Abstract
Levofloxacin was recently (May 2008) approved by the U.S. Food and Drug Administration as a treatment for children following inhalational exposure to anthrax. Given that no clinical trials to assess the efficacy of a chosen dose was conducted, the basis for the dose recommendation was based upon pharmacometric analyses. The objective of this paper is to describe the basis of the chosen pediatric dose recommended for the label. Pharmacokinetic (PK) data from 90 pediatric patients receiving 7 mg/kg of body weight levofloxacin and two studies of 47 healthy adults receiving 500 and 750 mg/kg levofloxacin were used for the pharmacometric analyses. Body weight was found to be a significant covariate for levofloxacin clearance and the volume of distribution. Consistently with developmental physiology, clearance also was found to be reduced in pediatric patients under 2 years of age due to immature renal function. Different dosing regimens were simulated to match adult exposure (area under the concentration-time curve from 0 to 24 h at steady state, maximum concentration of drug in serum at steady state, and minimum concentration of drug in serum at steady state) following the approved adult dose of 500 mg once a day. The recommended dose of 8 mg/kg twice a day was found to match the exposure of the dose approved for adults in a manner that permitted confidence that this dose in children would achieve efficacy comparable to that of adults.
Anthrax is a rare bacterial infection that is associated largely with contact with infected animals or their by-products. In children, as in adults, the most severe anthrax infections occur after the inhalation of Bacillus anthracis spores. In these circumstances, spores entering the child germinate in the lower respiratory tract, and this subsequently results in a toxin-mediated, necrotizing pneumonia that often leads to progressive respiratory failure and death. The potential for using anthrax as a biological weapon to cause respiratory disease has demanded that antimicrobial agents with known in vitro activity against B. anthracis be assessed for their potential to abort the progression of disease after inhalation exposure (6).
Fluoroquinolones, including levofloxacin and ciprofloxacin, have demonstrated consistently good activity against B. anthracis, and both have been considered an alternative for the treatment of adults after inhalation exposure. Based on assessing the exposure required to be effective in preventing the progression of disease after anthrax inhalation in animal models and the pharmacodynamics of these drugs, ciprofloxacin and, subsequently, levofloxacin were approved for use in humans to treat anthrax inhalation exposure (Cipro label [http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20780S08lbl.pdf] and Levaquin label [http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf]). Although it is generally accepted that fluoroquinolones should not be used in children, largely because these drugs have been shown to cause lesions in the cartilage of juvenile animals (10), the benefits of using fluoroquinolones as a potential life-saving therapy for children following anthrax inhalation has been determined to outweigh these risks. This benefit may be substantially greater when infection due to beta-lactamase-producing anthrax, which are resistant to penicillin-G or ampicillin, is not known. With infections suspected of being associated with bioterrorist attacks, it is generally recommended not to assume susceptibility to beta-lactam agents. For this reason, three agents (ciprofloxacin, levofloxacin, and doxycycline) that are not routinely used as first-line therapies in children have been recommended as empirical therapy for treating children with bioterrorism-associated infections (5).
The objective of this paper is to describe the approach for developing an optimized pediatric dosing regimen of levofloxacin for this indication by pharmacometric analyses. The recommended dosing regimen was calculated based on the pharmacokinetics of levofloxacin in children ranging from 6 months to 17 years old. Furthermore, we assessed pediatric dosing that would best approximate the exposures associated with the approved dosing regimen (500 mg once a day [q.d.]) for this indication in adults.
MATERIALS AND METHODS
Data.
Levofloxacin pharmacokinetic (PK) and demographic data from five phase I studies (three with children and two with adults) were used for pharmacometric analyses and dose determination.
Pediatric studies.
Study I was an open-label, single-intravenous (i.v.)-dose (7 mg/kg of body weight) study to evaluate the safety and PK of levofloxacin in children aged 6 months to 12 years. Each subject received a single i.v. levofloxacin dose of 7 mg/kg (not to exceed 500 mg) at a constant infusion rate during a 1-h period. Pharmacokinetic profiles were evaluated in 19 subjects (10 male [M], 9 female [F]) with sufficient PK samples.
Study II was an open-label, single-dose study to evaluate the safety and PK of levofloxacin in children aged 8 to 16 years. Subjects were enrolled into one of four cohorts, which were stratified by age (12 to 16 years inclusive in cohorts 1 and 2, 8 to 11 years inclusive in cohort 3, and 10 to 12 years in cohort 4). Subjects in cohorts 1, 3, and 4 were administered a single i.v. dose of levofloxacin at 7 mg/kg (not to exceed 500 mg) at a constant infusion rate during a 60-min period. Subjects in cohort 2 were administered a single oral dose of levofloxacin as a 250-mg tablet (not to exceed 7 mg/kg). A total of 31 subjects (12 M, 19 F) were included in the study.
Study III was an open-label, single-dose study to evaluate the safety and PK of the levofloxacin oral suspension formulation in children aged 6 months to 16 years. Levofloxacin was administered as a single oral 7 mg/kg dose (not to exceed 500 mg) using a 50 mg/ml suspension formulation. A total of 40 subjects (17 M, 23 F) were included for PK analysis.
Adult studies.
Study IV analyzed the bioavailability of levofloxacin from a 500-mg clinical tablet, a 500-mg tablet, and a 500-mg i.v. solution, each administered as a single 500-mg dose in the fasted state to healthy male subjects. Twenty-four subjects were enrolled, and 23 were included in the PK analysis.
Study V was an open-label, single-center, randomized, complete three-way crossover study. The bioavailability of levofloxacin was assessed comparatively from an oral dose of one 750-mg tablet, an oral dose of one 250-mg tablet, one 500-mg tablet, and a 750-mg i.v. dose of solution for injection, each administered as a single 750-mg dose in the fasted state to healthy subjects. Twenty-four healthy subjects (21 M, 3 F) were enrolled in the study.
Methods.
The PK of levofloxacin in adults is linear for doses ranging from 50 to 1,500 mg (Levaquin label [http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf]). The absolute oral bioavailability of levofloxacin tablets is approximately 99%, suggesting no difference between i.v. and oral doses in PK properties. The mean volume of distribution (V) of levofloxacin ranges between 74 and 112 liters, indicating widespread distribution into body tissues.
Levofloxacin undergoes limited metabolism, and the major route of clearance (CL) is renal excretion, where 87% of the dose is excreted unchanged in urine within 48 h after dosing (Levaquin label [http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf]). Therefore, the elimination of levofloxacin is dependent on the renal function. According to the theory of development pharmacology (9) and previous experience (11), the clearance of such drugs in children is correlated with body weight as well as age for younger patients due to immature renal function.
Total drug in plasma was determined by high-performance liquid chromatography methods. Adult CL and V were calculated from the concentration-time profiles by employing noncompartment methods (WinNolin 5.1; Pharsight, Mountain View, CA). Pediatric CL and V were obtained from the PK study reports submitted by Johnson and Johnson. The relationships between levofloxacin PK parameters (CL and V) and demographic covariates (body weight and age) were explored using the nonlinear regression procedure Proc NLIN in SAS 9.1.
RESULTS
Clearance models.
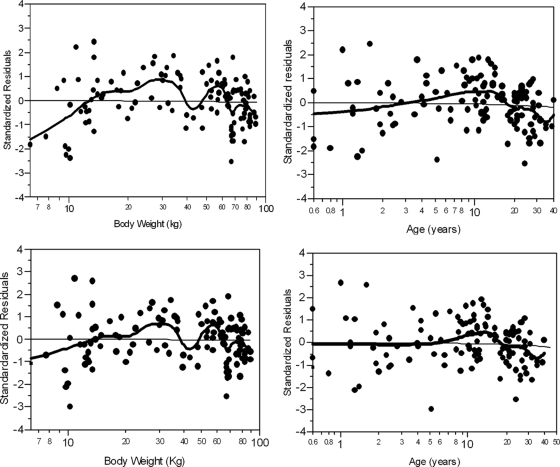
Two clearance models were explored: an allometric model using body weight (WT) as a covariate (model 1 in Table 1), and an allometric model with body weight together with an age factor to account for renal maturation (model 2 in Table 1). The residual plots for model 1 (Fig. 1, top) exhibited a clear negative trend at low body weights, suggesting the influence of other covariates on CL.
TABLE 1.
Tested clearance models
| Model | Description | Parameter | Estimate (SE) | Between subject variability (% coefficient of variance) | Residual sum of squares |
|---|---|---|---|---|---|
| 1 | CL = α·WTβ·exp(η) | α | 1.01 (0.12) ml/min/kg | 26.6 | 8.52 |
| β | 0.53 (0.03) | ||||
| 2 | CL = α·WTβ·[Age/(Age+A50)]·exp(η) | α | 1.50 (0.34) ml/min/kg | 26.2 | 8.17 |
| β | 0.43 (0.06) | ||||
| A50 | 0.32 (0.18) yr |
FIG. 1.
Standardized residual error for clearance model 1 (top) and model 2 (bottom) versus body weight (left) and age (right). The solid black line is a local smoothing line, and the solid black dots are the standardized residuals (raw residuals divided by the corresponding standard errors) for each individual.
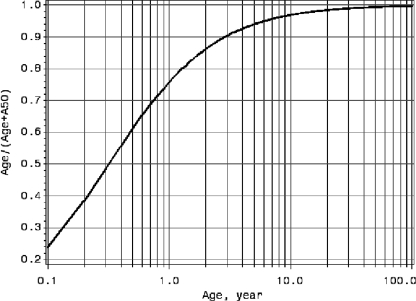
Developmental pharmacology suggests that the renal function of children is not fully developed until 2 to 3 years of age (9). Therefore, a renal function maturation factor, age/(age + A50), where A50 is the age required for achieving 50% of full renal maturation, was added to the allometric clearance model (model 2 in Table 1) to account for such effects. The A50 estimate using the levofloxacin data was 0.32 years (∼4 months). The renal function maturation plot in Fig. 2 suggests that the renal function is 75% matured for a 1-year-old subject, whereas a 6-month-old subject has 60% matured renal function.
FIG. 2.
Estimated renal function maturation curve versus age.
As demonstrated in Fig. 1 (bottom), after including both weight and age in the clearance model (model 2 in Table 1), there was no apparent trend in the residuals. This model then was chosen as the final model based on the visual inspection of residual plots and physiological knowledge. The model-predicted clearance shows good agreement with the calculated clearance from noncompartment analysis.
The volume-of-distribution model also was explored. The relationship between V and body weight was described adequately using an allometric model (data not shown). The value of V was used to calculate pediatric and adult maximum concentrations of drug in serum (Cmax), a target to match for pediatric dosing.
Pediatric dose selection.
The final clearance model was employed to determine the pediatric dosing regimen aimed at matching adult exposure (the area under the time-concentration curve [AUC]) and keeping the Cmax under that observed in adults. As indicated in the Levaquin package insert (http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf), the steady-state levofloxacin AUC from 0 to 24 h (AUC0-24,ss) (means ± standard deviations) after multiple i.v. dosings of 500 mg q.d. is 54.6 ± 11.1 μg·h/ml. To match pediatric exposure to adult exposure, the proposed pediatric dosing was calculated using the following formula:
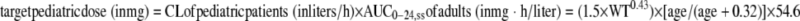 |
(1) |
When the age is >2 years, the renal function is close to being fully developed and the renal maturation factor [age/(age + 0.32)] approaches 1. Equation 1 then can be simplified to the following:
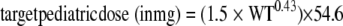 |
(2) |
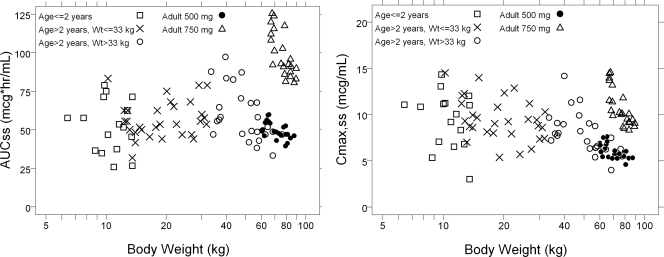
Based on equation 2, a dose of 15 mg/kg q.d. to a maximum dose of 500 mg q.d. (the adult dose) for children >2 years old was identified as best matching the total exposure achieved in adults given a 500-mg dose. With this dose of 15 mg/kg q.d., children weighing more than 33 kg will be given a dose of 500 mg. For children from 6 months to 2 years of age, a dose of 15 mg/kg q.d. was predicted to result in considerably (∼40%) higher exposure than that achieved in children older than 2 years because of the effect of maturing renal function. However, 15 mg/kg q.d. still was considered adequate for this group of pediatric patients, because the total exposures fell within what has been observed in adults, and the risk of higher exposure was considered to be outweighed by the benefit of treatment for children exposed to anthrax. The predicted steady-state exposure (AUC0-24,ss = dose/CL) and the Cmax at steady state {Cmax,ss = dose/[V(1 − e−kt)]}, where k is the elimination rate constant and t is time, is demonstrated in Fig. 3.
FIG. 3.
Predicted AUC0-24,ss (left) and Cmax,ss (right) following 15 mg/kg q.d. levofloxacin (not exceeding 500 mg) versus body weight.
The predicted pediatric AUC0-24,ss following 15 mg/kg q.d. was found to match that of the observed values in adults receiving 500 mg q.d. in all age groups (Fig. 3, left). However, the Cmax,ss for children following 15 mg/kg q.d. was found to be substantially higher than that observed in adults receiving 500 mg and was more in the range of that observed in adults receiving 750 mg (Fig. 3, right).
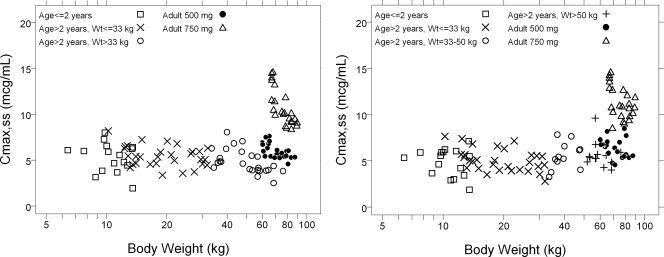
With the goal of aligning the Cmax,ss for children with that observed with the 500 mg dose in adults, a revised dose of 7.5 mg/kg twice a day (b.i.d.) was assessed. A plot of Cmax,ss by age for this dosing regimen was shown to best approximate the Cmax,ss observed in adults given the 500-mg dose (Fig. 4, left). In addition, for children who were >50 kg, it was shown that 500 mg q.d. (i.e., the adult dosing regimen) was acceptable, because it approximates the Cmax,ss achieved in adults following 500 mg q.d. (Fig. 4, right).
FIG. 4.
Predicted Cmax,ss versus body weight following 7.5 mg/kg b.i.d. levofloxacin (not exceeding 250 mg/dose) for all children (left) as well as 7.5 mg/kg b.i.d. levofloxacin (not exceeding 250 mg/dose) for children <50 kg and 500 mg q.d. for children ≥50 kg (right).
The assessment of the minimum concentration of drug in serum at steady state (Cmin,ss) associated with the 7.5-mg/kg b.i.d. regimen indicated that this regimen would achieve Cmin,ss values at least as high as, and in many instance substantially higher than, Cmin,ss values observed in adults following 500 mg q.d.
The predicted AUC0-24,ss, Cmax,ss, and Cmin,ss for children following 7.5 mg/kg b.i.d. are summarized in Table 2. Across all age groups, the AUC0-24,ss and Cmax,ss match those observed in adults after multiple doses of 500 mg q.d., while the Cmin,ss values for children are higher than those for adults.
TABLE 2.
Comparison of predicted AUC0-24,ss, Cmax,ss, and Cmin,ss from children following dosing regimen of 7.5 mg/kg b.i.d.a
| Age | PK parameter |
||
|---|---|---|---|
| AUC0-24,ss (μg.h.ml) | Cmax,ss (μg/ml) | Cmin,ss (μg/ml) | |
| 6 mo to < 2 yr | 51.7 (26.8-75) | 5.6 (3.2-7.3) | 0.6 (0.26-1.2) |
| 2 to <5 yr | 50 (41.7-65.2) | 5.4 (4.2-6.6) | 0.6 (0.25-1.1) |
| 5 to <10 yr | 55.6 (46.9-83.3) | 5.4 (3.7-7.1) | 0.9 (0.38-1.6) |
| 10 to 18 yr | 55.7 (42.0-83.5) | 6.3 (4.6-8.1) | 0.6 (0.2-1.4) |
| Adultb | 47.7 (41.8-55.1) | 5.5 (5.0-6.8) | 0.4 (0.3-0.55) |
Data are presented as the median (10th percentile to 90th percentile). The dosage regimen was 7.5 mg/kg b.i.d (not exceeding 250 mg/dose), 500 mg q.d. for children more than 50 kg.
The adult dose of 500 mg q.d was based on observed data from study IV.
As a final consideration in recommending a dose for children, the 7.5-mg/kg dose that was identified by modeling as the optimized dose was rounded up to 8 mg/kg to minimize errors in prescription doses (i.e., mistaking 7.5 mg/kg as 75 mg/kg) and still remained within the observed adult exposure following 500 mg q.d.
DISCUSSION
The dose of levofloxacin recommended for the treatment of children exposed to inhalational anthrax is based on pharmacometric analyses that utilized the pharmacokinetics of levofloxacin across an age range of children, from 6 months to 17 years, and it targeted the exposure based on animal experiments that were shown to prevent the progression of pulmonary anthrax after inhalation exposure (7). Pharmacometric analyses demonstrated that renal function in clearing levofloxacin was not mature until 2 years of age; after that, body weight is the only factor that affects levofloxacin clearance. Given the severity of infection, these analyses were designed to be especially sensitive to meeting the target exposure predicted by animal experiments at the same time that they considered the risks of using doses of levofloxacin that best approximated exposures in adults. Taken together, these considerations have led to conclusions that the recommended dose regimen for children is 8 mg/kg b.i.d. for children <50 kg and 500 mg q.d. for children weighing ≥50 kg.
The process leading to the recommendation for using levofloxacin in children exposed to levofloxacin aligns with published FDA guidance for industry (4) that provides for the regulatory approval of a drug based on extrapolating the experience with adults to children. Based on the guidance, the FDA can approve a drug for pediatric use based on adequate and well-controlled studies of adults, “provided that FDA is satisfied and concluded that the course of the disease and the effects of the drug, both beneficial and adverse, are sufficiently similar in the pediatric and adult populations to permit extrapolation from the adult efficacy data to the pediatric patients” (4). It is important to recognize that this process was focused on exposure targets that were associated with preventing the progression of inhalation anthrax that were further estimated for adults based on animal model experimentation. Therefore, it would not be appropriate to consider the approach as having identified dose regimens for children outside of those used to treat children exposed to inhalation anthrax. In considering this dose regimen, it was appreciated that the safety and tolerability of an 8-mg/kg b.i.d. regimen for children older than 5 years exceeded the exposure associated with dosing regimens of 10 mg/kg q.d. that were studied in a clinical trial involving children with community-acquired pneumonia (3, 8). Furthermore, there is no prospective clinical trial experience that has defined the safety and tolerability of levofloxacin used in children for more than 14 days.
In conclusion, we have demonstrated how quantitative analysis can be used to determine pediatric dosing that will match adult exposure. More examples that employed pharmacometric analyses in new drug approval and labeling decisions at the U.S. Food and Drug Administration can be found in other publications (1, 2).
Acknowledgments
We thank Joga Gobburu, Director of Division of Pharmacometrics, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, for his coordination and contribution to the preparation of the manuscript.
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Bhattaram, V. A., C. Bonapace, D. M. Chilukuri, J. Z. Duan, C. Garnett, J. V. Gobburu, S. H. Jang, L. Kenna, L. J. Lesko, R. Madabushi, Y. Men, J. R. Powell, W. Qiu, R. P. Ramchandani, C. W. Tornoe, Y. Wang, and J. J. Zheng. 2007. Impact of pharmacometric reviews on new drug approval and labeling decisions—a survey of 31 new drug applications submitted between 2005 and 2006. Clin. Pharmacol. Ther. 81:213-221. [DOI] [PubMed] [Google Scholar]
- 2.Bhattaram, V. A., B. P. Booth, R. P. Ramchandani, B. N. Beasley, Y. Wang, V. Tandon, J. Z. Duan, R. K. Baweja, P. J. Marroum, R. S. Uppoor, N. A. Rahman, C. G. Sahajwalla, J. R. Powell, M. U. Mehta, and J. V. Gobburu. 2005. Impact of pharmacometrics on drug approval and labeling decisions: a survey of 42 new drug applications. AAPS J. 7:E503-E512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley, J. S., A. Arguedas, J. L. Blumer, X. Saez-Llorens, R. Melkote, and G. J. Noel. 2007. Comparative study of levofloxacin in the treatment of children with community-acquired pneumonia. Pediatr. Infect. Dis. J. 26:868-878. [DOI] [PubMed] [Google Scholar]
- 4.Center for Drug Evaluation and Research, United States Food and Drug Administration. Accessed 12 December 2008. Guidance for industry. Exposure-response relationships—study design, data analysis, and regulatory applications. http://www.fda.gov/cder/guidance/5341.fnl.pdf.
- 5.Committee on Infections. 2009. Anthrax, p. 212-213. In Red book. Report of the Committee on Infectious Diseases, 30th ed. American Academy of Pediatrics, Elk Grove Village, IL.
- 6.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 7.Kao, L. M., K. Bush, R. Barnewall, J. Estep, F. W. Thalacker, P. H. Olson, G. L. Drusano, N. Minton, S. Chien, A. Hemeryck, and M. F. Kelley. 2006. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrob. Agents Chemother. 50:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noel, G. J., J. S. Bradley, R. E. Kauffman, C. M. Duffy, P. G. Gerbino, A. Arguedas, P. Bagchi, D. A. Balis, and J. L. Blumer. 2007. Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders. Pediatr. Infect. Dis. J. 26:879-891. [DOI] [PubMed] [Google Scholar]
- 9.Milsap, R. L., M. R. Hill, and S. J. Szefler. 1992. Pharmacokinetic considerations in children. In W. E. Evans, J. J. Schentag, and W. J. Jusk (ed.), Applied pharmacokinetics principles of therapeutic drug monitoring, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 10.Schadd, U. B. 2000. Use of quinolones in pediatrics, p. 455-476. In V. T. Andriole (ed.), The quinolones, 3rd ed. Academic Press, New York, NY.
- 11.Tornøe, C. W., J. J. Tworzyanski, M. A. Imoisili, J. J. Alexander, J. M. Korth-Bradley, and J. V. Gobburu. 2007. Optimising piperacillin/tazobactam dosing in paediatrics. Int. J. Antimicrob. Agents 30:320-324. [DOI] [PubMed] [Google Scholar]