Abstract
Ceftobiprole, a broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus (MRSA) (P. Hebeisen et al., Antimicrob. Agents Chemother. 45:825-836, 2001), was evaluated in a subcutaneous skin infection model with Staphylococcus aureus Smith OC 4172 (methicillin-susceptible S. aureus [MSSA]), S. aureus OC 8525 (MRSA), Pseudomonas aeruginosa OC 4351 (having an inducible AmpC β-lactamase), and P. aeruginosa OC 4354 (overproducing AmpC β-lactamase). In the MSSA and MRSA infection models, ceftobiprole, administered as the prodrug ceftobiprole medocaril, was more effective in reducing CFU/g skin (P < 0.001) than were cefazolin, vancomycin, or linezolid based on the dose-response profiles. Skin lesion volumes in MSSA-infected animals treated with ceftobiprole were 19 to 29% lower than those for cefazolin-, vancomycin-, or linezolid-treated animals (P < 0.001). In MRSA infections, lesion size in ceftobiprole-treated mice was 34% less than that with cefazolin or linezolid treatment (P < 0.001). Against P. aeruginosa, ceftobiprole at similar doses was as effective as meropenem-cilastatin in reductions of CFU/g skin, despite 8- and 32-fold-lower MICs for meropenem; both treatments were more effective than was cefepime (P < 0.001) against the inducible and overproducing AmpC β-lactamase strains of P. aeruginosa. Ceftobiprole was similar to meropenem-cilastatin and 47 to 54% more effective than cefepime (P < 0.01) in reducing the size of the lesion caused by either strain of P. aeruginosa in this study. These studies indicate that ceftobiprole is effective in reducing both bacterial load and lesion volume associated with infections due to MSSA, MRSA, and P. aeruginosa in this murine model of skin and soft tissue infection.
Antimicrobial resistance is becoming more problematic in the health care setting, with infections mediated by drug-resistant pathogens being associated with increased morbidity and mortality and corresponding increased health care costs and longer hospital stays (12). β-Lactams, including cefazolin, have been used for many years to treat methicillin-susceptible Staphylococcus aureus (MSSA) skin and soft tissue infections (9, 52). The rising proportion of methicillin resistance in staphylococcal infections in hospitals and in the community has compromised the effectiveness of this antibiotic class for staphylococci and resulted in the increased use of vancomycin to treat patients with these infections (21, 41). As a consequence, vancomycin-intermediate S. aureus (VISA) strains continue to increase in prevalence, and vancomycin-resistant S. aureus (VRSA) strains have begun to appear (24, 33, 34, 49, 51). Linezolid, an oxazolidinone antibiotic with a gram-positive antibacterial spectrum of activity including methicillin-resistant S. aureus (MRSA) (27), was introduced in 2000 to address the growing problem of MRSA, but safety concerns have limited its utility (4, 29).
Prior to the advent of methicillin resistance (and therein resistance to all approved β-lactams), cephalosporins were considered the drugs of choice to treat staphylococcal infections (45). Ceftobiprole is a new cephalosporin that, unlike currently marketed β-lactams, has high binding affinity for penicillin-binding protein 2a (PBP2a) (19), resulting in potent in vitro and in vivo activity against MRSA. The prodrug form of this agent, ceftobiprole medocaril, has completed two phase III complicated skin and skin structure infection (cSSSI) clinical trials in which efficacy was demonstrated against a broad spectrum of bacteria, including MRSA (1, 44).
Antimicrobial resistance in gram-negative organisms is also a serious problem that has limited the number of effective antimicrobial agents available to physicians to treat infection in the hospital setting. While skin infections with Pseudomonas aeruginosa are found with less frequency (6.2%) than are staphylococcal skin infections (19.1%), they remain a common cause of morbidity in surgical site and burn infections (2) and with immunocompromised patients. (26, 52). Antipseudomonal cephalosporins are frequently used for the treatment of P. aeruginosa; however, the overproduction of AmpC β-lactamases reduces the antibiotic choices available for treatment (40).
Ceftobiprole binds tightly to PBP3 in P. aeruginosa, as do other cephalosporins (20), and consequently has also been shown to have in vitro and in vivo activity against this bacterium. MICs for ceftobiprole against recent clinical isolates are similar to those of cefepime and meropenem (10, 25). The in vivo antipseudomonal activity of ceftobiprole has previously been characterized in the mouse septicemia and the neutropenic thigh infection models (23, 30, 32), but the effect of AmpC β-lactamases on in vivo activity has not been reported.
In this study, ceftobiprole medocaril was evaluated in a murine skin and soft tissue infection model utilizing methicillin-susceptible and -resistant strains of S. aureus and strains of P. aeruginosa that express various levels of AmpC. In these models, the broad-spectrum (gram-positive and gram-negative) activity of ceftobiprole was demonstrated against these important skin pathogens.
MATERIALS AND METHODS
Bacterial strains and in vitro susceptibility testing.
S. aureus OC 4172 was obtained from the American Type Culture Collection (ATCC) as Staphylococcus aureus subsp. aureus ATCC 13709. MRSA strain OC 8525, a community-acquired MRSA strain, was obtained from Barry Kreiswirth of the Public Health Research Institute, Newark, NJ. P. aeruginosa OC 4351 and OC 4354, an AmpC-derepressed mutant of OC 4351, were obtained from SynPhar Laboratories, Inc. (Edmonton, Alberta, Canada). OC 4354 was produced by the serial passage of OC 4351 (parent) on plates with increasing concentrations of ceftazidime. This selection resulted in a strain with a 128-fold ceftazidime MIC increase (to 128 μg/ml) and a 350-fold increase in AmpC production as measured spectrophotometrically by nitrocefin hydrolysis (48). Broth microdilution MIC determinations were performed according to CLSI guidelines (14).
Antimicrobial agents.
Ceftobiprole medocaril was obtained from Basilea Pharmaceutica (Basel, Switzerland). Cefazolin was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Cefepime, meropenem, and cilastatin were obtained from USP (Rockville, MD). Meropenem was combined with cilastatin due to meropenem's instability to murine renal DHP-1 (35). Vancomycin was purchased from MP Biomedicals, Inc. (Irvine, CA), and linezolid was obtained from Pfizer (New York, NY).
Dextrin microcarrier bead preparation.
Stock suspensions (2%, wt/vol) of Cytodex 1 microcarrier dextrin beads, 131 to 220 μm (Sigma-Aldrich Chemical Company, St. Louis, MO), were prepared in 0.85% phosphate-buffered saline (PBS) and sterilized by autoclaving.
Inoculum preparation.
Overnight cultures of S. aureus Smith OC 4172 (MSSA), S. aureus OC 8525 (MRSA), P. aeruginosa OC 4351, or P. aeruginosa OC 4354 were inoculated from frozen glycerol stocks and shaken for 18 h at 37°C. To prepare the Cytodex bead inoculum, the overnight culture was centrifuged (10 min, 3,450 × g), and the cells were washed with 0.5 volume of saline. The cells were recentrifuged, and the pellet was resuspended to half the original culture volume in brain heart infusion (BHI). A 1:200 dilution was made in BHI, and the Cytodex 1 bead suspension was added to a final concentration of 0.1% (vol/vol). This method resulted in a range of inocula of 6.7 to 7.0 log10 CFU/mouse for S. aureus and 5.9 to 6.8 log10 CFU/mouse for P. aeruginosa.
Pharmacokinetic studies.
To study the plasma and skin exposures of ceftobiprole and its comparators, female Crl:SKH1-hrBr hairless, immunocompetent or immunocompromised mice (Charles River Laboratories, Wilmington, MA) were anesthetized with 3% isoflurane (Abbott, Chicago, IL). Studies with S. aureus strains were performed with immunocompetent mice. Studies with P. aeruginosa were performed in mice rendered neutropenic using a modified method described by Zuluaga and colleagues (56) by administering cyclophosphamide monohydrate (Sigma-Aldrich Chemical Company, St. Louis, MO) intraperitoneally 5 days (at a dose of 150 mg/kg of body weight) and 1 day (at a dose of 100 mg/kg) prior to the initiation of the infection. The animals were then given an 0.2-ml subcutaneous (s.c.) injection of the Cytodex bead suspension containing MRSA strain OC 8525 or P. aeruginosa OC 4354 on the left flank. On the right flank, the animals were given 0.2 ml of sterile Cytodex bead suspension in BHI. After 24 h, the animals were treated subcutaneously at the nape of the neck with 18.2 or 73 mg/kg of ceftobiprole medocaril (equivalent to 12.5 and 50 mg/kg, respectively, of ceftobiprole); 12.5 or 50 mg/kg of meropenem-cilastatin (individual compound dose, 1:1 [wt/wt] ratio of meropenem and cilastatin); 50 mg/kg of cefazolin, vancomycin, and cefepime; or 50 mg/kg orally with linezolid. At selected time points the animals were euthanized by CO2 asphyxiation and blood was collected by cardiac puncture into Microtainer plasma separator tubes with lithium heparin (Becton Dickinson and Company, Franklin Lakes, NJ). The blood was centrifuged immediately, and the plasma was removed and stored on ice for no longer than 1 hour before analysis. Following blood collection, noninfected and infected skin samples were excised, weighed, and homogenized in 1 ml dimethyl sulfoxide (DMSO; 35,000 rpm for 0.5 min; Omni TH tissue homogenizer and Omni Tip disposable rotor stator generator probes; Omni International, Marietta, GA). Skin homogenates were centrifuged and analyzed by high-performance liquid chromatography (HPLC). Each experiment was performed twice for a total of at least 8 animals/time point. All animal studies were reviewed and approved by the Johnson & Johnson Pharmaceutical Research and Development Institutional Animal Care and Usage Committee.
Concentrations of compounds in plasma and skin extracts were determined by HPLC methodology using an Agilent 1100 HPLC system with diode array detection. To remove plasma proteins from samples prior to analysis, (i) plasma samples containing ceftobiprole or cefazolin were diluted 20% (vol/vol) with acetonitrile and 20% (vol/vol) with a solution of 50% (wt/vol) trichloroacetic acid and centrifuged, (ii) plasma samples containing cefepime were diluted 35% (vol/vol) with acetonitrile and 5% (vol/vol) with a solution of 50% (wt/vol) trichloroacetic acid and centrifuged, and (iii) plasma samples containing vancomycin and linezolid were diluted with an equal volume of acetonitrile and centrifuged. Supernatants from extracts of skin samples were analyzed by HPLC without further processing. The limits of detection were 0.2 μg/ml for cefazolin and 0.1 μg/ml for ceftobiprole, linezolid, meropenem, cefepime, and vancomycin. The pharmacokinetic parameters (area under the concentration curve from time zero to infinity [AUC0-∞], elimination half-life [t1/2], average maximum concentration [Cmax], and the time to maximum concentration [Tmax]) were calculated from the exposure curves using a noncompartmental model in WinNonlin version 4.0.1 software (Pharsight Corporation, Mountain View, CA). In separate studies evaluating the capacity of the Cytodex beads to sequester the antibiotics in mouse plasma, no measurable binding of any of the antibiotics to the beads could be detected (data not shown), indicating that the beads are not likely to impact the localized concentration of antibiotic in the lesion.
Efficacy studies.
Efficacy was evaluated in a skin infection model with Cytodex beads as characterized by Bunce et al. (11). To study the effect of ceftobiprole, cefazolin, vancomycin, or linezolid on S. aureus infections, female SKH1 mice were anesthetized and given an 0.2-ml s.c. injection of the Cytodex bead inoculum containing S. aureus Smith OC 4172 on the left flank. On the right flank, the animals were given 0.2 ml of Cytodex bead suspension containing MRSA strain OC 8525. The animals received ceftobiprole medocaril, cefazolin, vancomycin, or linezolid 1, 3, 25, and 27 h postinfection at a dose of 1.6, 6.2, 25, or 100 mg/kg/day. All drugs were delivered subcutaneously except for linezolid, which was given orally because of the equivalent bioavailability of linezolid (subcutaneous versus oral) against MSSA and MRSA in a mouse septicemia model (31).
To study the effect of ceftobiprole, cefepime, and meropenem-cilastatin against pseudomonal skin infections, immunocompromised female SKH1 mice were anesthetized and each mouse was infected with a Cytodex bead suspension containing P. aeruginosa OC 4351 and OC 4354, on the left and right flanks, respectively. The animals received indicated doses of freshly prepared ceftobiprole medocaril, cefepime, or meropenem-cilastatin (1:1 [wt/wt] ratio) subcutaneously at 1, 3, 25, and 27 h postinfection.
The animals were euthanized by CO2 asphyxiation 48 h after infection, and the lesions on each flank were measured with electronic calipers (Mitutoyo Corporation, Aurora, IL). Evaluation of treatment effect on lesion size was not blinded. A lesion volume score was calculated from the following equation: LV = (π/6)(L × W2), where LV = lesion volume, L = length of the lesion in mm, and W = width of the lesion in mm (11). For determination of CFU/g skin, the skin from the infected areas was disinfected with chlorhexidine diacetate (Nolvasan; Fort Dodge Animal Health, Fort Dodge, IA), excised, weighed, and homogenized in 1 ml of saline (4°C, 35,000 rpm for 0.5 min). Serial 100-fold dilutions in saline (0.85% saline; Remel, Lenexa, KS) were plated (Autoplate 4000; Spiral Biotech, Inc., Norwood, MA) on tryptic soy agar (TSA) plates. The plates were incubated for 18 h at 37°C and analyzed (Q-Count; Spiral Biotech, Inc., Norwood, MA) for the number of CFU. Each experiment, consisting of 5 animals/dose group, was performed twice, and results were combined for analysis.
Statistical analysis.
A test for a dose-response trend was performed using a Cochran-Armitage test for each bacterial strain and lesion size separately (53). This was followed by a comparison of the treatment dose-response profiles using the analysis of covariance (43). This analysis was performed on the logarithmic-transformed data and restricted to the region of dose response that supported parallelism as identified by preliminary exploratory modeling. The corresponding log-linear model intercepts were then tested for a significant difference using a linear contrast (43) in the analysis of covariance model, which contained terms for treatment and dose means.
RESULTS
In vitro susceptibility.
The MIC for ceftobiprole against S. aureus Smith OC 4172 was 0.25 μg/ml, twofold more potent than cefazolin and four and eight times more potent than vancomycin and linezolid, respectively (Table 1). Against MRSA strain OC 8525, the ceftobiprole MIC was fourfold higher than its MIC observed with the MSSA strain; as expected, the MIC of cefazolin against the MRSA strain increased 32-fold. The MICs of vancomycin and linezolid against the MRSA strain compared to MSSA were equivalent and twofold higher, respectively.
TABLE 1.
In vitro susceptibilities
| Agent | MIC (μg/ml) against strain |
|||
|---|---|---|---|---|
|
S. aureus |
P. aeruginosa |
|||
| OC 4172 (MSSA) | OC 8525 (MRSA) | OC 4351 | OC 4354a | |
| Ceftobiprole | 0.25 | 1 | 4 | 4 |
| Cefazolin | 0.5 | 16 | NTb | NT |
| Vancomycin | 1 | 1 | NT | NT |
| Linezolid | 2 | 4 | NT | NT |
| Meropenem | NT | NT | 0.12 | 0.5 |
| Cefepime | NT | NT | 4 | 32 |
AmpC-overproducing strain.
NT, not tested.
Against P. aeruginosa the MICs of ceftobiprole were equivalent for the wild-type (OC 4351) strain and the mutant (OC 4354) of the wild type overproducing AmpC. Notably, meropenem and cefepime MICs increased four and eight times, respectively, for OC 4354 compared to OC 4351.
Pharmacokinetic studies.
The free-drug pharmacokinetic parameters of ceftobiprole and comparators in immunocompetent mice with S. aureus skin infections are summarized in Table 2. In plasma, at ceftobiprole s.c. doses of 12.5 or 50 mg/kg, free-drug exposure levels were roughly proportional to the dose, with fCmaxs of 17 and 66 μg/ml and fAUC values of 481 and 2,336 min·μg/ml, respectively. The fCmaxs at these doses were approximately 17- and 66-fold higher, respectively, than the ceftobiprole MIC for the MRSA strain. With immunocompetent mice, the t1/2 values were similar in plasma and in skin (infected and uninfected).
TABLE 2.
Free-drug exposures of ceftobiprole, cefazolin, linezolid, and vancomycin in plasma and noninfected and S. aureus-infected skin of immunocompetent SKH1 mice
| Drug | Dose (mg/kg) | Route of administration | Source | t1/2 (min) | Tmax (min) | fCmax (μg/ml or g)a,b | fAUC0-∞ (min·μg/ml or g)a,c |
|---|---|---|---|---|---|---|---|
| Ceftobiprole | 12.5d | s.c. | Plasma | 17 | 7.5 | 17 | 481 |
| 50e | s.c. | Plasma | 13 | 7.5 | 66 | 2,336 | |
| 50e | s.c. | Uninfected skin | 19 | 15 | 17 | 668 | |
| 50e | s.c. | Infected skin | 15 | 15 | 13 | 652 | |
| Cefazolin | 50 | s.c. | Plasma | 14 | 7.5 | 19 | 565 |
| Linezolid | 50 | p.o.f | Plasma | 102 | 30 | 14 | 1,795 |
| Vancomycin | 50 | s.c. | Plasma | 22 | 10 | 40 | 2,070 |
Corrected for protein binding in mouse plasma: 16% ceftobiprole (data on file), 81% cefazolin, 32% linezolid, and 25% vancomycin (28, 37, 54).
Micrograms per milliliter for plasma and micrograms per gram for skin.
Minutes per microgram per milliliter for plasma and minutes per microgram per gram for skin.
18.2 mg/kg of ceftobiprole medocaril (prodrug) equals 12.5 mg/kg of ceftobiprole (parent).
73 mg/kg of ceftobiprole medocaril (prodrug) equals 50 mg/kg of ceftobiprole (parent).
p.o., oral.
At the 50-mg/kg dose, the fCmax of cefazolin (19 μg/ml) was 38-fold higher than its MIC for the MSSA strain but similar to the MRSA MIC. In contrast, in animals treated with ceftobiprole at the same dose, the plasma fCmaxs were 264 and 66 times higher than the MIC of ceftobiprole to the MSSA and the MRSA strains, respectively. The fCmaxs for all drugs exceeded the MICs at least 3.5-fold against both staphylococcus strains used, except for cefazolin with MRSA. This latter result is consistent with cefazolin's poor activity against mecA-expressing S. aureus (MRSA) (8). At comparable doses, fAUC values for cefazolin were approximately fourfold lower than those for ceftobiprole, whereas fAUC values for linezolid and vancomycin were similar. The t1/2s of ceftobiprole and cefazolin were similar, while vancomycin and linezolid t1/2 values were 1.7- and 7.8-fold longer, respectively, than that of ceftobiprole.
The pharmacokinetic properties of ceftobiprole and comparators in immunocompromised mice used in the P. aeruginosa skin infection model are summarized in Table 3. All three drugs displayed similar free-drug plasma exposures (fCmax, fAUC) following a single 50-mg/kg subcutaneous dose. The plasma fCmaxs of ceftobiprole, meropenem, and cefepime were 69, 72, and 66 μg/ml, respectively, which were 16.5 to 18 times higher than the MIC for P. aeruginosa used in this study. While the t1/2s in plasma with ceftobiprole were similar in immunocompetent and in immunocompromised mice (∼15 min), the t1/2s in the uninfected and infected skin were two to six times longer in immunocompromised mice. The t1/2s of meropenem and cefepime were slightly shorter (9 min) than that of ceftobiprole in immunocompromised mice.
TABLE 3.
Free-drug exposures of ceftobiprole, meropenem, and cefepime in plasma and noninfected and P. aeruginosa-infected skin of immunocompromised SKH1 mice following a single subcutaneous dose
| Drug | Dose (mg/kg) | Source | t1/2 (min) | Tmax (min) | fCmax (μg/ml or g)a,b | fAUC0-∞ (min·μg/ml or g)a,c |
|---|---|---|---|---|---|---|
| Ceftobiprole | 50d | Plasma | 13 | 7.5 | 69 | 2,011 |
| 50d | Uninfected skin | 30 | 15 | 17 | 690 | |
| 50d | Infected skin | 76 | 15 | 9 | 704 | |
| Meropenem | 12.5 | Plasma | 13.6 | 7.5 | 11 | 268 |
| 50 | Plasma | 8.6 | 7.5 | 72 | 1,837 | |
| Cefepime | 50 | Plasma | 9 | 7.5 | 66 | 1,790 |
Corrected for protein binding in mouse plasma: 16% ceftobiprole (data on file), 19% meropenem, and 20% cefepime (35, 38).
Micrograms per milliliter for plasma and micrograms per gram for skin.
Minutes per microgram per milliliter for plasma and minutes per microgram per gram for skin.
73 mg/kg of ceftobiprole medocaril (prodrug) equals 50 mg/kg of ceftobiprole (parent).
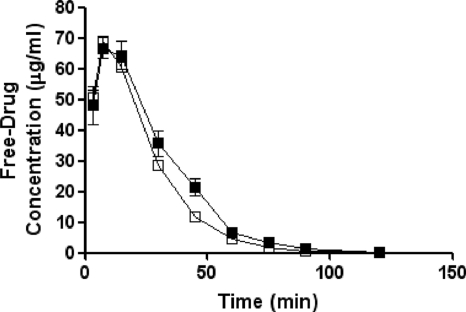
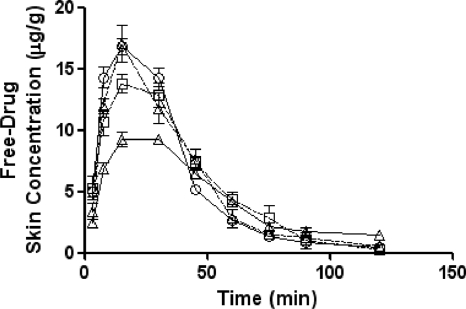
The free-drug pharmacokinetics of ceftobiprole in the plasma and skin of both normal and immunocompromised mice are shown in Fig. 1 and 2, respectively.
FIG. 1.
Free-drug plasma pharmacokinetics of ceftobiprole in normal (solid squares) and immunocompromised (open squares) SKH1 mice following a single 73-mg/kg subcutaneous dose. Each symbol represents the mean ± standard error for 8 mice.
FIG. 2.
Free-drug pharmacokinetics of ceftobiprole in the skin of infected (open squares) or uninfected (open triangles, dashed lines) normal SKH1 mice and infected (open triangles, solid lines) or uninfected (open circles) immunocompromised SKH1 mice following a single 73-mg/kg subcutaneous dose. Each symbol represents the mean ± standard error for 8 mice.
Efficacy studies.
The efficacies of ceftobiprole, cefazolin, vancomycin, and linezolid against S. aureus Smith OC 4172 (MSSA) are summarized in Table 4 and Fig. 3. In untreated control animals, S. aureus Smith OC 4172 grew 1.6 log10 CFU beyond the inoculum level, to a maximum value of 7.9 log10 CFU/g skin in 48 h. In contrast, treatment of mice with ceftobiprole at doses of 1.6 to 100 mg/kg/day resulted in decreases of 1.4 to 2.4 log10 CFU below the initial inoculum for every dose tested and achieved maximal killing with the 25-mg/kg/day dose. With cefazolin, slight growth over 48 h was observed at the 1.6-mg/kg dose, with CFU increasing to 7.3 log10 CFU/g skin. At cefazolin doses of 6.2 to 100 mg/kg/day, dose-dependent reduction in CFU was observed, with maximal killing occurring at 25 mg/kg/day. Treatment with lower doses (1.6 and 6.2 mg/kg/day) of cefazolin resulted in 2.0 and 1.2 log10 more CFU, respectively, than those in ceftobiprole-treated animals. The two higher doses (25 and 100 mg/kg/day) of each agent were similar with respect to their abilities to reduce bacterial load. Treatment with vancomycin at doses of 1.6 and 6.2 mg/kg/day resulted in increases of 1.7 and 1.8 log10 CFU/g skin, respectively, over the inoculum level after 48 h; stasis was achieved at 25 mg/kg/day. Only at 100 mg/kg/day did the skin titers of S. aureus Smith decrease, with 3.1 log10 fewer CFU/g skin at 48 h, the largest decrease in CFU observed for all the agents tested. Linezolid treatment resulted in growth of OC 4172 above the inoculum level at doses of 1.6 and 6.2 mg/kg/day, with growth at the lowest linezolid dose approximating that for the untreated control. At linezolid doses of 25 and 100 mg/kg/day, CFU of S. aureus in the skin remained proximal to the inoculum level. At their respective maximal effective doses (ceftobiprole, 25 mg/kg/day; linezolid, 100 mg/kg/day), ceftobiprole achieved a 1.6-log10 CFU-greater reduction of S. aureus Smith OC 4172 CFU than did linezolid. Overall, the ceftobiprole dose-response profile with S. aureus Smith OC 4172 indicated significantly greater reductions (P < 0.001) of bacterial cells than did those of the other treatment regimens.
TABLE 4.
Effect of treatment (Δlog CFU/g skin [from infecting inoculum]) with ceftobiprole, cefazolin, vancomycin, or linezolid on CFU of S. aureus in skina
| Dose (mg/kg/day) | ΔLog CFU of strain/g skin (from infecting inoculum) with agent |
|||||||
|---|---|---|---|---|---|---|---|---|
| MSSA |
MRSA |
|||||||
| BPRb | CFZ | VAN | LZD | BPRc | CFZ | VAN | LZD | |
| 0 | 1.6 | 1.6 | 1.6 | 1.6 | 0.8 | 0.8 | 0.8 | 0.8 |
| 1.6 | −1.0 | 0.9 | 1.5 | 1.4 | 0.3 | 0.8 | 1.5 | 0.4 |
| 6.2 | −1.7 | −0.6 | 1.6 | 0.6 | −0.8 | 0.6 | 1.2 | 0 |
| 25 | −2.0 | −1.7 | 0.2 | 0.1 | −1.3 | −0.3 | −0.7 | −0.4 |
| 100 | −1.7 | −1.9 | −3.1 | −0.4 | −1.4 | −0.9 | −1.4 | −0.4 |
Abbreviations: BPR, ceftobiprole; CFZ, cefazolin; VAN, vancomycin; LZD, linezolid. MICs (μg/ml) of the drugs against MSSA and MRSA were as follows: ceftobiprole, 0.25 and 1; cefazolin, 0.5 and 16; vancomycin, 1 and 1; and linezolid, 2 and 4, respectively.
P < 0.038 (versus other treatment regimens [Cochran-Mantel-Haenszel test]).
P < 0.061 (versus other treatment regimens [Cochran-Mantel-Haenszel test]).
FIG. 3.
The effect of ceftobiprole (solid squares), cefazolin (open squares), vancomycin (solid triangles), or linezolid (open triangles) on S. aureus Smith OC 4172 in a murine skin infection model. Each symbol represents the mean ± standard error for 10 mice. The starting inoculum was 6.3 log10 CFU/mouse, and the untreated control value (48 h) was 7.9 log10 CFU/g skin tissue. P was <0.001 for ceftobiprole versus other treatment regimens (Cochran-Armitage test).
The efficacies of ceftobiprole, cefazolin, vancomycin, and linezolid against MRSA strain OC 8525 are summarized in Table 4 and Fig. 4. In control animals, the bacteria grew 0.8 log10 CFU from the initial inoculum to 7.2 log10 CFU/g skin tissue over the 48-h treatment period. In animals treated with 1.6 to 100 mg/kg/day of ceftobiprole, skin levels of MRSA strain OC 8525 decreased 0.8 to 1.4 log10 CFU/g skin tissue (doses from 6.2 to 100 mg/kg/day) below that of the starting inoculum. Maximal killing with ceftobiprole was achieved at doses of 25 and 100 mg/kg/day. In contrast, cefazolin at the same doses had 1 and 0.4 log10 more CFU than did ceftobiprole in skin, achieving a maximal effective dose at 100 mg/kg/day. Animals treated with vancomycin at 100 mg/kg/day demonstrated decreases in MRSA skin levels similar to those of animals treated with ceftobiprole at this dose; however, lower doses (1.6 to 25 mg/kg/day) resulted in 0.5 to 1.9 log10 more CFU/g skin tissue than did ceftobiprole. Treatment of animals with linezolid had a static effect on MRSA growth, with the colony counts similar to the initial inoculum level across all doses, with maximal reduction observed at 25 mg/kg/day. In comparison, ceftobiprole at the same dose (25 mg/kg/day) resulted in the MRSA skin burden being reduced by 0.5 log10 CFU more than with linezolid. Overall, the reduction in CFU of MRSA strain OC 8525/g skin tissue in animals treated with ceftobiprole was significantly greater (P < 0.001) than in those treated with linezolid or cefazolin based on the dose-response profiles. Differences in the activities of ceftobiprole and vancomycin were not considered statistically significant (P < 0.17).
FIG. 4.
The effect of ceftobiprole (solid squares), cefazolin (open squares), vancomycin (solid triangles), or linezolid (open triangles) on MRSA strain OC 8525 in a murine skin infection model. Each symbol represents the mean ± standard error for 10 mice. The starting inoculum was 6.3 log10 CFU/mouse, and the untreated control value (48 h) was 7.2 log10 CFU/g skin tissue. P was <0.001 for ceftobiprole versus other treatment regimens (Cochran-Armitage test).
Control animals infected with S. aureus Smith OC 4172 or MRSA strain OC 8525 had mean lesion volumes of 1,181 mm3 and 1,535 mm3, respectively, 48 h postinfection (Table 5). Over this time period, treatment with ceftobiprole resulted in dose-proportional decreases in lesion volume for all doses ranging from 21% to 53% against OC 4172 and from 31% to 68% with OC 8525, relative to untreated controls. In contrast, for cefazolin at the same respective doses, the decreases in lesion volumes were smaller, ranging from 8% to 34% against OC 4172 and 10% to 40% against OC 8525. With vancomycin and linezolid treatment, lesion volumes from OC 4172 increased at the 1.6- and 6.2-mg/kg/day doses relative to no-drug controls but decreased at the 25- and 100-mg/kg/day doses by 22% and 63% (vancomycin) or 14% and 42% (linezolid), respectively. Similarly, against OC 8525, treatment with vancomycin or linezolid resulted in lesion volume increases at the lowest dose (1.6 mg/kg/day) and decreases of 9% to 60% (vancomycin) or 17% to 44% (linezolid) for the remaining doses. Thus, for either S. aureus strain, the magnitude of the decrease in lesion volume was smaller for the comparators than for ceftobiprole at all doses tested, except vancomycin at the 100-mg/kg/day dose. Based on the dose-response profiles, this relative decrease in lesion volume with ceftobiprole-treated animals was significantly greater (P < 0.001) than the decreases observed with the other treatment regimens.
TABLE 5.
Effect of treatment on lesion volume (48 h postinfection)
| Agent and dose (mg/kg/day) | Lesion vol [mm3 (SE)] for strain |
|||
|---|---|---|---|---|
|
S. aureus |
P. aeruginosa |
|||
| OC 4172 (MSSA) | OC 8525 (MRSA) | OC 4351 | OC 4354 | |
| Ceftobiprole | ||||
| 0 | 1,181 (62)b | 1,535 (93)c | 2,234 (145)d | 2,195 (126)e |
| 1.6 | 937 (40)b | 1,058 (69)c | 1,593 (151)d | 1,833 (198)e |
| 6.2 | 885 (89)b | 896 (95)c | 847 (124)d | 1,028 (239)e |
| 25 | 640 (49)b | 642 (53)c | 552 (139)d | 580 (136)e |
| 100 | 555 (45)b | 484 (47)c | 389 (83)d | 373 (107)e |
| Cefazolin | ||||
| 0 | 1,181 (62) | 1,535 (93) | NTa | NT |
| 1.6 | 1,090 (69) | 1,383 (142) | ||
| 6.2 | 987 (79) | 1,300 (108) | ||
| 25 | 877 (60) | 1,070 (94) | ||
| 100 | 781 (74) | 912 (82) | ||
| Vancomycin | ||||
| 0 | 1,181 (62) | 1,535 (93) | NT | NT |
| 1.6 | 1,386 (70) | 1,637 (110) | ||
| 6.2 | 1,211 (52) | 1,392 (107) | ||
| 25 | 916 (88) | 927 (49) | ||
| 100 | 437 (84) | 606 (96) | ||
| Linezolid | ||||
| 0 | 1,181 (62) | 1,535 (93) | NT | NT |
| 1.6 | 1,407 (80) | 1,644 (125) | ||
| 6.2 | 1,234 (39) | 1,277 (48) | ||
| 25 | 1,018 (54) | 932 (95) | ||
| 100 | 567 (40) | 858 (63) | ||
| Meropenem | ||||
| 0 | NT | NT | 1,866 (193) | 1,769 (158) |
| 1.6 | 1,190 (87) | 1,316 (86) | ||
| 6.2 | 992 (93) | 1,030 (71) | ||
| 25 | 862 (70) | 926 (51) | ||
| 100 | 602 (93) | 810 (157) | ||
| Cefepime | ||||
| 0 | NT | NT | 2,234 (145) | 2,195 (126) |
| 1.6 | 2,319 (247) | 2,250 (201) | ||
| 6.2 | 1,685 (104) | 2,248 (202) | ||
| 25 | 1,805 (314) | 2,054 (123) | ||
| 100 | 566 (64) | 1,696 (172) | ||
NT, not tested.
P < 0.001 versus all other treatment regimens (Cochran-Armitage test).
P < 0.001 versus the cefazolin and linezolid treatment regimens (Cochran-Armitage test); P < 0.17 versus the vancomycin treatment regimen.
P < 0.01 versus the cefepime treatment regimen (Cochran-Armitage test); P < 0.07 versus the meropenem-cilastatin treatment regimen.
P < 0.01 versus the cefepime treatment regimen (Cochran-Armitage test); P < 0.03 versus the meropenem-cilastatin treatment regimen.
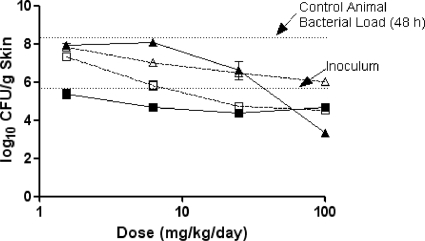
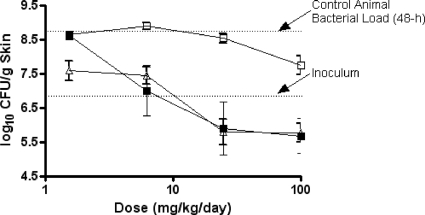
The efficacies of ceftobiprole, meropenem-cilastatin, and cefepime against P. aeruginosa OC 4351 in immunocompromised mice are summarized in Table 6 and Fig. 5. In untreated control animals, P. aeruginosa OC 4351 grew nearly 2 log10 CFU from the initial inoculum to 8.7 log10 CFU/g skin in 48 h. In contrast, during the same 48-h period, CFU in animals treated twice daily with ceftobiprole medocaril displayed a dose-dependent reduction in CFU, with maximal killing at 25 to 100 mg/kg/day. Compared to ceftobiprole, treatment with meropenem-cilastatin at 6.2 to 100 mg/kg/day resulted in similar reductions in bacterial load, with maximal effectiveness occurring at 25 to 100 mg/kg/day. The observed small differences between the ceftobiprole and the meropenem-cilastatin treatment groups were not considered significant. Treatment with cefepime (1.6 to 25 mg/kg/day) had little effect on the growth of OC 4351 in skin lesions, with CFU values at the end of 48 h similar to those of untreated control animals. Only at 100 mg/kg/day of cefepime was there a nearly 1-log10 CFU/g skin tissue reduction from the value for control animals. In comparison, ceftobiprole at this dose reduced the P. aeruginosa skin burden by an additional 2.1 log10 CFU/g skin tissue. The bacterial response to ceftobiprole and meropenem-cilastatin treatment was significantly different (P < 0.01) from that to the cefepime treatment.
TABLE 6.
Effect of treatment (Δlog CFU/g skin [from infecting inoculum]) with ceftobiprole, cefepime, or meropenem on CFU of P. aeruginosa in skina
| Dose (mg/kg/day) | ΔLog CFU of strain/g skin (from infecting inoculum) with agent |
|||||
|---|---|---|---|---|---|---|
| OC 4351 |
OC 4354 |
|||||
| BPR | FEP | MEM | BPR | FEP | MEM | |
| 0 | 2.1 | 2.1 | 1.5 | 1.8 | 1.8 | 1.5 |
| 1.6 | 1.9 | 2.1 | 0.2 | 1.7 | 1.7 | 0.7 |
| 6.2 | 0.2 | 2.1 | 0.2 | −0.4 | 2.9 | 0.5 |
| 25 | −0.9 | 1.8 | −0.9 | −1.3 | 1.7 | −1.1 |
| 100 | −1.1 | 1.0 | −1.0 | −1.4 | 1.7 | −1.1 |
Abbreviations: BPR, ceftobiprole; FEP, cefepime; MEM, meropenem. MICs (μg/ml) of drugs against OC 4351 and OC 4354 were as follows: ceftobiprole, 4 and 4; cefepime, 4 and 32; and meropenem, 0.12 and 0.5, respectively. Ceftobiprole was administered as ceftobiprole medocaril, and meropenem was administered as meropenem-cilastatin.
FIG. 5.
The effect of ceftobiprole (solid squares), cefepime (open squares), or meropenem-cilastatin (open triangles) on P. aeruginosa OC 4351 in a murine skin infection model. Each symbol represents the mean ± standard error for 10 mice. The starting inoculum was 6.8 log10 CFU/mouse, and the untreated control value (48 h) was 8.7 log10 CFU/g skin tissue. P was <0.01 for ceftobiprole versus cefepime (Cochran-Armitage test).
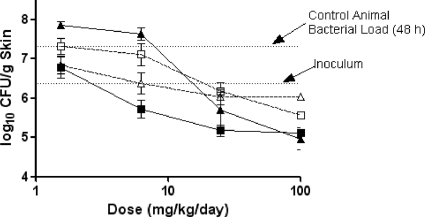
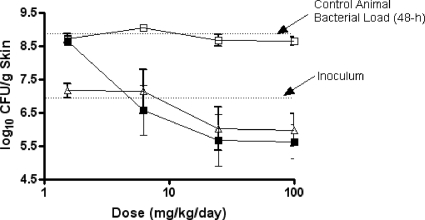
The efficacies of ceftobiprole, meropenem-cilastatin, and cefepime against P. aeruginosa OC 4354, an AmpC-overproducing strain, are summarized in Table 6 and Fig. 6. In control animals, bacteria grew 1.8 log10 CFU/g skin, to a final density of 8.8 log10 CFU/g skin, in the 48-h testing period. Treatment with 6.2 to 100 mg/kg/day of ceftobiprole medocaril resulted in reductions in the concentration of OC 4354 below the inoculum level, with maximal killing occurring at 25 to 100 mg/kg/day. Treatment with meropenem-cilastatin at the same doses resulted in similar bacterial decreases and was not significantly different from the ceftobiprole treatment. Maximal killing was also observed at the 25- to 100-mg/kg/day doses for meropenem-cilastatin. Compared to control animals, cefepime had little effect on the growth of OC 4354 in skin lesions. Ceftobiprole treatment was significantly different (P < 0.01) from cefepime treatment against P. aeruginosa OC 4354 skin infections. Overall, the overproduction of AmpC did not have any significant effect on the in vitro or in vivo efficacy of ceftobiprole against these isogenic strains.
FIG. 6.
The effect of ceftobiprole (solid squares), cefepime (open squares), or meropenem-cilastatin (open triangles) on P. aeruginosa OC 4354 in a murine skin infection model. Each symbol represents the mean ± standard error for 10 mice. The starting inoculum was 6.9 log10 CFU/mouse, and the untreated control value (48 h) was 8.8 log10 CFU/g skin tissue. P was <0.01 for ceftobiprole versus cefepime (Cochran-Armitage test).
The size of the skin lesions (lesion volume) that resulted from the subcutaneous infection with P. aeruginosa in immunocompromised mice is summarized in Table 5. Animals infected with P. aeruginosa OC 4351 or OC 4354 had mean lesion volumes ranging from 1,769 to 2,234 mm3 48 h postinfection. Treatment with 1.6 to 100 mg/kg/day of ceftobiprole medocaril resulted in dose-proportional decreases in lesion volume ranging from 29% to 82% against OC 4351 and 16% to 83% against OC 4354 relative to untreated controls. Treatment with meropenem-cilastatin at the same doses resulted in dose-proportional decreases in lesion volume ranging from 36% to 68% against OC 4351 and 26% to 54% against OC 4354 relative to untreated controls. Treatment with meropenem-cilastatin resulted in lesions 15% to 36% larger than those in ceftobiprole-treated animals infected with OC 4351 (P < 0.07) and lesions 37% to 54% larger than those in ceftobiprole-treated animals (P < 0.03) infected with the AmpC-overproducing strain, OC 4354. Treatment with cefepime resulted in lesion volume scores that were 31% to 69% and 18% to 78% higher than those with ceftobiprole treatment against OC 4351 (P < 0.01) and OC 4354 (P < 0.01), respectively, at the same doses.
The observed decreases in lesion volume and CFU at the respective ceftobiprole doses were similar in the wild-type (OC 4351) and AmpC-overproducing (OC 4354) strains, suggesting that the elevated AmpC levels had no effect on lesion volume or efficacy in ceftobiprole-treated animals. Conversely, there was a trend toward higher lesion volumes caused by the AmpC-overproducing strain in animals treated with meropenem-cilastatin or cefepime.
Pharmacodynamic studies.
The pharmacodynamic parameters were calculated using the free-drug fraction following administration of ceftobiprole medocaril, cefazolin, linezolid, vancomycin, meropenem, and cefepime (Table 7). Against staphylococci, two 73-mg/kg doses of ceftobiprole medocaril (50 mg/kg ceftobiprole) resulted in a free-drug plasma concentration that was above the MIC (fT>MIC) for 3.3 to >4 h, 9.1% to >17% of the 24-h dosing interval). In contrast, treatment with cefazolin at the same dose resulted in a %fT>MIC of 1.3 for MRSA; this correlated with poor in vivo efficacy. However, when the %fT>MIC was 10.2 with MSSA, the efficacy improved and was similar to that of ceftobiprole. In general, for concentration-dependent drugs, an AUC/MIC ratio of ≥25 has been associated with optimal efficacy in less severe infections or in immunocompetent hosts, while ratios of ≥100 are recommended for severe infections or for use with immunocompromised patients (36). In our studies, vancomycin when administered at 100 mg/kg/day achieved an fAUC/MIC of 34.5 (calculated with AUC values converted from μg·min/ml to μg·h/ml), resulting in good efficacy against the MSSA and MRSA strains. However, when linezolid was tested at the same dose, the fAUC/MIC ratios were two- to fourfold less, correlating with a smaller reduction in CFU.
TABLE 7.
Pharmacodynamics of ceftobiprole and comparator antibiotics in the murine skin infection model
| Drug | Organism | MIC (μg/ml) | fT>MICa,b (h) | %fT>MICa,c | fAUC/MICa |
|---|---|---|---|---|---|
| Ceftobiprole | S. aureus OC 4172 | 0.25 | >4 | >17 | NAd |
| MRSA OC 8525 | 1 | 3.3 | 13.8 | NA | |
| P. aeruginosa OC 4351 | 4 | 2.2 | 9.1 | NA | |
| P. aeruginosa OC 4354 | 4 | 2.2 | 9.1 | NA | |
| Cefazolin | S. aureus OC 4172 | 0.5 | 2.4 | 10.2 | NA |
| MRSA OC 8525 | 16 | 0.3 | 1.3 | NA | |
| Linezolid | S. aureus OC 4172 | 2 | NA | NA | 898 |
| MRSA OC 8525 | 4 | NA | NA | 449 | |
| Vancomycin | S. aureus OC 4172 | 1 | NA | NA | 2,070 |
| MRSA OC 8525 | 1 | NA | NA | 2,070 | |
| Meropenem | P. aeruginosa OC 4351 | 0.12 | 2 | 8.5 | NA |
| P. aeruginosa OC 4354 | 0.5 | 1.9 | 8.0 | NA | |
| Cefepime | P. aeruginosa OC 4351 | 4 | 1.5 | 6.4 | NA |
| P. aeruginosa OC 4354 | 32 | 0.8 | 3.3 | NA |
Corrected for protein binding in mouse plasma: 16% ceftobiprole (data on file), 81% cefazolin, 32% linezolid, 25% vancomycin, 19% meropenem, and 20% cefepime (28, 35, 37, 38, 54).
Free drug following two 50-mg/kg doses, 1 and 3 h after infection.
For the 24-h dosing interval.
NA, not applicable.
In pseudomonal infections, two 73-mg/kg doses of ceftobiprole medocaril (50 mg/kg ceftobiprole) resulted in a free-drug plasma concentration that was above the MIC (fT>MIC) for 2.2 h (9.1% of the 24-h dosing interval), which resulted in a greater than 3-log10 CFU/g decrease in skin tissue. In contrast, animals treated with meropenem-cilastatin had fT>MICs that were 7 to 12% shorter than those in animals treated with ceftobiprole and bacterial decreases in skin that were 0.5 log10 CFU less than those in animals treated with ceftobiprole at the same dose. A decrease in efficacy was noted with cefepime-treated animals which was concordant with the smaller %fT>MIC (3.3 to 6.6) of cefepime.
DISCUSSION
Several skin and soft tissue murine infection models have been described in the literature (7, 11, 30, 46). Protocols vary widely in terms of the mouse strain employed, the introduction of foreign material, and/or the length of the study. Studies using 5% mucin (46), BHI agar (30), or Cytodex beads (7, 11) in the infecting inoculum have been reported. We chose to evaluate our compounds in an acute model (48 h) using Cytodex beads and SKH1 mice, based on the consistency of the infection and the advantages of hairless mice in the evaluation of skin infections. This acute model provides some insight into the ability of a compound to “protect” the host from infection.
Although there are no other reports describing the efficacy of ceftobiprole in skin infection animal models, the antistaphylococcal activity of ceftobiprole has been studied in vivo by other investigators in septicemia, endocarditis, foreign body, and subcutaneous abscess models (13, 22, 30, 55). Similar to our study, Hebeisen and colleagues reported that ceftobiprole and vancomycin were highly effective, whereas linezolid was only slightly effective, against an MRSA abscess infection in mice (30). Our study expands on this previous work by evaluating ceftobiprole in MSSA and MRSA skin infection models, contrasting the efficacy, pharmacokinetics, and pharmacodynamics with those of comparator antibiotics. In the MSSA and MRSA models, ceftobiprole (6.2 to 100 mg/kg/day) showed comparable reductions in bacterial burdens, suggesting that ceftobiprole is similarly effective regardless of the presence of PBP2a. The in vitro improvement in the MIC of ceftobiprole over linezolid correlated with lower bacterial loads in the skin of mice when ceftobiprole was administered at 6.2 to 100 mg/kg/day. Also, lesion volume was lower at every dose tested for ceftobiprole compared to that with linezolid, consistent with the lower CFU recovered from infected skin following ceftobiprole treatment. even compared to vancomycin, an agent with in vitro potency equal to that of ceftobiprole and often employed as a standard of care therapy for treating MRSA infections, ceftobiprole achieved greater killing at doses of 1.6 to 25 mg/kg/day and similar killing at 100 mg/kg/day. Our study shows that ceftobiprole was generally more effective at lowering bacterial counts and lesion volumes in our murine infection models than were current therapies available to treat MRSA infections.
In P. aeruginosa, we expanded the limited in vivo animal experience with ceftobiprole by comparing it to the clinically relevant comparators, cefepime and meropenem-cilastatin, against strains with basal (OC 4351) and elevated (OC 4354) AmpC β-lactamase levels. In these mouse pseudomonal skin infections, at the same dose (50 mg/kg) and similar plasma exposures of ceftobiprole or meropenem-cilastatin, similar reductions in bacterial burden and %fT>MIC were observed, in spite of the 8- and 32-fold-lower MICs of meropenem. Also, lesion volumes were lower for the ceftobiprole-treated mice than for the meropenem-cilastatin-treated mice.
AmpC β-lactamases in P. aeruginosa have been reported to reduce the susceptibility to some β-lactams (40); however, ceftobiprole and cefepime are poor inducers and are hydrolyzed slowly by AmpC (47, 48). In studies described in this paper, elevation of AmpC levels 350-fold had no detectable effect on ceftobiprole MICs, while cefepime MICs increased eightfold (Table 1). Thus, the poor in vivo efficacy of cefepime against P. aeruginosa OC 4354 was expected due to the high MIC in the elevated-AmpC-producing strain. In addition, at the 50-mg/kg dose, cefepime achieved a relatively low %fT>MIC of 3.3% for the 24-h dosing interval, twofold lower than that observed for cefepime with P. aeruginosa OC 4351. One would expect that if the dose were optimized for cefepime in this model, improved efficacy could have been achieved. Elevated AmpC levels in P. aeruginosa did not affect the in vitro and in vivo efficacy of ceftobiprole. Our in vivo findings are in agreement with the earlier kinetic work in which a low rate of hydrolysis of ceftobiprole by the pseudomonal AmpC enzyme was observed (48).
Reductions in lesion volume resulted in decreases in staphylococcal bacterial density in the skin. The magnitude of the lesion volume score was generally in agreement with the magnitude of the decrease in CFU, except for the 100-mg/kg/day doses of ceftobiprole and linezolid, where similar lesion volume scores were achieved, but greater bacterial decreases were attained with ceftobiprole. In skin infections caused by P. aeruginosa, greater bacterial growth (0.6 log10 CFU/g) was observed in control animals than in the staphylococcal controls. Corresponding lesion volume scores were 33% higher in the Pseudomonas controls. In addition, lesion volume reductions were predictive of decreases in bacterial load in P. aeruginosa-infected animals. Lesion volume may be a useful surrogate marker for bacterial load in this skin infection model.
T>MIC has been previously shown to be the primary driver of in vivo efficacy for β-lactam antibiotics (16, 17, 42). In mouse pharmacodynamic studies, the length of time that the free-drug concentration needs to be above the MIC to confer efficacy varied between 16 and >60% of the dosing interval, with target values of 19 to 28% T>MIC for staphylococci for cephalosporin antibiotics (16, 17). According to results reported by Craig and Andes (18), the ceftobiprole T>MIC required for a static effect in a mouse neutropenic thigh model ranged from 14 to 28% (S. aureus). Human studies with β-lactams have generated somewhat different results, with T>MICs of approximately 40 to 50% reported to be necessary for efficacy with various pathogens and indications (5, 15). In studies by Azoulay-Dupuis et al. with ceftobiprole in an immunocompromised mouse pneumonia model, T>MICs ranging from 9 to 18% were shown to be efficacious (6). To our knowledge, no detailed experiments investigating the pharmacodynamics of β-lactams in a mouse skin infection model have been reported. The ceftobiprole fT>MICs obtained in our studies ranged from 9 to >17% (100-mg/kg/day dose); these values are similar to those described by Craig and Andes and by Azoulay-Dupuis et al. for the mouse thigh (S. aureus) and pneumonia (Streptococcus pneumoniae) models, respectively. At this ceftobiprole dose of 100 mg/kg/day, CFU decreases in our skin model ranged from 1.1 to 1.7 log10 for the staphylococcal and P. aeruginosa isolates, indicating that a fT>MIC of 9 to >17% was sufficient for a decrease in CFU of at least 1 log10. With cefazolin (100-mg/kg/day dose), a fT>MIC of 10.2% with the MSSA strain S. aureus OC 4172 was associated with a CFU decrease of 1.9 log10, similar to values for ceftobiprole. In contrast, with the MRSA strain the cefazolin fT>MIC was 1.3%, and CFU decreased to a lesser extent (0.9 log10) relative to initial inoculum levels. With P. aeruginosa, cefepime fT>MICs of 6.4% (OC 4351) and 3.3% (OC 4354) were associated with poor outcomes in skin lesions, with CFU increases of 1 to 1.7 log10 over inoculum levels. Meropenem fT>MICs for these isolates were slightly higher (8.5% and 8.0%, respectively) and were associated with a CFU decrease of approximately 1 log10. With the exception of cefepime and the MRSA strain, fT>MICs of at least 8% were associated with CFU decreases of ≥1 log10 over 48 h, while fT>MICs of less than 8% had little or no impact on growth in skin lesions. The effective minimum fT>MIC of 8% observed for β-lactams in these studies is thus consistent with other mouse pharmacodynamic analyses of ceftobiprole but somewhat less than lower target limits for β-lactams in general, for mouse (16 to >60%) and human (40% to 50%) studies.
The pharmacodynamic parameter predictive of efficacy with vancomycin and linezolid has been determined to be AUC/MIC (3, 50). In a recent publication (39), vancomycin (360-mg/kg/day dose) and linezolid (400-mg/kg/day dose) were bacteriostatic for community-acquired MRSA mouse thigh infections, with fAUC/MICs ranging from 136 to 272 (vancomycin) and 50 to 99 (linezolid) (estimated from data in this paper by these authors). In S. aureus mouse neutropenic thigh studies with linezolid by Craig et al., AUC/MIC ratios ranging from 39 to 167 were needed for a bacteriostatic effect. AUC/MICs obtained in this skin infection model (calculated with AUC values converted from μg·min/ml to μg·h/ml) of 35 (vancomycin) and 7.5 to 15 (linezolid) were thus lower than those determined for these agents in other pharmacodynamic studies. Although fT>MIC and fAUC/MICs obtained in this skin infection model were in some cases lower than target values identified by other investigators, these pharmacodynamic parameters generally remained predictive of trends in efficacy for the respective compounds.
Clinical doses for the agents discussed in this paper range from 500 mg to 2 g (7 mg/kg to 28 mg/kg when adjusted for body weight). Similarly, the doses used in our mouse studies ranged from 1.56 mg/kg to 50 mg/kg, providing some basis for allometric scaling of our results to humans. Nonetheless, because of differences in mouse and human metabolism, some caution should be exercised in the extrapolation of specific efficacy or pharmacokinetic/pharmacodynamic (PK/PD) values.
In summary, ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity, was effective in vivo against MSSA, MRSA, and P. aeruginosa isolates in this murine model of skin and soft tissue infection. Ceftobiprole effectively reduced bacterial load and the lesion size associated with infection due to MSSA, MRSA, and P. aeruginosa and was significantly different from treatment with comparator antibiotics with proven antistaphylococcal or antipseudomonal clinical usage.
Acknowledgments
We thank Alfred M. Barron for his support with the statistical analysis, Anne Marie Queenan for her molecular characterization of the pseudomonas strains, Barbara Foleno and Ellyn Wira for their assistance with the MIC determinations, and Anthony Simon Lynch for his critical reading of the manuscript.
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Amsler, K. M., T. A. Davies, W. Shang, M. R. Jacobs, and K. Bush. 2008. In vitro activity of ceftobiprole against pathogens from two phase 3 clinical trials of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 52:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaya, D. A., and E. P. Dellinger. 2007. Necrotizing soft-tissue infection: diagnosis and management. Clin. Infect. Dis. 44:705-710. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attassi, K., E. Hershberger, R. Alam, and M. J. Zervos. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695-698. [DOI] [PubMed] [Google Scholar]
- 5.Auckenthaler, R. 2002. Pharmacokinetics and pharmacodynamics of oral beta-lactam antibiotics as a two-dimensional approach to their efficacy. J. Antimicrob. Chemother. 50(Suppl.):13-17. [DOI] [PubMed] [Google Scholar]
- 6.Azoulay-Dupuis, E., J. P. Bedos, J. Mohler, A. Schmitt-Hoffmann, M. Schleimer, and S. Shapiro. 2004. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob. Agents Chemother. 48:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barg, N. 1998. Comparison of four antibiotics in a murine model of necrotizing cutaneous infections caused by toxigenic Streptococcus pyogenes and Staphylococcus aureus. J. Antimicrob. Chemother. 42:257-260. [PubMed] [Google Scholar]
- 8.Bogdanovich, T., L. M. Ednie, S. Shapiro, and P. C. Appelbaum. 2005. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:4210-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bratzler, D. W., and D. R. Hunt. 2006. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin. Infect. Dis. 43:322-330. [DOI] [PubMed] [Google Scholar]
- 10.Brown, N. P., M. E. Jones, D. C. Draghi, M. K. Aranza, K. Murfitt, C. Thornsberry, and D. Sahm. 2006. Baseline surveillance profiles of ceftobiprole (BPR) activity against Enterobacteriaceae and P. aeruginosa (PA), abstr. E-0112. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 11.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbon, C. 1999. Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J. Antimicrob.Chemother. 44(Suppl. A):31-36. [DOI] [PubMed] [Google Scholar]
- 13.Chambers, H. F. 2005. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. 2006. Performance standards for antimicrobial susceptibility testing; sixteenth informational supplement. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 15.Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 3):S233-S237. [DOI] [PubMed] [Google Scholar]
- 16.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 17.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 18.Craig, W. A., and D. R. Andes. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies, T. A., M. G. P. Page, W. Shang, T. Andrew, M. Kania, and K. Bush. 2007. Binding of ceftobiprole and comparators to the penicillin-binding proteins of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 51:2621-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, T. A., W. Shang, R. K. Flamm, and K. Bush. 2006. Binding of ceftobiprole and comparators to penicillin-binding proteins in Pseudomonas aeruginosa, abstr. C1-0933. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 21.Ena, J., R. W. Dick, R. N. Jones, and R. P. Wenzel. 1993. The epidemiology of intravenous vancomycin usage in a university hospital. A 10-year study. JAMA 269:598-602. [PubMed] [Google Scholar]
- 22.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez, J., J. J. Hilliard, J. L. Melton, D. Abbanat, R. K. Flamm, and K. Bush. 2007. In vivo activity of ceftobiprole in a pseudomonal murine skin infection model, abstr. 437. Abstr. 45th Annu. Meet. Infect. Dis. Soc. Am. (IDSA), San Diego, CA.
- 24.Fridkin, S. K., J. Hageman, L. K. McDougal, J. Mohammed, W. R. Jarvis, T. M. Perl, F. C. Tenover, and Vancomycin-Intermediate Staphylococcus aureus Epidemiology Study Group. 2003. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997-2001. Clin. Infect. Dis. 36:429-439. [DOI] [PubMed] [Google Scholar]
- 25.Fritsche, T. R., H. S. Sader, and R. N. Jones. 2006. In vitro activity of ceftobiprole tested against a recent collection of North American Pseudomonas aeruginosa (PSA), abstr. E-0115. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC.
- 26.Fung, H. B., J. Y. Chang, and S. Kuczynski. 2003. A practical guide to the treatment of complicated skin and soft tissue infections. Drugs 63:1459-1480. [DOI] [PubMed] [Google Scholar]
- 27.Fung, H. B., H. L. Kirschenbaum, and B. O. Ojofeitimi. 2001. Linezolid: an oxazolidinone antimicrobial agent. Clin. Ther. 23:356-391. [DOI] [PubMed] [Google Scholar]
- 28.Gill, C. J., G. K. Abruzzo, A. M. Flattery, A. S. Misura, K. Bartizal, and E. J. Hickey. 2007. In vivo efficacy of a novel oxazolidinone compound in two mouse models of infection. Antimicrob. Agents Chemother. 51:3434-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291. [DOI] [PubMed] [Google Scholar]
- 30.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilliard, J. J., J. Fernandez, J. Melton, M. J. Macielag, R. Goldschmidt, K. Bush, and D. Abbanat. 2009. In vivo activity of the pyrrolopyrazolyl-substituted oxazolidinone RWJ-416457. Antimicrob. Agents Chemother. 53:2028-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilliard, J. J., W. Zhang, J. Melton, J. Fernandez, R. K. Flamm, and K. Bush. 2006. In vivo anti-pseudomonal acivity of ceftobiprole, abstr. 198. Abstr. 44th Annu. Meet. Infect. Dis. Soc. Am. (IDSA), Toronto, Ontario, Canada.
- 33.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 34.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 35.Hori, T., M. Nakano, Y. Kimura, and K. Murakami. 2006. Pharmacokinetics and tissue penetration of a new carbapenem, doripenem, intravenously administered to laboratory animals. In Vivo 20:91-96. [PubMed] [Google Scholar]
- 36.Jacobs, M. R. 2001. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7:589-596. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen, J. D., K. Fuursted, F. Espersen, and N. Frimodt-Moller. 1997. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to pharmacokinetic parameters in the mouse peritonitis model. Antimicrob. Agents Chemother. 41:1910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knudsen, J. D., K. Fuursted, N. Frimodt-Moller, and F. Espersen. 1997. Comparison of the effect of cefepime with four cephalosporins against pneumococci with various susceptibilities to penicillin, in vitro and in the mouse peritonitis model. J. Antimicrob. Chemother. 40:679-686. [DOI] [PubMed] [Google Scholar]
- 39.LaPlante, K. L., S. N. Leonard, D. R. Andes, W. A. Craig, and M. J. Rybak. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob. Agents Chemother. 52:2156-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livermore, D. M. 2001. Of Pseudomonas, porins, pumps and carbapenems. J. Antimicrob. Chemother. 47:247-250. [DOI] [PubMed] [Google Scholar]
- 41.McDonald, L. C. 2006. Trends in antimicrobial resistance in health care-associated pathogens and effect on treatment. Clin. Infect. Dis. 42(Suppl. 2):S65-S71. [DOI] [PubMed] [Google Scholar]
- 42.McNabb, J. J., and K. Q. Bui. 2002. B-lactam pharmacodynamics, vol. 28. Marcel Dekker, Inc., New York, NY.
- 43.Milliken, G. A., and D. E. Johnson. 2001. Analysis of messy data, vol. III. Analysis of covariance. CRC Press, Danvers, MA.
- 44.Noel, G. J., R. S. Strauss, K. Amsler, M. Heep, R. Pypstra, and J. S. Solomkin. 2008. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 52:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panlilio, A. L., D. H. Culver, R. P. Gaynes, S. Banerjee, T. S. Henderson, J. S. Tolson, and W. J. Martone. 1992. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975-1991. Infect. Control Hosp. Epidemiol. 13:582-586. [DOI] [PubMed] [Google Scholar]
- 46.Patel, M. V., N. J. De Souza, S. V. Gupte, M. A. Jafri, S. S. Bhagwat, Y. Chugh, H. F. Khorakiwala, M. R. Jacobs, and P. C. Appelbaum. 2004. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob. Agents Chemother. 48:4754-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Queenan, A. M., and K. Bush. 2005. Ceftobiprole: effect on AmpC Β-lactamase induction and resistance frequency in gram-negative bacteria. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-55, p. 57.
- 48.Queenan, A. M., W. Shang, M. Kania, M. G. P. Page, and K. Bush. 2007. Interactions of ceftobiprole with beta-lactamases from molecular classes A to D. Antimicrob. Agents Chemother. 51:3089-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert, J., R. Bismuth, and V. Jarlier. 2006. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large French teaching hospital, 1983-2002. J. Antimicrob. Chemother. 57:506-510. [DOI] [PubMed] [Google Scholar]
- 50.Rybak, M. J., B. M. Lomaestro, J. C. Rotscahfer, R. C. Moellering, W. A. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 49:325-327. [DOI] [PubMed] [Google Scholar]
- 51.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 52.Stevens, D. L., A. L. Bisno, H. F. Chambers, E. D. Everett, P. Dellinger, E. J. C. Goldstein, S. L. Gorbach, J. V. Hirschmann, E. L. Kaplan, J. G. Montoya, J. C. Wade, and Infectious Diseases Society of America. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373-1406. (Erratum, 41:1830.) [DOI] [PubMed] [Google Scholar]
- 53.Stokes, M., C. S. Davis, and G. G. Koch. 1995. Categorical data analysis using the SAS system. SAS Institute, Inc., Cary, NC.
- 54.Tawara, S., S. Matsumoto, T. Kamimura, and S. Goto. 1992. Effect of protein binding in serum on therapeutic efficacy of cephem antibiotics. Antimicrob. Agents Chemother. 36:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaudaux, P., A. Gjinovci, M. Bento, D. Li, J. Schrenzel, and D. P. Lew. 2005. Intensive therapy with ceftobiprole medocaril of experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3789-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuluaga, A. F., B. E. Salazar, C. A. Rodriguez, A. X. Zapata, M. Agudelo, and O. Vesga. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]