Abstract
Determination of the attributable hospital cost and length of stay (LOS) are of critical importance for patients, providers, and payers who must make rational and informed decisions about patient care and the allocation of resources. The objective of the present study was to determine the additional total hospital cost and LOS attributable to health care-associated infections (HAIs) caused by antibiotic-resistant, gram-negative (GN) pathogens. A single-center, retrospective, observational comparative cohort study was performed. The study involved 662 patients admitted from 2000 to 2008 who developed HAIs caused by one of following pathogens: Acinetobacter spp., Enterobacter spp., Escherichia coli, Klebsiella spp., or Pseudomonas spp. The attributable total hospital cost and LOS for HAIs caused by antibiotic-resistant GN pathogens were determined by comparison with the hospital costs and LOS for a control group with HAIs due to antibiotic-susceptible GN pathogens. Statistical analyses were conducted by using univariate and multivariate analyses. Twenty-nine percent of the HAIs were caused by resistant GN pathogens, and almost 16% involved a multidrug-resistant GN pathogen. The additional total hospital cost and LOS attributable to antibiotic-resistant HAIs caused by GN pathogens were 29.3% (P < 0.0001; 95% confidence interval, 16.23 to 42.35) and 23.8% (P = 0.0003; 95% confidence interval, 11.01 to 36.56) higher than those attributable to HAIs caused by antibiotic-susceptible GN pathogens, respectively. Significant covariates in the multivariate analysis were age ≥12 years, pneumonia, intensive care unit stay, and neutropenia. HAIs caused by antibiotic-resistant GN pathogens were associated with significantly higher total hospital costs and increased LOSs compared to those caused by their susceptible counterparts. This information should be used to assess the potential cost-efficacy of interventions aimed at the prevention of such infections.
During the last few decades, the increasing rates of resistance among common bacterial pathogens have become a major threat to human health (1, 14, 18, 32, 34, 38). Research involved with the development of new antibiotics has not progressed in parallel with the increasing rates of resistance, which leaves clinicians with fewer options for the treatment of some infections (1, 39). Infections caused by antibiotic-resistant bacteria are believed to result in higher mortality rates, longer durations of hospital stays, and higher health care costs compared to those that result from infections with their antibiotic-susceptible counterparts (16). Over 50% of health care-associated infections (HAIs) are caused by resistant strains (18). The trends of increasing resistance are most critical in intensive care unit (ICU) patients, a population extremely susceptible to HAIs (5, 25). Although gram-negative (GN) bacteria comprise the major source of HAIs, the main focus of recent research and development has been on resistant gram-positive multidrug-resistant (MDR) organisms, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci (3, 9, 11). The GN bacteria causing HAIs are mainly Klebsiella spp., Pseudomonas aeruginosa, Acinetobacter baumannii, and Escherichia coli (14). All of these organisms, as well as Enterobacter spp., have been shown to have increasing rates of resistance (14, 30, 33), and MDR within these bacteria is becoming a problem (31, 40). The incidence of HAIs caused by the resistant pathogens Pseudomonas aeruginosa and A. baumannii are high and are a cause for concern (14).
HAIs are one of the most serious patient safety issues in health care today; indeed, they are the fifth leading cause of death in acute-care hospitals (20). Between 5% and 15% of hospital inpatients develop an infection during their admission, and critically ill, ICU patients are 5 to 10 times more likely to acquire an HAI than those in general wards. In the United States, approximately 2 million people per year acquire a bacterial infection while they are in the hospital. Of these, 50 to 70% are caused by antimicrobial-resistant strains of bacteria and 77,000 to 90,000 infected patients die. Prior research has shown that antibiotic resistance, in general, leads to additional costs, lengths of stay (LOSs), morbidity, and mortality, presumably as a result of inappropriate or suboptimal therapy (8). Although it is known that HAIs due to resistant GN bacteria, in particular, have been associated with negative patient outcomes, the additional cost associated with infections with these pathogens has not been fully elucidated. In 2002, the Centers for Disease Control and Prevention conducted a systematic audit to investigate economic evidence linking HAIs caused by resistant bacteria with increased costs. The attributable cost of HAIs, in general, was estimated to be $13,973 (42), but interpretation of the findings of the studies considered was difficult because of various methodological issues.
Studies that appropriately assess attributable costs could further clarify the financial burden of HAIs caused by antibiotic-resistant bacteria and thus enable decision makers to weigh and justify the allocation of resources to control this growing problem. Some studies have been designed to clarify the financial impact of nosocomial infections caused by drug-resistant GN pathogens (4, 7, 12, 13, 21-24, 36), but the studies' scope and methods varied widely. Therefore, the goal of the retrospective investigation described here was to appropriately determine the extra cost and LOS attributable to HAIs caused by resistant GN pathogens compared to the cost and LOS of infections caused by their susceptible counterparts at the Medical University of South Carolina hospital in Charleston, SC. The study was approved by the university's Institutional Review Board.
MATERIALS AND METHODS
Design.
This was a single-center, retrospective, observational comparative cohort study that included patients with HAIs due to GN bacteria. The study cohort comprised a sample of 662 patients from the age of newborn to 93 years admitted to an ICU or general hospital ward between January 2000 and June 2008 and diagnosed with a nosocomial infection due to a GN bacterium.
Data collection.
The database used for this analysis was created from a query of a larger database of all patients diagnosed with an HAI in our hospital between 1998 and 2008. All isolates had undergone testing for antimicrobial susceptibility by standard test methods in the hospital's Clinical Microbiology Laboratory by the use of a standard methodology and definitions. The database represented records for 1,236 HAIs caused by GN pathogens. Patients were excluded from the analysis if they had multiple infections during the same period of admission, incomplete financial data, or missing susceptibility data, leaving data for a total of 662 patients for evaluation. Data from the original database were collected for patients in both the ICU and general wards and was divided on the basis of the five pathogenic bacteria of interest: Acinetobacter spp., E. coli, Enterobacter spp., Klebsiella spp., and Pseudomonas spp.
Candidate risk factors and covariates included MDR, age, gender, a diagnosis of pneumonia, ICU stay, neutropenia, use of a central venous line, receipt of chemotherapy, use of a Foley catheter, receipt of total parenteral nutrition (TPN), use of mechanical ventilation, transplantation, and the HAIs of interest: bloodstream infection (BSI), surgical site infection (SSI), other infection (including urinary tract infection [UTI]). Antibiotic susceptibility was denoted susceptible, intermediate, or resistant and not tested.
Financial data included total hospital charges, which were provided through the hospital's patient accounting system. The total hospital cost for each patient's entire admission was calculated. The overall hospital cost for patients with HAIs caused by GN pathogens included the costs for drugs, laboratory and medical tests, ICU stay, as well as other patient care procedures (28). All costs are reported in 2008 U.S. dollars. Estimates of the cost of a hospital episode were determined by adjusting UB-92 and UB-04 (from the Uniform Billing Act of 1982, revised 1992 and 2004) hospital billing (charge) information by using the hospitalwide cost-to-charge ratio (27). By this approach, the cost of each hospitalization was calculated as the product of the billed charges during the hospital episode found in the hospital billing database and the hospital's overall cost-to-charge ratio for that year, available from the Medicare cost report. The total cost was general and was not attributable solely to the costs associated with each infection. The statistical multivariate methodology, presented below, provides a description of the attributable cost assessment.
Definitions.
HAIs were defined according to the criteria of the Centers for Disease Control and Prevention. The definition of resistance used in the current study was that of Hidron et al. (15). Bacteria were considered resistant if they were not susceptible to one of the following antibiotic groups: fluoroquinolones (ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin, or gatifloxacin), piperacillin (piperacillin or piperacillin-tazobactam), carbapenems (imipenem or meropenem), or extended-spectrum cephalosporins (ceftriaxone, ceftazidime, cefotaxime, or cefepime). Organisms were considered MDR if they were resistant to antibiotics in two or more of these same groups.
Statistical analysis.
The effect that each patient characteristic had on the total hospital cost and LOS for our sample of patients was initially assessed by univariate analysis. The normality of the distribution for total hospital cost and LOS was tested by use of the Kolmogorov-Smirnov test statistic. Because it was determined that cost and LOS data were each non-normally distributed, the univariate analysis assessing cost and LOS across categorical variables employed the Kruskal-Wallis nonparametric test. To assess attributable total hospital cost and LOS, multivariate analyses were conducted. To correct for the non-normal distribution of the total hospital cost and LOS, a gamma distribution and logarithmic transformation (29) were specified for the dependent variable through the use of the PROC GENMOD module in SAS statistical software (version 9.0; SAS Institute, Inc., Cary, NC). The interpretation of the result when a dependent (outcome) variable assumes a gamma distribution and has been log transformed and the independent variables (covariates) have not been log transformed is that the dependent variable changes by the coefficient (valued in percent) for a 1-unit increase in the value of the independent variable, controlling for the remaining independent covariate variables in the model.
Thus, for the primary analysis of the effect of resistance (yes or no) on the total hospital cost and LOS, the implication would suggest that infection with a resistant pathogen would result in an x percent change in the total hospital cost or LOS compared to infection with a nonresistant pathogen. This was the methodology used to capture attributable cost. For the multivariate analysis, multicollinearity was assessed by the use of Pearson correlation coefficients. If independent variables were found to be highly correlated, they were dropped from the multivariate analysis. The levels and the amounts (the numbers of correlated variables) of correlations, as well as clinical input, determined this decision process. Finally, a backward selection process was used to determine the final multivariate model. Variables with significance at the 0.10 level were included in the model. SAS computer software (version 9.0; SAS Institute, Inc.) was used for all analyses included in this study, and statistical significance was determined at a level of 0.05.
RESULTS
Patient characteristics.
Table 1 provides the demographic information, frequencies of resistance, sites of infection, LOSs, and the total hospital cost for the sample of patients. The gender distribution was 65% to 35% males to females. Age was divided into three groups for the purposes of analysis, and the majority of patients were either younger than 1 year of age (27.4%) or at least 12 years of age (68.8%). Of the 662 patients, 29.2% were infected by a resistant GN pathogen, and almost 16% of the total were infected with MDR GN bacteria. The most common types of infections were BSIs and pneumonia. It should be kept in mind that UTIs were inconsistently noted in the database and were therefore included in the group of “other.” Overall, the patients had an average LOS of 43.2 days. Of the 662 patients, 498 (74%) patients had an ICU stay (average LOS, 37.1 days). The average total hospital cost for our sample of patients was $151,512.
TABLE 1.
Patient demographics
| Variable (na = 662) | Mean | SD |
|---|---|---|
| % Male | 63.1 | 0.48 |
| Age (% of patients) | ||
| <1 yr | 27.4 | 0.45 |
| ≥1 to <12 yr | 3.80 | 0.19 |
| ≥12 yr | 68.8 | 0.46 |
| Race (% of patients) | ||
| White | 54.5 | 0.50 |
| Black | 37.6 | 0.48 |
| Hispanic | 5.0 | 0.22 |
| Asian | 1.1 | 0.10 |
| Other | 1.5 | 0.12 |
| Type of pathogen resistance (% of patients) | ||
| Resistant | 29.2 | 0.45 |
| MDR | 15.5 | 0.36 |
| Site of infection (% of patients) | ||
| BSI | 31.7 | 0.47 |
| Pneumonia | 34.0 | 0.47 |
| SSI | 26.9 | 0.44 |
| Other | 7.4 | 0.26 |
| LOS (days) | ||
| Overall | 43.2 | 40 |
| ICU | 37.1b | 36 |
| Total (range) costc ($) | 151,512 (152-1,056,054) | 144,944 |
n = 662 (for gender, n = 661; for age, n = 605; for ICU stay, n = 498).
Median, 26.1 days.
The total cost is adjusted to 2008 constant dollars.
Distributions of organisms and frequency of resistance.
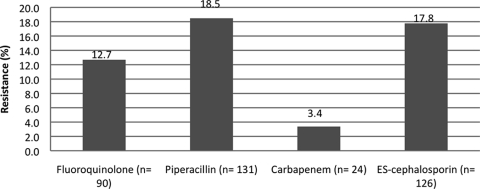
A total of 709 isolates were collected from the 662 patients with HAIs caused by GN pathogens. The distribution of pathogens of interest in the entire cohort was as follows: 26% Pseudomonas spp., 25% Enterobacter spp., 21% Klebsiella spp., 21% E. coli, and 7% Acinetobacter spp. The combined rates of resistance to each antibiotic group for all pathogens is presented in Fig. 1. The percentage of pathogens isolated by each type of infection is presented in Table 2. Of the 662 patients, 182 Pseudomonas infections were documented, which made this bacterium the most common pathogen in the population. Among the Pseudomonas isolates, the rates of resistance to the antibiotics of interest ranged from 10 to 16.5%. In contrast, Acinetobacter spp. caused infections at the lowest frequency (n = 51), but they had the highest rates of resistance to extended-spectrum cephalosporins, piperacillin, and the fluoroquinolones (31 to 39%).
FIG. 1.
Distribution of resistance to fluoroquinolones (ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin, or gatifloxacin), piperacillin or piperacillin-tazobactam, carbapenems (imipenem or meropenem), or extended-spectrum cephalosporins (ceftriaxone, ceftazidime, cefotaxime, or cefepime).
TABLE 2.
Frequency of resistance by organism and infection site
| Organism | No. (%) of pathogens |
||||
|---|---|---|---|---|---|
| BSI | SSI | Pneumonia | Other | Total | |
| Acinetobacter spp. (n = 51) | 5 (9.8) | 2 (3.9) | 16 (31.4) | 1 (2.0) | 24 (47.1) |
| E. coli (n = 151) | 12 (7.9) | 17 (11.3) | 11 (7.3) | 4 (2.6) | 44 (29.1) |
| Enterobacter spp. (n = 179) | 24 (13.4) | 12 (6.7) | 14 (7.8) | 8 (4.5) | 58 (32.4) |
| Klebsiella spp. (n = 146) | 12 (8.2) | 2 (1.4) | 12 (8.2) | 3 (2.1) | 29 (19.9) |
| Pseudomonas spp. (n = 182) | 6 (3.3) | 11 (6.0) | 35 (19.2) | 3 (1.6) | 55 (30.2) |
Univariate analysis: hospital costs and LOSs.
The results of the univariate analysis assessing the effect of each patient characteristic on hospital cost are presented in Table 3. Patients infected with resistant bacteria had a median total cost $38,121 higher than that for patients infected with nonresistant bacteria ($144,414 and $106,293, respectively; P < 0.0001). Other variables with a strong positive association with cost (P < 0.0001 for all variables) included MDR, receipt of care in an ICU, age of <1 year, pneumonia, and ventilator use. Variables positively associated with cost with a P value of <0.05 included Foley catheter use, receipt of TPN, and transplantation. Variables that were negatively associated with cost (compared to the association for all other patients in the sample) were age of ≥12 years (P < 0.0001), SSI (P < 0.0001), and the receipt of chemotherapy (P = 0.0140).
TABLE 3.
Effect of patient characteristics on hospital costa by univariate analysis
| Variable (no. of patients no/no. of patients yes) | Median ± SD (range) total hospital costa ($) |
P valueb | |
|---|---|---|---|
| No | Yes | ||
| Resistant pathogen (469/193) | 106,293 ± 125,018 (152-924,661) | 144,414 ± 178,528 (5,191-1,056,054) | <0.0001 |
| MDR pathogen (559/103) | 106,293 ± 128,447 (152-924,662) | 178,359 ± 198,247 (5,192-1,056,054) | <0.0001 |
| ICU stay (164/498) | 22,904 ± 39,715 (152-230,384) | 155,209 ± 146,719 (17,192-1,056,054) | <0.0001 |
| Gender (244/417) | 108,294 ± 140,070 (152-1,017,789) | 121,819 ± 147,405 (174-1,056,054) | 0.0990 |
| Age < 1 yr (439/166) | 101,476 ± 150,439 (152-1,056,054) | 159,035 ± 131,029 (2,680-825,512) | <0.0001 |
| Age ≥1 but <12 yr (582/23) | 115,311 ± 145,611 (152-1,056,054) | 95,262 ± 173,630 (5,712-624,182) | 0.2961 |
| Age ≥ 12 yr (189/416) | 150,610 ± 136,886 (2,680-825,512) | 101,753 ± 149,279 (152-1,056,054) | <0.0001 |
| BSI (452/210) | 113,115 ± 157,462 (152-1,056,054) | 116,471 ± 113,619 (2,956-614,990) | 0.2757 |
| Pneumonia (437/225) | 82,861 ± 112,403 (152-680,777) | 175,214 ± 171,641 (36,047-1,056,054) | <0.0001 |
| SSI (484/178) | 152,852 ± 147,997 (182-1,056,054) | 30,452 ± 89,415 (152-680,777) | <0.0001 |
| Otherc (613/48) | 113,580 ± 147,277 (152-1,056,054) | 158,559 ± 112,584 (181-624,181) | 0.1192 |
| Chemotherapy (650/12) | 116,471 ± 145,569 (152-1,056,054) | 45,821 ± 70,149 (4,309-215,382) | 0.0140 |
| Central venous line (517/145) | 113,580 ± 153,676 (152-1,056,054) | 116,628 ± 108,460 (4,308-614,990) | 0.3351 |
| Foley catheter (648/14) | 113,352 ± 145,921 (152-1,056,054) | 208,164 ± 71,808 (95,503-381,758) | 0.0033 |
| Neutropenia (652/10) | 115,842 ± 145,690 (152-1,056,054) | 113,722 ± 83,670 (15,985-305,952) | 0.9230 |
| TPN (584/78) | 112,947 ± 145,008 (152-1,056,054) | 149,661 ± 142,223 (17,192-825,512) | 0.0047 |
| Transplantation (658/4) | 114,420 ± 136,721 (152-924,662) | 661,870 ± 443,232 (236,583-1,056,054) | 0.0031 |
| Ventilator (441/221) | 82,861 ± 112,032 (152-680,777) | 177,585 ± 172,440 (36,046-1,056,054) | <0.0001 |
All costs are expressed in 2008 constant dollars.
Significance was determined at the 0.05 level.
Other includes UTIs.
The results of the univariate analysis of the categorical variables related to LOS are presented in Table 4. Patients infected with resistant bacteria had a longer median LOS of 5 days than patients infected with nonresistant bacteria (36 and 31 days, respectively; P < 0.0060). Other variables with a strong positive association with LOS (all P < 0.0001) included the presence of an MDR pathogen, ICU stay, age of <1 year, BSI, pneumonia, receipt of TPN, ventilator use, Variables that had P values of <0.05 and that were positively associated with LOS, included other infections, central venous line, Foley catheter use, and transplantation. Variables that were negatively associated with total hospital cost (compared to all other patients in the sample) were age of ≥12 years (P < 0.0001), SSI (P < 0.0001), and chemotherapy (P = 0.0185).
TABLE 4.
Effect of patient characteristics on LOS by univariate analysis
| Variable (no. of patients no/no. of patients yes) | Median ± SD (range) LOS (days) |
P valuea | |
|---|---|---|---|
| No | Yes | ||
| Resistant pathogen (469/193) | 31 ± 38.75 (1-270) | 36 ± 42.96 (1-278) | 0.0060 |
| MDR pathogen (559/103) | 30 ± 37.69 (1-270) | 47 ± 49.00 (1-278) | <0.0001 |
| ICU stay (164/498) | 8 ± 14.66 (1-101) | 42 ± 40.92 (2-287) | <0.0001 |
| Gender (244/417) | 31 ± 37.22 (1-206) | 34 ± 42.21 (1-278) | 0.2066 |
| Age < 1 yr (439/166) | 26 ± 31.53 (1-206) | 58 ± 49.95 (3-278) | <0.0001 |
| Age ≥1 but <12 yr (582/23) | 33 ± 40.64 (1-278) | 21 ± 40.58 (5-147) | 0.2715 |
| Age ≥ 12 yr (189/416) | 52 ± 49.85 (3-278) | 26.5 ± 31.00 (1-206) | <0.0001 |
| BSI (452/210) | 29.5 ± 39.12 (1-278) | 38.5 ± 41.52 (1-216) | <0.0001 |
| Pneumonia (437/225) | 24 ± 37.20 (1-216) | 43 ± 42.99 (7-278) | <0.0001 |
| SSI (484/178) | 42 ± 41.38 (1-278) | 10 ± 21.27 (1-137) | <0.0001 |
| Otherb (613/48) | 31 ± 40.43 (1-278) | 47 ± 32.18 (1-121) | 0.0239 |
| Central venous line (517/145) | 31 ± 40.83 (1-278) | 37 ± 37.72 (1-206) | 0.0408 |
| Chemotherapy (650/12) | 33 ± 40.36 (1-278) | 14 ± 18.25 (1-48) | 0.0185 |
| Foley catheter (648/14) | 32 ± 40.40 (1-278) | 52.5 ± 25.80 (15-101) | 0.0183 |
| Neutropenia (652/10) | 32.5 ± 40.41 (1-278) | 37.5 ± 17.72 (4-61) | 0.8742 |
| TPN (584/78) | 30 ± 37.33 (1-270) | 58.5 ± 50.38 (7-278) | <0.0001 |
| Transplantation (658/4) | 32 ± 39.81 (1-278) | 101 ± 63.71 (34-168) | 0.0331 |
| Ventilator (441/221) | 24 ± 37.04 (1-216) | 43 ± 43.29 (7-278) | <0.0001 |
Significance was determined at the 0.05 level.
Other includes UTIs.
Multivariate analysis of cost and LOS: attributable cost and LOS.
To assess the data for the presence of multicollinearity, Pearson correlation coefficients between each independent variable were first calculated. Table 5 presents those variables with correlations of >40%. To correct for multicollinearity, one of the two highly correlated variables had to be left out of the multivariate analysis. Thus, the resulting variables included in the multivariate analysis were resistance (primary effect), age of ≥12 years, pneumonia, ICU stay, neutropenia, and transplantation. Multivariate analysis was conducted by utilizing a backward stepwise selection. Variables significant at the 0.10 level were included in the final model, since they demonstrated some statistical effect (trend) in the model, allowing more robust results to be obtained. The multivariate analysis revealed that the cost attributable to an infection with a resistant GN pathogen represented an additional 29.3% (P < 0.0001) over the total hospital cost of an infection with a nonresistant GN pathogen (Table 6). A positive association with LOS was also seen in patients with HAIs due to resistant pathogens, with the increase being 23.8% (P = 0.0003). This implies that for this sample of patients, when all other independent covariates are held constant, having an HAI caused by a resistant GN pathogen was associated with a 29.3% higher total hospital cost for each admission and a 23.8% increase in the LOS than those for patients with HAIs caused by nonresistant pathogens.
TABLE 5.
Highly correlated variables
| Correlated variables (absolute correlation > 40%) |
Pearson coefficient (%) | |
|---|---|---|
| Variable 1 | Variable 2 | |
| Pneumonia | Ventilator | 98.0 |
| Age ≥ 12 yr | Age <1 yr | −91.2 |
| BSI | CVLa | 76.9 |
| Resistant | MDR | 66.9 |
| TPN | Age <1 yr | 51.7 |
| Pneumonia | BSI | −48.9 |
| BSI | Ventilator | −48.3 |
| Foley catheter | Other | 48.0 |
| TPN | Age ≥12 yr | −47.8 |
| Ventilator | SSI | 42.9 |
| BSI | Age ≥12 yr | −42.2 |
CVL, central venous line.
TABLE 6.
Effect of patient characteristics on hospital costa and LOS by multivariate analysis
| Variable | Total hospital cost (log) (n = 605) |
LOS (log) (n = 605) |
||
|---|---|---|---|---|
| Parameter estimate (95% CI) | P valueb | Parameter estimate (95% CI) | P valueb | |
| Intercept | 10.5624 (10.3801, 10.7448) | <0.0001 | 3.0290 (2.8503, 3.2077) | <0.0001 |
| Resistant | 0.2929 (0.1623, 0.4235) | <0.0001 | 0.2379 (0.1101, 0.3656) | 0.0003 |
| Age ≥ 12 yr | −0.2634 (−0.4126, −0.1142) | 0.0005 | −0.6681 (−0.8146, −0.5217) | <0.0001 |
| Pneumonia | 0.4382 (0.2888, 0.5875) | <0.0001 | 0.3817 (0.2353, 0.5281) | <0.0001 |
| ICU | 1.4208 (1.2532, 1.5884) | <0.0001 | 1.0595 (0.8956, 1.2233) | <0.0001 |
| Neutropenia | 0.8356 (0.3455, 1.3256) | 0.0008 | 0.7092 (0.2320, 1.1863) | 0.0036 |
| Transplanta-tion | 1.1582 (0.3210, 1.9954) | 0.0067 | 0.7443 (−0.0722, 1.5608) | 0.0740 |
All costs are expressed in 2008 constant dollars.
Significance was determined at the 0.05 level.
Other variables positively associated with total hospital cost and LOS were pneumonia, ICU stay, neutropenia, and transplantation. Pneumonia was associated with 43.8% and 38.2% increases in total hospital cost and LOS, respectively (P < 0.0001 for both), compared to the total hospital costs and LOSs for other types of infections (BSIs, SSIs, and others). ICU stay was associated with a 142% increase in total hospital cost (P < 0.0001) and an almost 106% increase in LOS (P < 0.0001) compared to the cost and LOS for patients without ICU stays. Neutropenia significantly contributed to an increase in total hospital cost of 83.5% (P = 0.0008) and a 70.9% increase in LOS (P = 0.0036) compared to the cost and LOS for nonneutropenic patients. The transplant patients in the sample had a significantly higher total hospital cost of 115.8% compared to that for nontransplant patients and a trend for an increased LOS of 74% compared to that for nontransplant patients (P = 0.0740). The patients in the sample who were at least 12 years of age had a 26.3% lower total hospital cost and a 66.8% lower LOS compared to those for patients younger than 12 years of age (P = 0.0005 and P < 0.0001, respectively).
DISCUSSION
HAIs have been associated with a number of negative consequences for patients, including increased LOS, morbidity, mortality, and hospital costs. Those associated with antibiotic-resistant pathogens, including GN pathogens, magnify the effect to an even higher level (37). In the current study, 29.2% of the 662 patients had an HAI caused by a resistant GN pathogen, and approximately half of those patients (15.5%) were infected with an MDR pathogen. This is similar to the findings presented in a recent National Healthcare Safety Network annual update, which reported that as many as 16% of HAIs were caused by MDR pathogens, although that study included both gram-positive and GN organisms (15). The main results of the present study illustrating increased hospital costs and LOSs confirm the prior observations made by other investigators and provide a context in which to weigh the potential cost utility of preventative interventions or programs.
The additional financial burden of antimicrobial resistance to health care organizations has been an intense area of study over the last two decades. Studies focusing on the impacts of costs associated with antibiotic-resistant GN bacteria have tended to include multiple pathogens, although some were either organism or resistance mechanism specific. Two of three studies focusing on infections with antibiotic-resistant Pseudomonas aeruginosa described increased rates of mortality, LOSs, and hospital costs or charges (13, 22), while the third found no effect on hospital charges (4). These have also described increased hospital costs and/or increased rates of mortality, LOSs, and antibiotic costs (23, 43). Similar to the work of Carmeli et al. (4), Cosgrove et al. studied the effects of resistance development during therapy, but in that case, the study was performed in the context of expanded-spectrum cephalosporin resistance in Enterobacter spp. (7). In that controlled analysis, patients in whom resistance developed during therapy had higher hospital charges than control patients in whom resistance did not develop ($79,323 and $40,406, respectively; P < 0.001). The development of resistance had an attributable median hospital stay of 9 days and an attributable hospital charge of $29,379. The authors concluded that measures to prevent this type of resistance, which costs up to an average of $2,900 per patient, would be cost saving at their institution. Other investigators have studied two or more drug-resistant pathogens, most commonly extended-spectrum β-lactamase producers (21, 24, 36). Those studies also found increased LOSs, hospital charges or costs, and, in one case, increased rates of mortality and a delay in the onset of the appropriate antibiotic therapy (36). Thus, the preponderance of evidence indicates a positive association between HAIs with antibiotic-resistant GN bacteria and increased hospital costs or charges, an observation that the present study corroborates.
Although there is overwhelming agreement among the studies in terms of the effect of resistance in GN pathogens on costs, caution should be exercised in comparing results across studies. Studies estimating the attributable cost of resistance can be difficult to interpret, as the statistical distributions of cost and LOS may be normally distributed in some circumstances and non-normally distributed in others. Appropriate tests must be performed to confirm the statistical distribution, as was done in the current study and a study by Lee et al. (24). In addition, it is important to control for confounding factors in order to minimize the influence of these variables on cost (19). In the current study, it appeared that having an ICU stay might affect the outcome. This variable was accounted for by controlling for ICU stay in our analysis. Of the 662 patients, 498 had an ICU stay (74%), and in the multivariate analysis it was noted that ICU stays were positively associated with cost and LOS, as one might expect. Given the strength of association between ICU stay and outcomes in our analysis, it is possible that studies that have not taken the number of days in the ICU into account may likely overestimate the impact of resistance on outcomes.
Numerous methodological issues involving studies of this type are clearly reviewed elsewhere by Cosgrove and Carmeli (8). In that context, a number of such issues, including the limitations of this study, merit comment. When attributable LOS is estimated, it has been suggested that the duration of hospitalization prior to infection should be controlled to prevent an overestimation from being made (6, 26). This was not considered in the current study, primarily because the increased cost in relation to contracting the infection may well begin even before any diagnostic test may be performed or antibiotic treatment is initiated. The subjectivity specifying an attributable cost related to the date of infection would vary and may make this factor too inconclusive. Furthermore, it is known that patients with HAIs caused by resistant pathogens have been admitted for a longer time prior to the infection than patients with HAIs caused by susceptible pathogens (35). The time required to establish the right initial treatment for an infection may also affect the cost, as described by Lautenbach et al. (21). The importance of controlling for confounding factors was also exemplified in the same retrospective matched-cohort study in which the APACHE score and the time at risk had major influences on estimates of costs and LOSs. Without controlling for these factors, the authors suggested that an overestimation of attributable cost and LOS could possibly result. It is also important to consider the impact of mortality on outcomes (17). Three other similar studies have found mortality to be a predictor of increased cost (7, 23, 36). For the current study, APACHE score, time at risk, and mortality data were not available in the database. For that reason, it was not possible to examine whether these factors significantly affected resistance and whether they perhaps needed to be controlled for in the multivariate analysis. If the APACHE score, time at risk, and mortality were measured and included as confounding factors in our study, it is possible that our estimate of the attributable outcomes (total hospital cost and LOS) of resistance would be dampened to some extent.
It is also known that hospital costs are dependent upon infection sites (3, 36, 42). A comprehensive review from 2005 included 70 studies related to the cost of various infections and concluded that nosocomial UTIs have the lowest attributable cost ($1,006), whereas nosocomial BSIs have the highest attributable cost ($36,441) (42). We also found the infection site to be positively associated with the total hospital cost and LOS. It should be noted that the attributable outcomes related to other infection sites could be underestimated in the current study, since UTIs had not been consistently included in the database during the time period evaluated. For example, if data for several cases of UTIs had been collected and analyzed, these would likely cause pneumonia to be associated with a higher attributable cost and LOS, since UTIs are known to be less expensive cases (10, 41, 42). In addition, cost may depend not only on infection sites but also on the organism (7); thus, the correct way to conduct such analyses would be not only by infection site but also by organism.
Similar to comparable studies that included data for more than one type of bacterium, we pooled the data for all GN organisms of interest for our analysis. As described above, other investigations included data for only one type of bacterium, while others have distinguished between resistance mechanisms. Only one study distinguished the outcome of resistance between three types of bacteria (2). The impact of resistance on cost and LOS is perhaps dependent upon the prevalence of both the infection type and the bacterium. Since the distribution of infection sites and organisms varies from institution to institution, it would be preferable to conduct separate analyses by infection site and by type of bacterium, which would provide information on the attributable cost of a specific bacterium at a specific infection site. Due to our relatively small study population, such an analysis was not possible in the present study.
There is a lack of standardization in the definition of resistance. Thus, comparing the impact of resistance across studies becomes difficult. Our definition is fairly similar to that of others but takes into consideration the antibiotics present on our formulary during the time period of interest. Since the utilization of antibiotics varies among hospitals and given that the patterns and the extent of antibiotic use affect the resistance rates, resistance rates differ among hospitals and other health care settings, and thus relevant definitions of resistance vary locally. Nonetheless, common definitions for use in the research arena are needed in order to accurately assess and then independently confirm findings relevant to the consequences of HAIs with antibiotic-resistant bacteria. Finally, the current study involved a single institution evaluated over a limited time period, and the findings may not reflect the impact of resistance in other institutions. It is for this reason that we chose to stress the percent differences in LOS and cost as opposed to numerical differences. This should allow extrapolation to other, similar institutions. Since the current study has examined data reflecting 8 years of experience, variations in data collection throughout this period may have occurred. Furthermore, since it is known that the prevalence and mechanisms of resistance among bacteria have changed over the last 20 years (1, 38), our results could have been affected by changing patterns of resistance.
We have clearly shown that HAIs with antibiotic-resistant GN bacteria have a measurable and significant attributable cost. Resistance was associated with a 29.3% higher total hospital cost and a 23.8% increased LOS compared to the cost and LOS for HAIs caused by susceptible GN pathogens. Future attributable outcomes analyses regarding resistance should incorporate standardized economic evaluation methods. The use of such a methodology will ensure reliable results, which will be reproducible and comparable. Lastly, a global definition of resistance is desirable to assess the impact of resistance in and across multiple settings. Additional studies are needed to improve the quality and the scope of the evidence. Finally, it should be emphasized that the perspective of the current study reflects that of the hospital. Other perspectives, such as those of the patient, payer, and society, may be as relevant or even more relevant, since they may include factors such as lost wages and morbidity. Economic evaluations from these additional perspectives would provide valuable information for decision making. Nonetheless, our results provide information that should be useful in planning and justifying the cost of measures aimed at preventing HAIs with antibiotic-resistant GN bacteria.
Acknowledgments
This work was funded, in part, by an investigator-initiated research grant from AstraZeneca Pharmaceuticals LP, Wilmington, DE.
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, Jr., D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 2.The Brooklyn Antibiotic Resistance Task Force. 2002. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa on length of hospital stay. Infect. Control Hosp. Epidemiol. 23:106-108. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli, Y., G. Eliopoulos, E. Mozaffari, and M. Samore. 2002. Health and economic outcomes of vancomycin-resistant enterococci. Arch. Intern. Med. 162:2223-2228. [DOI] [PubMed] [Google Scholar]
- 4.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127-1132. [DOI] [PubMed] [Google Scholar]
- 5.Clark, N. M., J. Patterson, and J. P. Lynch III. 2003. Antimicrobial resistance among gram-negative organisms in the intensive care unit. Curr. Opin. Crit. Care 9:413-423. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove, S. E. 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 42(Suppl. 2):S82-S89. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulous, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove, S. E., and Y. Carmeli. 2003. The impact of antimicrobial resistance on health and economic outcomes. Clin. Infect. Dis. 36:1433-1437. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove, S. E., Y. Qi, K. S. Kaye, S. Harbarth, A. W. Karchmer, and Y. Carmeli. 2000. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 26:166-174. [DOI] [PubMed] [Google Scholar]
- 10.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engemann, J. J., Y. Carmeli, S. E. Cosgrove, V. G. Fowler, M. Z. Bronstein, S. L. Trivette, J. P. Briggs, D. J. Sexton, and K. S. Kaye. 2003. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 36:592-598. [DOI] [PubMed] [Google Scholar]
- 12.Evans, H. L., S. N. Lefrak, J. Lyman, R. L. Smith, T. W. Chong, S. T. McElearney, A. R. Schulman, M. G. Hughes, D. P. Raymond, T. L. Pruett, and R. G. Sawyer. 2007. Cost of gram-negative resistance. Crit. Care Med. 35:89-95. [DOI] [PubMed] [Google Scholar]
- 13.Gasink, L. B., N. O. Fishman, M. G. Weiner, I. Nachamkin, W. B. Bilker, and E. Lautenbach. 2006. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am. J. Med. 119:526e19-526e25. [DOI] [PubMed] [Google Scholar]
- 14.Gaynes, R., and J. R. Edwards. 2005. Overview of healthcare associated infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 15.Hidron, A. I., J. R. Edwards, J. Patel, T. C. Horan, D. M. Sievert, D. A. Pollock, and S. K. Fridkin. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996-1011. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg, S. D., S. L. Solomon, and P. A. Blake. 1987. Health and economic impacts of antimicrobial resistance. Rev. Infect. Dis. 9:1065-1078. [DOI] [PubMed] [Google Scholar]
- 17.Howard, D., R. Cordell, J. E. McGowan, Jr., R. M. Packard, R. D. Scott II, and S. L. Solomon. 2001. Measuring the economic costs of antimicrobial resistance in hospital settings: summary of the Centers for Disease Control and Prevention-Emory Workshop. Clin. Infect. Dis. 33:1573-1578. [DOI] [PubMed] [Google Scholar]
- 18.Jones, R. N. 2001. Resistance patterns among nosocomial pathogens: trends over the past few years. Chest 119(2 Suppl.):397S-404S. [DOI] [PubMed] [Google Scholar]
- 19.Kaye, K. S., J. J. Engemann, E. Mozaffari, and Y. Carmeli. 2004. Reference group choice and antibiotic resistance outcomes. Emerg. Infect. Dis. 10:1125-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klevens, R. M., J. Edwards, C. Richards, T. C. Horan, R. P. Gaynes, D. A. Pollock, and D. M. Cardo. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 122:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lautenbach, E., J. B. Patel, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2001. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162-1171. [DOI] [PubMed] [Google Scholar]
- 22.Lautenbach, E., M. G. Weiner, I. Nachamkin, W. B. Bilker, A. Sheridan, and N. O. Fishman. 2006. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 27:893-900. [DOI] [PubMed] [Google Scholar]
- 23.Lee, N. Y., H. C. Lee, N. Y. Ko, C. M. Chang, H. I. Shih, C. J. Wu, and W. C. Ko. 2007. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect. Control Hosp. Epidemiol. 28:713-719. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. Y., S. Kotapati, J. L. Kuti, C. H. Nightingale, and D. P. Nicolau. 2006. Impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect. Control Hosp. Epidemiol. 27:1226-1232. [DOI] [PubMed] [Google Scholar]
- 25.Lim, S. M., and S. A. Webb. 2005. Nosocomial bacterial infections in intensive care units. I. Organisms and mechanisms of antibiotic resistance. Anaesthesia 60:887-902. [DOI] [PubMed] [Google Scholar]
- 26.Maragakis, L. L., E. N. Perencevich, and S. E. Cosgrove. 2008. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti Infect. Ther. 6:751-763. [DOI] [PubMed] [Google Scholar]
- 27.Mauldin, P. D., C. D. Salgado, V. L. Durkalski, and J. A. Bosso. 2008. Healthcare associated infections due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: relationships with antibiotic use and cost drivers. Ann. Pharmacother. 42:317-326. [DOI] [PubMed] [Google Scholar]
- 28.Mauldin, P. D., W. S. Weintraub, and E. R. Becker. 1994. Predicting hospital costs for first-time coronary artery bypass grafting from preoperative and postoperative variables. Am. J. Cardiol. 74:772-775. [DOI] [PubMed] [Google Scholar]
- 29.Montez-Rath, M., C. L. Christiansen, S. L. Ettner, S. Loveland, and A. K. Rosen. 2006. Performance of statistical models to predict mental health and substance abuse cost. BMC Med. Res. Methodol. 6:53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Nosocomial Infections Surveillance System Report. 2004. Data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 31.Paterson, D. L. 2006. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 34(5 Suppl. 1):S20-S28. [DOI] [PubMed] [Google Scholar]
- 32.Rahal, J. J., C. Urban, and S. Segal-Maurer. 2002. Nosocomial antibiotic resistance in multiple gram-negative species: experience at one hospital with squeezing the resistance balloon at multiple sites. Clin. Infect. Dis. 34:499-503. [DOI] [PubMed] [Google Scholar]
- 33.Rhomberg, P. R., and R. N. Jones. 2007. Contemporary activity of meropenem and comparator broad-spectrum agents: MYSTIC program report from the United States component (2005). Diagn. Microbiol. Infect. Dis. 57:207-215. [DOI] [PubMed] [Google Scholar]
- 34.Rice, L. B. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197:1079-1081. [DOI] [PubMed] [Google Scholar]
- 35.Roghmann, M., D. D. Bradham, M. Zhan, S. K. Fridkin, and T. M. Perl. 2005. Measuring impact of antimicrobial resistance. Emerg. Infect. Dis. 11:982-983. [Google Scholar]
- 36.Schwaber, M. J., S. Navon-Venezia, K. S. Kaye, R. Ben-Ami, D. Schwartz, and Y. Carmeli. 2006. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 50:1257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shorr, A. F. 2009. Review of studies on the impact on gram-negative bacterial resistance on outcomes in the intensive care unit. Crit. Care Med. 37:1463-1469. [DOI] [PubMed] [Google Scholar]
- 38.Siegel, R. E. 2008. Emerging gram-negative antibiotic resistance: daunting challenges, declining sensitivities, and dire consequences. Respir. Care 53:471-479. [PubMed] [Google Scholar]
- 39.Spellberg, B., R. Guidos, D. Gilbert, J. Bradley, H. W. Boucher, W. M. Scheld, J. G. Bartlett, and J. Edwards, Jr. 2008. The epidemic of antibiotic resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155-164. [DOI] [PubMed] [Google Scholar]
- 40.Slama, T. G. 2008. Gram-negative antibiotic resistance: there is a price to pay. Crit. Care 12(Suppl. 4):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone, P. W., D. Braccia, and E. Larson. 2005. Systematic review of economic analyses of health care-associated infections. Am. J. Infect. Control 33:501-509. [DOI] [PubMed] [Google Scholar]
- 42.Stone, P. W., E. Larson, and L. N. Kawar. 2002. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990-2000. Am. J. Infect. Control 30:145-152. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, S. J., C. J. Knipe, M. J. Zieger, K. M. Gabehart, J. E. Goodman, H. M. Volk, and R. Sood. 2004. Direct costs of multidrug-resistant Acinetobacter baumannii in the burn unit of a public teaching hospital. Am. J. Infect. Control 32:342-344. [DOI] [PubMed] [Google Scholar]