following conditions: drying gas flow rate, 12.0 liters/min; gas temperature, 350°C; fragmentor voltage, 150 V; nebulizer pressure, 50 lbs/in2; capillary voltage, 4,500 V; positive ion mode; scanning range, 1,000 to 3,000 m/z; ion source, API-ES. The limit of detection of the LC-MS method was 0.6 μM.
Pharmacokinetic studies in rats and monkeys.
Ten male Wistar rats (n = 5 per test compound) were administered a single dose of either FB006M or FB006 (4.8 mg/kg in phosphate buffer) intravenously via the tail vein. Serial blood samples were collected from each animal before injection and at 0.08, 0.5, 1, 2, 4, 8, 24, 48, 72, 96, and 120 h postinjection in tubes containing EDTA. The samples then were centrifuged (2,500 rpm for 10 min at 4°C), and the plasma was aliquoted and kept frozen until analysis.
Two male rhesus monkeys (n = 1 per test compound) were administered a single dose of either FB006M or FB006 (5.0 mg/kg in phosphate buffer) intravenously via a lower-limb vein. Serial blood samples were collected from monkeys that received FB006 at predose (before injection) and at 0.08, 0.5, 1, 4, 6, 8, 12, and 24 h for FB006, and from monkeys that received FB006M at predose and at 0.08, 1, 3, 6, 12, 24, 48, 72, 96, 144, 192, and 288 h in tubes containing EDTA. The samples then were centrifuged (2,500 rpm for 10 min at 4°C), and the plasma was aliquoted and kept frozen until analysis.
Chronic toxicity study and immunogenicity analysis in rats and monkeys.
One hundred four Wistar rats of specific-pathogen-free grade, half of which were male and half female and with body weights of 130 to 150 g, were supplied by Shanghai Laboratory Animal Co. (Shanghai, China). Three doses of FB006M (4.2, 8.3, and 27.8 mg/kg) were administered intravenously daily for 30 days.
Twenty-four healthy rhesus monkeys (Macacca mulatta), half of which were female and half male and with body weights of 3.5 to 6 kg, were supplied by Pingan Animal Breeding and Research Base (Chengdu, China). Intravenous bolus injections of FB006M at 2.8, 8.3, and 25 mg/kg were administered every 5 days for 3 months.
Cageside and clinical observations were made daily, and the clinical pathology testing was conducted before and after the recovery period. Electrocardiography was performed on rhesus monkeys. Systematic necropsies and histopathological assessments of tissues and organs were performed in end-of-life studies by a qualified toxicology pathologist.
Antibody against FB006M was tested with an indirect enzyme-linked immunosorbent assay (ELISA) on days 30, 60, and 90 for rhesus monkeys and on days 15 and 30 for rats after the first dosing, as well as on day 30 for rhesus monkeys and on day 15 for rats after the last dosing. FB006M was immobilized onto the wells of a microplate to which serum samples were added and incubated. A second goat antibody against rat or monkey antibody labeled with horseradish peroxidase (HRP) was added and incubated. Following color development by 3,3′,5,5′-tetramethylbenzide (TMB) solution, the optical densities (ODs) of the wells were read on a plate reader to determine if there was any antibody formation. The indirect ELISA was characterized by a cutoff rOD (ratio of the OD of sample to the OD of negative serum) of 2.1 for positive samples. If the rOD of a sample was less than 2.1, the sample was considered to contain no antibody against FB006M.
Other immunological indices, including C3 and C4 complements (immunoturbidimetry), circulating immune complexes (immunoprecipitation), immunoglobulins (IgA, IgE, IgG, and IgM; immunoturbidimetry), and CD3, CD8, and CD20 immunocytes (flow cytometry), also were determined at the time points described above. Histopathology examination also was performed on the immune organs (thymus, spleen, and lymphoid nodes) by a qualified veterinary pathologist at WCFP.
ELISA method for pharmacokinetic analysis of plasma samples.
Rabbit polyclonal antibodies of FB006 and HRP-labeled FB006M (FB006M-HRP) were utilized in this competitive ELISA. A 96-well polystyrene microplate was coated by incubation overnight at 4°C with anti-FB006 polyclonal antibody in carbonate buffer, pH 9.6. After being coated, the plate was washed three times with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T) to remove excess antibody. The remaining active sites on the plate were blocked by the addition of 200 μl of blocking buffer, followed by three washes with PBS-T. The serially diluted FB006M or FB006 standard solution, negative control, and diluted unknown samples were added to appropriate wells; each sample, including controls, was tested in duplicate; and antigen-enzyme conjugate FB006M-HRP then was added to wells and incubated at 37°C for 1 h. After the plate was washed as described above, a solution of TMB reagent was added and incubated for 30 min, resulting in the development of a blue color. The color development was stopped by the addition of stop solution (2 mol/liter H2SO4). The plate was read at 450 nm using a microplate reader (Bio-Rad Benchmark Plus). Levels of FB006M and FB006 in the unknown samples were calculated by interpolation from the FB006M and FB006 standard curves, respectively. The ELISA was characterized by a limit of detection (LOD) of 1.5 nM, a linear range of 2 to 64 nM, and a limit of quantitation (LOQ) of 3.1 nM. The inter- and intraday variability were 3.7 and 4.5%, respectively.
PBMC assay to test anti-HIV activity of FB006M.
The PBMC assays were performed at the Southern Research Institute under NIH contract NO1-AI-05415 (22). HIV replication in PBMC cultures was determined by the measurement of supernatant reverse transcriptase activity, and in MDM cultures it was determined by ELISA for p24 antigen (Coulter HIV-1 p24 antigen assay; Beckman Coulter) expression 6 days postinfection. Cell viability by formazan dye reduction was determined using CellTiter reagent (Promega, Madison, WI). All determinations were performed in triplicate. The HIV reverse transcriptase inhibitor 3′-azido-3′-deoxythymidine (AZT) was used as a positive control for all assays.
Drug preparation.
FB006M first was solubilized in dimethylsulfoxide (DMSO) at a concentration of 10 mM. A 1 mM FB006M-albumin conjugate then was prepared by making a 10-fold dilution of the 10 mM DMSO solution of FB006M into a 25% human serum albumin solution in PBS buffer. The mixture was incubated at 37°C for 4 h to allow the conjugation to complete and subsequently was stored at 4°C until the day of the assay. On the day of assay setup, this 1 mM stock was used to generate the working drug dilutions used in the assays. Working dilutions are made fresh for each experiment and are not stored for reuse in subsequent experiments performed on different days. The completion of albumin conjugation was verified by LC-MS analysis to assure that no unreacted peptide was detected.
In vivo efficacy study in SCID/hu mouse model.
The efficacy study was performed at the Gladstone Institute of Virology and Immunology, University of California at San Francisco. Details of the model were published previously (14). Male C.B-17 SCID (model no. CB17SC-M, homozygous, C.B-Igh-1b/IcrTac-Prkdcscid) mice were obtained at 5 weeks of age from Taconic (Germantown, NY) and were implanted with human fetal tissue. Mice were treated prophylactically with trimethoprim-sulfamethoxazole pellets (SCID's MD's; Bio-Serv, Frenchtown, NJ) in the food bin to prevent opportunistic infection with Pneumocystis carinii. For surgical procedures, 9-week-old mice were anesthetized with 100 mg/kg ketamine plus 8 mg/kg xylazine by intraperitoneal injection. The implantation of fragments of human fetal liver and human fetal thymus under the mouse kidney capsule was performed to create SCID/hu Thy/Liv mice, and a cohort of 55 mice was produced with organs from the same donor. Fifteen randomly chosen mice were anesthetized and examined 13 weeks after implantation to evaluate the growth of the Thy/Liv implants.
Inoculations were performed on anesthetized mice in a restricted animal barrier facility under biosafety level 3 guidelines. Using the ink mark as a guide, each Thy/Liv implant was injected with 50 μl (∼1,000 50% tissue culture infectious doses [TCID50]) of NL4-3 batch JK WS1 D3 (diluted 1:2) or with RPMI 1640 medium in one to three places with a 250-μl Hamilton glass syringe and a 30-gauge, 0.5-inch blunt needle. For this study, implants were inoculated 21 weeks after tissue implantation. All implants were collected 21 days after inoculation.
Mice were euthanized by CO2 inhalation followed by cervical dislocation, and the Thy/Liv implants were surgically excised and transferred into 6-well tissue culture plates containing cold sterile PBS-2% fetal bovine serum (FBS). A single-cell suspension was made by placing the implant into a sterile nylon mesh bag, submerging the bag in PBS-2% FBS in a 60-mm tissue culture dish, and dispersing the tissue between the nylon layers with forceps. The cells were counted with a Coulter counter, and appropriate numbers of cells were aliquoted for each assay. For p24 ELISA, pellets of 2.5 × 106 cells were resuspended in 400 μl of p24 lysis buffer, rotated overnight at 4°C, and stored at −20°C. For RNA quantitation by branched DNA (bDNA) assay, dry pellets of 5 × 106 cells were frozen and stored at −80°C. For fluorescence-activated cell sorter analysis, 106 cells per well were placed in a 96-well plate for fixation and staining and were analyzed on the same day.
Cells were disrupted with sterile disposable pestles and a cordless motor grinder (Kontes, Vineland, NJ) in 8 M guanidine HCl with 0.5% sodium N-lauroylsarcosine. The RNA was extracted by adding 0.5 ml 100% ethanol, and each sample was vortexed and pelleted at 12,000 × g for 20 min at 4°C. Supernatants were aspirated to remove DNA, and RNA pellets were washed with 0.5 ml 70% ethanol, placed on dry ice, and digested with reagents supplied by the manufacturer (Versant HIV-1 RNA 3.0 assay; Bayer Diagnostics, Norwood, MA). The implant HIV-1 RNA load is expressed as copies per 106 implant thymocytes, and the log10 values were used for the calculation of geometric means. The limit of detection was 101.48 RNA copies per 106 cells, and this lower-limit value was used for the mean calculation of implants with undetectable viral RNA.
RESULTS
In vivo protein binding.
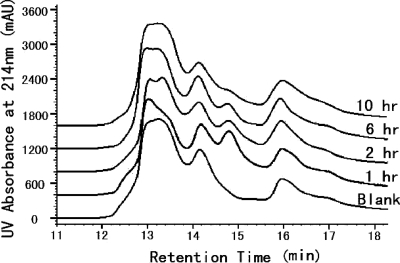
The in vivo protein binding of FB006M was tested by the intravenous injection of the peptide in rats. Plasma samples taken at different time points were subjected to LC-MS analysis (Fig. 2). Upon injection, unbound FB006M was not observed in plasma at any time point. Compared to that of the control sample, a single newly formed product was seen at 14.8 min, with a molecular mass of 70.53 kDa. This corresponds to the sum of rat serum albumin (65.9 kDa) and FB006M (4.67 kDa). Within the detection limit of this method, there were no other reaction products observed.
FIG. 2.
In vivo albumin binding in rats. FB006M was injected intravenously in two male Sprague-Dawley rats at 63.0 mg/kg. Serial blood samples were collected from each animal at predose (before injection) and at 1, 2, 6, and 10 h after injection. The plasma samples were analyzed by LC-MS. Data from the two animals are very similar. Data for animal 1 are shown.
Pharmacokinetic studies in rat and monkeys.
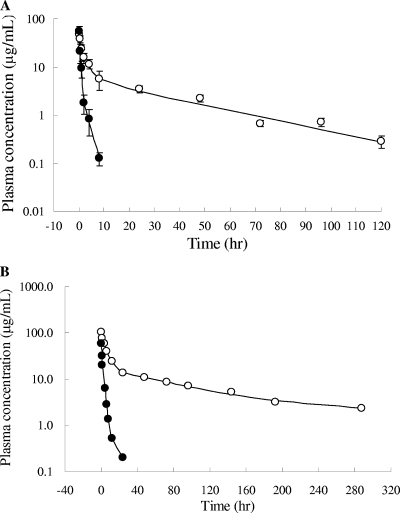
The plasma samples of albumin-FB006M contained a high protein background, resulting in a low sensitivity of detection in the LC-MS analysis. To determine the plasma concentration of FB006 (the unmodified peptide) and albumin-FB006M conjugate with high sensitivity, we developed and validated an ELISA using rabbit polyclonal antibodies against FB006. Pharmacokinetic studies were performed in rats and rhesus monkeys. Single doses of FB006M and FB006 were administered intravenously. In rats, FB006 exhibited a terminal half-life (t1/2β) of 1.67 h, a systematic clearance (CL) of 0.11 liters/kg/h, and an apparent volume of distribution of 0.28 liters/kg. In contrast, FB006M-albumin showed a significantly prolonged half-life (Fig. 3A). The terminal half-life, systematic clearance, and apparent volume of distribution were 25.8 h, 0.015 liters/kg/h, and 0.64 liters/kg, respectively. The difference between FB006 and FB006M pharmacokinetic profiles was demonstrated further in monkeys (Fig. 3B). In rhesus monkeys receiving 5.0 mg/kg FB006M, the mean terminal half-life was 102.4 h, which was 9.4-fold longer than that of FB006. The mean area under the concentration-time curve from 0 h to infinity (AUC0-∞) of FB006M was 25-fold higher than that of FB006 (2,429.1 and 97.1 mg/liter·h, respectively).
FIG. 3.
Plasma concentration of FB006M and FB006 following single intravenous administration. (A) The compounds were administered at 4.8 mg/kg. Each time point is represented by the mean values and standard deviations for five male Sprague-Dawley rats. (B) FB006 and FB006M were administered individually at 5.0 mg/kg in one male rhesus monkey. The concentrations were determined by a validated ELISA using rabbit polyclonal antibodies against FB006. Solid circle, FB006; open circle, FB006M.
Chronic toxicity and immunogenicity assessment.
To examine the potential immunogenicity and toxicity of the molecule and using the same intended route of administration in humans, we injected FB006M intravenously in rats, which is the same route of administration as that intended for humans, at 4.2, 8.3, and 27.8 mg/kg once daily for 30 days and in rhesus monkeys at 2.8, 8.3, and 25.0 mg/kg once every 5 days for 90 days. The dosing frequency was set to the length of one half-life of the molecule. There were no deaths or other observable drug-related adverse effect in any dose group. Blood samples were taken to examine antibody formation using an indirect ELISA during the dosing period and 15 days for rat and 30 days for monkey after the last dosing. No antibody against FB006M was detected. No significant changes in other immunological indices, such as C3 and C4 complements, circulating immune complexes, immunoglobulins (IgA, IgE, IgG, and IgM), and immunocytes (CD3, CD8, and CD20), were detected during the dosing period and 30 days after the last dosing in the monkey chronic toxicity study. The histopathological evaluation of the immune organs (thymus, spleen, and lymphoid nodes) did not reveal any abnormal changes compared to results with a saline control.
Anti-HIV activity in vitro and in vivo.
After demonstrating the in vivo albumin conjugation of FB006M and its long half-life, we further examined the anti-HIV activity of albumin-FB006M conjugate. We first examined the in vitro anti-HIV activities of FB006 and FB006M-HSA conjugate in a PBMC assay (Table 1). The assay used subtype B, A, C, G, and EA HIV-1 viruses, utilizing either CXCR4 or CCR5 coreceptor. The activity against HIV-1MDR 769, a clinical isolate resistant to most of the HIV reverse transcriptase and protease inhibitors (15), also was determined. Both FB006 and FB006M-HSA conjugate exhibited potent activity, with average 50% effective concentrations (EC50s) of 1.4 and 2.7 nM, respectively. Interestingly, the FB006M-HSA conjugate, in which FB006 was linked to albumin at the 13th lysine residue by a linker molecule, showed anti-HIV activities similar to those of free peptide FB006.
TABLE 1.
Anti-HIV activity of FB006M-HSA conjugate against a panel of HIV-1 isolates in fresh human PBMCsa
| HIV-1 isolate | FB006 |
FB006M-HSA |
AZT |
|||
|---|---|---|---|---|---|---|
| EC50 (nM) | SI | EC50 (nM) | SI | EC50 (nM) | SI | |
| IIIB (subtype B, SI) | 1.4 | 11,277b | 3.9 | >6,345 | 1.83 | >13,661 |
| SLKA (presumed B, NSI) | 3.8 | >262 | 4.8 | >208 | 0.20 | >5000 |
| RW/92/016 (subtype A/A, R5) | 1.2 | >826 | 0.9 | >1149 | 0.47 | >2128 |
| BR/92/025 (subtype C/C, R5, NSI) | 0.2 | >4000 | 0.5 | >1961 | 0.95 | >1053 |
| JV1083 (subtype G, R5) | 0.8 | >1190 | 4.2 | >240 | 0.67 | >1493 |
| 302056 (subtype B, R5, NSI) | 1.5 | >676 | 0.8 | >1235 | 0.45 | >2222 |
| CMU02 (subtype EA, X4, SI) | 0.5 | >1887 | 1.6 | >621 | 2.20 | >455 |
| MDR 769 (multidrug resistant) | 1.5 | >654 | 5.0 | >202 | >1000 | NA |
| Avg | 1.4 | NA | 2.7 | NA | NA | NA |
The selectivity index, SI, was calculated from the ratio of EC50 to TC50, the half cytotoxic concentration. The greater-than sign indicates that no cytotoxicity was observed at the highest test concentration. All determinations were performed in triplicate, and EC50s were determined from the average reverse transcriptase activities of the triplicate using an in-house computer program. NA, not applicable.
The TC50 of FB006 against HIV-1IIIB was determined to be 15.9 μM.
The in vivo efficacy of FB006M was studied in a SCID/hu Thy/Liv mouse model of HIV-1 infection (14). Homozygous immunodeficient C.B-17-SCID mice were implanted under the left kidney capsule with human fetal thymus and liver tissues from a single donor 20 weeks before inoculation with HIV-1. FB006M was administered by subcutaneous injection at 10 mg/kg per day, beginning 1 day before the direct inoculation of each implant with 1,000 TCID50 of HIV-1NL4-3. FB006 and lamivudine (3TC) were used as controls. The dose selected for the study was based on the observed effective dose of approved AIDS drugs in this model (19, 21). Because mouse albumin has a half-life of approximately 12 h and the albumin-FB006M conjugate was expected to show a half-life similar to that of albumin, the dosing frequency was set to once daily and once every 2 days. When given by once-daily injection, both FB006 and FB006M showed potent antiviral activity (Table 2). No p24 antigen was detected in the implants of seven mice treated with 10 mg/kg/day FB006M, and viral RNA was reduced by 3.0 log10 compared to results for untreated mice. When the treatment was once every other day, FB006M reduced p24 by 95% and HIV-1 RNA by 1.5 log10, while FB006 showed no statistically significant activity.
TABLE 2.
Antiviral activity in the SCID/hu Thy/Liv mouse modela
| Group | No. of mice/group | Virus | Drug | Dose (mg/kg/day) | Dosing frequencyd | p24 |
HIV-1 RNA (log10 copies/106 cells) | |
|---|---|---|---|---|---|---|---|---|
| Amt in pg/106 cells | % of control | |||||||
| A | 7 | NL4-3 | FB006 | 10 | q.d. | 1.6 ± 1.6* | 0.5 ± 0.5 | 2.4 ± 0.39* |
| B | 6 | NL4-3 | FB006 | 10 | q.o.d. | 290 ± 83 | 89 ± 25 | 4.9 ± 0.64 |
| Cb | 7 | NL4-3 | FB006M | 10 | q.d. | Negative* | 0.0 ± 0.0 | 1.9 ± 0.31* |
| D | 6 | NL4-3 | FB006M | 10 | q.o.d. | 18 ± 16* | 5.5 ± 4.9 | 3.4 ± 0.47* |
| E | 7 | NL4-3 | 3TC | 100 | q.d. | Negative* | 0.0 ± 0.0 | 1.6 ± 0.09* |
| F | 7 | NL4-3 | 3TC | 100 | q.o.d. | 3.9 ± 3.9* | 1.2 ± 1.2 | 2.8 ± 0.45* |
| G | 7 | NL4-3 | None | Untreated | Untreated | 330 ± 130 | 100 ± 39 | 4.9 ± 0.27 |
| Hc | 4 | None | None | Untreated | Untreated | Negative | 0.0 ± 0.0 | Negative |
*, P ≤ 0.05 compared to untreated infected mice (group G) by Mann-Whitney U test.
Mouse 19 died on 14 April 2004 (thymic lymphoma). Mouse 21 was excluded from all analyses (thymic lymphoma and abnormal cell profile).
Mouse 51 was excluded from all implant analyses (abnormal cell profile).
q.d., once daily; q.o.d., once every 2 days.
DISCUSSION
Our results demonstrated that upon injection, an MPA-modified peptide rapidly reacted with albumin and formed a stable 1:1 molar ratio conjugate. When the modification, and the linkage site, were placed at the 13th lysine residue, the albumin-peptide conjugate exhibited longer half-life and similar anti-HIV activity compared to that of the unmodified peptide. The half-life of albumin is related to body weight in different species, being approximately 24 h in rats, 5 days in monkeys, and 19 days in humans (18). These values coincide with those determined for FB006M in rats and monkeys. Thus, we postulate that the half-life of FB006M in humans is 19 days. Interestingly, we did not observe immune response and antibody formation for FB006M after repeated challenges in rats and monkeys. In the current study, FB006M was injected intravenously and became conjugated to endogenous serum albumin in vivo. At a 1:1 molar ratio, the peptide was linked specifically to Cys34 of albumin, which is located at a hydrophobic crevice. This is in contrast to the traditional conjugation of small antigens to surface lysine residues of serum albumin and may explain the lack of the immunogenicity of FB006M.
There is great interest in developing a long-lasting, safe, and effective HIV-1 fusion inhibitor. Such a drug candidate could significantly reduce the cost of treatment and improve treatment compliance and patient quality of life. FB006M showed promising properties to address significantly some unmet medical needs in HIV/AIDS treatment.
Acknowledgments
This work was funded partially by NIH/NIAID contract NO1-AI-05415, by the China Ministry of Science and Technology 863 program grants (2003AA219033 and 2003AA001043), and by a Challenge grant of the Chongqing Science and Technology Commission.
The views of the authors do not necessarily reflect those of the funding agencies.
We thank D. Bridon, J. Erickson, and G. Krafft for helpful discussions and C. Stoddart for performing the SCID/hu mouse study.
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Abu Ajaj, K., R. Graeser, I. Fichtner, and F. Kratz. 2009. In vitro and in vivo study of an albumin-binding prodrug of doxorubicin that is cleaved by cathepsin B. Cancer Chemother. Pharmacol. 64:413-418. [DOI] [PubMed] [Google Scholar]
- 2.Balan, V., D. R. Nelson, M. S. Sulkowski, G. T. Everson, L. R. Lambiase, R. H. Wiesner, R. C. Dickson, A. B. Post, R. R. Redfield, G. L. Davis, A. U. Neumann, B. L. Osborn, W. W. Freimuth, and G. M. Subramanian. 2006. A phase I/II study evaluating escalating doses of recombinant human albumin-interferon-alpha fusion protein in chronic hepatitis C patients who have failed previous interferon-alpha-based therapy. Antivir. Ther. 11:35-45. [PubMed] [Google Scholar]
- 3.Buckheit, R. W., Jr., J. L. Roberson, C. Lackman-Smith, J. R. Wyatt, T. A. Vickers, and D. J. Ecker. 1994. Potent and specific inhibition of HIV envelope-mediated cell fusion and virus binding by G quartet-forming oligonucleotide (ISIS 5320). AIDS Res. Hum. Retroviruses 10:1497-1506. [DOI] [PubMed] [Google Scholar]
- 4.Buckheit, R. W., Jr., and R. Swanstrom. 1991. Characterization of an HIV-1 isolate displaying an apparent absence of virion-associated reverse transcriptase activity. AIDS Res. Hum. Retroviruses 7:295-302. [DOI] [PubMed] [Google Scholar]
- 5.Chan, D. C., C. T. Chutkowski, and P. S. Kim. 1998. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc. Natl. Acad. Sci. U. S. A. 95:15613-15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz, L. J., E. Iglesias, J. C. Aguilar, D. Quintana, H. E. Garay, C. Duarte, and O. Reyes. 2001. Study of different coupling agents in the conjugation of a V3-based synthetic MAP to carrier proteins. J. Pept. Sci. 7:511-518. [DOI] [PubMed] [Google Scholar]
- 7.Esmaeili, F., R. Dinarvand, M. H. Ghahremani, M. Amini, H. Rouhani, N. Sepehri, S. N. Ostad, and F. Atyabi. 2009. Docetaxel-albumin conjugates: preparation, in vitro evaluation and biodistribution studies. J. Pharm. Sci. 98:2718-2730. [DOI] [PubMed] [Google Scholar]
- 8.Hamburger, A. E., S. Kim, B. D. Welch, and M. S. Kay. 2005. Steric accessibility of the HIV-1 gp41 N-trimer region. J. Biol. Chem. 280:12567-12572. [DOI] [PubMed] [Google Scholar]
- 9.Hartung, G., G. Stehle, H. Sinn, A. Wunder, H. H. Schrenk, S. Heeger, M. Kranzle, L. Edler, E. Frei, H. H. Fiebig, D. L. Heene, W. Maier-Borst, and W. Queisser. 1999. Phase I trial of methotrexate-albumin in a weekly intravenous bolus regimen in cancer patients. Phase I study group of the Association for Medical Oncology of the German Cancer Society. Clin. Cancer Res. 5:753-759. [PubMed] [Google Scholar]
- 10.Jetté, L., R. Leger, K. Thibaudeau, C. Benquet, M. Robitaille, I. Pellerin, V. Paradis, P. van Wyk, K. Pham, and D. P. Bridon. 2005. Human growth hormone-releasing factor (hGRF)1-29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: identification of CJC-1295 as a long-lasting GRF analog. Endocrinology 146:3052-3058. [DOI] [PubMed] [Google Scholar]
- 11.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 12.Lalezari, J. P., K. Henry, M. O'Hearn, J. S. Montaner, P. J. Piliero, B. Trottier, S. Walmsley, C. Cohen, D. R. Kuritzkes, J. J. Eron, Jr., J. Chung, R. DeMasi, L. Donatacci, C. Drobnes, J. Delehanty, and M. Salgo. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 13.Liu, S., W. Jing, B. Cheung, H. Lu, J. Sun, X. Yan, J. Niu, J. Farmar, S. Wu, and S. Jiang. 2007. HIV gp41 C-terminal heptad repeat contains multifunctional domains. Relation to mechanisms of action of anti-HIV peptides. J. Biol. Chem. 282:9612-9620. [DOI] [PubMed] [Google Scholar]
- 14.McCune, J. M., R. Namikawa, H. Kaneshima, L. D. Shultz, M. Lieberman, and I. L. Weissman. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632-1639. [DOI] [PubMed] [Google Scholar]
- 15.Palmer, S., R. W. Shafer, and T. C. Merigan. 1999. Highly drug-resistant HIV-1 clinical isolates are cross-resistant to many antiretroviral compounds in current clinical development. AIDS 13:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel, I. H., X. Zhang, K. Nieforth, M. Salgo, and N. Buss. 2005. Pharmacokinetics, pharmacodynamics and drug interaction potential of enfuvirtide. Clin. Pharmacokinet. 44:175-186. [DOI] [PubMed] [Google Scholar]
- 17.Perno, C. F., G. Moyle, C. Tsoukas, W. Ratanasuwan, J. Gatell, and M. Schechter. 2008. Overcoming resistance to existing therapies in HIV-infected patients: the role of new antiretroviral drugs. J. Med. Virol. 80:565-576. [DOI] [PubMed] [Google Scholar]
- 18.Peters, T., Jr. 1985. Serum albumin. Adv. Protein Chem. 37:161-245. [DOI] [PubMed] [Google Scholar]
- 19.Rabin, L., M. Hincenbergs, M. B. Moreno, S. Warren, V. Linquist, R. Datema, B. Charpiot, J. Seifert, H. Kaneshima, and J. M. McCune. 1996. Use of standardized SCID-hu Thy/Liv mouse model for preclinical efficacy testing of anti-human immunodeficiency virus type 1 compounds. Antimicrob. Agents Chemother. 40:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stehle, G., H. Sinn, A. Wunder, H. H. Schrenk, S. Schutt, W. Maier-Borst, and D. L. Heene. 1997. The loading rate determines tumor targeting properties of methotrexate-albumin conjugates in rats. Anticancer Drugs 8:677-685. [PubMed] [Google Scholar]
- 21.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turpin, J. A., R. W. Buckheit, Jr., D. Derse, M. Hollingshead, K. Williamson, C. Palamone, M. C. Osterling, S. A. Hill, L. Graham, C. A. Schaeffer, M. Bu, M. Huang, W. M. Cholody, C. J. Michejda, and W. G. Rice. 1998. Inhibition of acute-, latent-, and chronic-phase human immunodeficiency virus type 1 (HIV-1) replication by a bistriazoloacridone analog that selectively inhibits HIV-1 transcription. Antimicrob. Agents Chemother. 42:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]