Abstract
Favipiravir (T-705 [6-fluoro-3-hydroxy-2-pyrazinecarboxamide]) and oseltamivir were combined to treat influenza virus A/NWS/33 (H1N1), A/Victoria/3/75 (H3N2), and A/Duck/MN/1525/81 (H5N1) infections. T-705 alone inhibited viruses in cell culture at 1.4 to 4.3 μM. Oseltamivir inhibited these three viruses in cells at 3.7, 0.02, and 0.16 μM and in neuraminidase assays at 0.94, 0.46, and 2.31 nM, respectively. Oral treatments were given twice daily to mice for 5 to 7 days starting, generally, 24 h after infection. Survival resulting from 5 days of oseltamivir treatment (0.1 and 0.3 mg/kg/day) was significantly better in combination with 20 mg/kg of body weight/day of T-705 against the H1N1 infection. Treatment of the H3N2 infection required 50 mg/kg/day of oseltamivir for 7 days to achieve 60% protection; 25 mg/kg/day was ineffective. T-705 was ≥70% protective at 50 to 100 mg/kg/day but inactive at 25 mg/kg/day. The combination of inhibitors (25 mg/kg/day each) increased survival to 90%. The H5N1 infection was not benefited by treatment with oseltamivir (≤100 mg/kg/day for 7 days). T-705 was 30 to 70% protective at 25 to 100 mg/kg/day. Survival improved slightly with combination treatments. Increased activity was seen against H5N1 infection by starting treatments 2 h before infection. Oseltamivir was ineffective at ≤40 mg/kg/day. T-705 was 100% protective at 40 and 80 mg/kg/day and inactive at 20 mg/kg/day. Combining ineffective doses (20 mg/kg/day of T-705 and 10 to 40 mg/kg/day of oseltamivir) afforded 60 to 80% protection and improved body weights during infection. Thus, synergistic responses were achieved with low doses of T-705 combined with oseltamivir. These compounds may be viable candidates for combination treatment of human influenza infections.
The emergence of swine influenza H1N1 virus infections in 2009 (2) highlights the need for effective antiviral therapy in a largely immune-naïve population. Treatment options for influenza are becoming more limited because viruses, including the 2009 swine H1N1 virus, are resistant to the antiviral drugs amantadine and rimantadine (3, 4, 11, 13, 20). Oseltamivir-resistant viruses are also becoming more common in the environment, particularly within the last 2 years (1, 5, 19). Thus, more potent and effective treatments are needed to combat these growing threats.
More potent antiviral therapy can be achieved by using drugs in combination, as demonstrated in mouse models (10, 14-17, 24, 26, 27). Such treatment can slow down the emergence of drug-resistant viruses (12). The reported animal studies have primarily focused on the known-active antiviral agents amantadine, rimantadine, oseltamivir, peramivir, zanamivir, and ribavirin. The kinds of studies that can be performed have been limited based upon the number of active antiviral compounds that are available.
In 2002, Furuta et al. reported a novel pyrazine molecule, T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide, now named favipiravir), as an inhibitor of influenza virus infections in cell culture and in mice (8). T-705 inhibits both influenza A and B viruses (8, 23, 29). The compound converts to nucleoside mono- (T-705 RMP [ribosylated, monophosphorylated]), di-, and triphosphate (T-705 RTP [ribosylated, triphosphorylated]) forms in cells (9). The mode of action of T-705 RTP is similar to that of ribavirin triphosphate as an inhibitor of influenza virus RNA polymerase (6, 9). Unlike ribavirin monophosphate, T-705 RMP is only weakly inhibitory to cellular inosine monophosphate (IMP) dehydrogenase (9, 28), and thus, it is less cytotoxic. These properties make T-705 a viable candidate for the treatment of influenza virus infections in humans. The compound is currently undergoing phase II clinical trials.
The use of T-705 in combination with other antiviral substances has not been reported. The purpose of the present work was to evaluate whether the combination of T-705 with the widely used antiviral drug oseltamivir is more beneficial than either substance used alone against influenza virus infections in mice. We chose three mouse-adapted influenza viruses for these comparisons, A/NWS/33 (H1N1), A/Victoria/3/75 (H3N2), and A/Duck/MN/1525/81 (H5N1). The A/NWS and A/Victoria viruses are of seasonal origin and are confined to the respiratory tract following infection. The A/Duck virus is a low-pathogenicity avian virus from the United States that also does not spread beyond the respiratory tract of mice. The experimental influenza A/Duck mouse infection does not fully reflect the type of pathogenesis of the highly pathogenic avian influenza H5N1 viruses from the Old World. This is because the A/Duck virus lacks the multibasic amino acid R-X-R/K-R motif in the hemagglutinin protein, whereas the highly pathogenic avian H5N1 viruses contain it (7). This motif allows for the highly pathogenic viruses to be proteolytically activated by ubiquitous subtilisin-like cellular proteases, allowing the virus to spread in vivo beyond the respiratory tract and to cause multiorgan failure. Nevertheless, the A/Duck virus induces rapid, severe lung infections that are difficult to treat with conventional antiviral therapy. Using these three models, H1N1, H3N2, and H5N1, in mice, we were able to demonstrate the benefits of using oseltamivir and T-705 in combination to treat influenza virus infections.
MATERIALS AND METHODS
Antiviral compounds.
Oseltamivir phosphate (Tamiflu) was purchased from a local pharmacy. Toyama Chemical Co. (Tokyo, Japan) provided T-705. Oseltamivir carboxylate, the active form of oseltamivir, and pure oseltamivir phosphate were kindly provided by Jack Nguyen (Adamas Pharmaceuticals, Emeryville, CA). Hereafter, oseltamivir phosphate will primarily be referred to as oseltamivir. Because oseltamivir from pharmaceutical capsules that contained other ingredients besides the drug as filler material was used, the contents of entire 75-mg capsules minus the shell were added to water to make up the highest mg/kg of body weight/day dose of drug. Lower doses of oseltamivir were made by dilution into water. T-705 was weighed out for each dose of compound and suspended in 0.4% carboxymethylcellulose (CMC). CMC served as the placebo control.
Viruses.
Influenza A/NWS/33 (H1N1) was originally obtained from Kenneth Cochran (University of Michigan, Ann Arbor, MI). The virus was passaged 9 times in MDCK cells. Influenza A/Victoria/3/75 (H3N2) virus was purchased from the American Type Culture Collection (Manassas, VA). The virus became lethal after seven serial passages in the lungs of mice. Influenza A/Duck/MN/1525/81 (H5N1) virus was provided by Robert Webster (St. Jude Children's Research Hospital, Memphis, TN). It was passaged three times in mice to enhance its virulence. Virus pools were pretitrated in mice prior to performing these studies to determine approximate 90% to 100% lethal doses (LD90s to LD100s).
Cell culture antiviral studies.
Antiviral activities of oseltamivir carboxylate and T-705 were determined in confluent cultures of Madin-Darby canine kidney (MDCK) cells. The assays were performed in 96-well microplates infected with approximately 50 cell culture infectious doses (CCID50s) of virus by quantifying virus-induced cytopathic effect (CPE) by neutral red dye uptake (25). Three microwells at each concentration of compound were infected. Two microwells were uninfected and served as toxicity controls. Microplates were examined after 3 days of infection and treated for 2 h with neutral red (0.011% final concentration) for 2 h. Excess dye was rinsed from cells with phosphate-buffered saline (PBS). The absorbed dye was eluted from the cells with 0.1 ml of 50% Sörensen's citrate buffer (pH 4.2)-50% ethanol. Plates were read for optical density determination at 560 nm. Readings were converted to the percentage of the result for the uninfected control using an Excel spread sheet developed for this purpose. Fifty-percent virus-inhibitory concentrations (50% effective concentrations [EC50s]) were determined by plotting percent CPE versus log10 inhibitor concentration. Toxicity at each concentration was determined in the same microplates by measuring dye uptake.
Viral neuraminidase inhibition assay.
The effects of compounds on viral neuraminidase activity were assayed using a commercially available kit (NA-Star influenza neuraminidase inhibitor resistance detection kit; Applied Biosystems, Foster City, CA) in 96-well solid white microplates following the manufacturer's instructions and as has been reported previously (22). Compounds in half-log dilution increments were incubated with virus (as the source of neuraminidase). The amount of virus in each microwell was approximately 500 cell culture infectious doses. Plates were preincubated for 10 min at 37°C prior to the addition of chemiluminescent substrate. Following the addition of substrate, plates were incubated for 30 min at 37°C. The neuraminidase activity was evaluated using a Centro LB 960 luminometer (Berthold Technologies, Oak Ridge, TN) for 0.5 s immediately after the addition of NA-Star accelerator solution. Fifty-percent inhibitory concentrations (IC50s) of viral neuraminidase activity were determined by plotting percent chemiluminescence counts versus log10 inhibitor concentration.
Animal experiment design.
Specific-pathogen-free BALB/c mice weighing approximately 17 to 19 g were purchased from Charles River Laboratories (Wilmington, MA). They were maintained on standard rodent chow and given water ad libitum with water bottles. The animals were quarantined 48 h prior to beginning of studies. Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and then infected intranasally with a 90-μl suspension of virus. The infecting virus titers were approximately 104.5 to 105.0 CCID50 per mouse to achieve 100% lethality. This corresponded to 50% mouse LD50s (MLD50s) of 3, 2, and 4 for the A/NWS (H1N1), A/Victoria (H3N2), and A/Duck (H5N1) virus infections, respectively. Groups of mice were treated orally (by gavage) with oseltamivir and T-705 alone or in combination. The compounds were given twice a day (at 12-h intervals) for 5 or 7 days, depending upon the experiment, starting 24 h after virus exposure for most studies. One experiment with the A/Duck virus started treatments 2 h before infection. The placebo was administered in parallel with antiviral treatments. Drug combinations were administered with treatment with one compound followed by treatment with the second compound. Ten to 12 compound-treated, infected mice and 20 placebo-treated controls were observed daily for death through 21 days. Some mice died during the treatment phase and were deemed to have died from improper treatment or treatment stress. Group sizes were reduced accordingly, and these dead animals were not included in the calculations of number of survivors/total number of mice in group or mean day of death. Mice were weighed every other day during the infection. Healthy controls (10 uninfected, untreated animals) were weighed in parallel with the infected groups.
Statistical analysis and determination of synergy for animal studies.
The survival curves for all groups together in each experiment were evaluated by the log-rank test. Because statistically significant differences were found among groups (P < 0.05), pairwise comparisons of numbers of survivors in groups of animals were performed by Fisher's exact test. Death hazard ratios were calculated by the Mantel-Haenzel method, with P values assigned using the Gehan-Breslow-Wilcoxon test. The two-tailed Mann-Whitney U test was used to analyze differences in the mean day of death. Comparisons of body weight curves were performed using two-way analysis of variance. Analyses were performed using Instat and Prizm software (GraphPad Software, San Diego, CA). Statistical comparisons were made between treated and placebo groups and between monotherapy and drug combination groups.
Drug-drug interactions were analyzed by the three-dimensional model of Prichard and Shipman (21), using the MacSynergy II software program at 95% confidence limits. Descriptions of additive, synergistic, and antagonistic interactions using this computer model have been described for influenza studies of mice (14). Briefly, 0 to 25, 25 to 50, 50 to 100, and >100 μm2 unit % calculated values in either a positive or negative direction using MacSynergy software are defined as insignificant synergy or antagonism, minor synergy or antagonism, moderate synergy or antagonism, or strong synergy or antagonism, respectively. The reported values represent the calculated volume of synergy minus the volume of antagonism for each set of study data.
Ethical treatment of laboratory animals.
This study was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University. The work was performed in the University's AAALAC-accredited Laboratory Animal Research Center. The research was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (20a).
RESULTS
Inhibition of viruses in cell culture.
The susceptibilities of influenza A/NWS/33 (H1N1), A/Victoria/3/75 (H3N2), and A/Duck/MN/1525/81 (H5N1) viruses to oseltamivir carboxylate and T-705 were evaluated in viral CPE inhibition assays (Table 1). Oseltamivir carboxylate was only weakly inhibitory to the H1N1 virus in culture (3.7 μM) but was highly inhibitory by neuraminidase assay (0.94 nM). The low potency of the compound against the A/NWS virus in cell culture has been reported previously (25) and suggests weak binding of the virus to MDCK cells that reduces the need for viral neuraminidase activity. More potent inhibition of viral CPE was seen against the H3N2 and H5N1 viruses (0.02 and 0.16 μM, respectively) and is in agreement with published results (25). Oseltamivir carboxylate was most inhibitory to the A/Victoria virus in culture. T-705 was inhibitory to the three viruses in the range of 1.4 to 4.3 μM, with the A/Duck virus being least sensitive and A/Victoria being most sensitive to inhibition. The in vitro activity of T-705 has been reported previously for the A/Duck/MN/1525 (H5N1) virus infection (23) but not for infections with the other two viruses. Both of the compounds were not toxic to uninfected cells at ≤1,000 μM.
TABLE 1.
Activities of oseltamivir carboxylate and T-705 against influenza A/NWS (H1N1), A/Victoria (H3N2), and A/Duck (H5N1) virus infections in cell culture and neuraminidase inhibition assays
| Compound | Mean result ± SD fora: |
|||||
|---|---|---|---|---|---|---|
| A/NWS (H1N1) |
A/Victoria (H3N2) |
A/Duck (H5N1) |
||||
| CPE EC50 | NA IC50 | CPE EC50 | NA IC50 | CPE EC50 | NA IC50 | |
| Oseltamivir carboxylate | 3.7 ± 3.4 | 0.94 ± 0.25 | 0.02 ± 0.01 | 0.46 ± 0.05 | 0.16 ± 0.05 | 2.31 ± 0.27 |
| T-705 | 2.0 ± 0.5 | — | 1.4 ± 0.8 | — | 4.3 ± 1.9 | — |
Results are from 3 independent experiments. CPE EC50, 50% effective concentration (μM) for inhibition of virus-induced cytopathic effect (CPE) in MDCK cells, determined by neutral red dye uptake; NA IC50, 50% inhibitory concentration (nM) of viral neuraminidase (NA) activity, determined by chemiluminescence assay; —, no inhibition at ≤100 μM.
Inhibition of viral neuraminidase activity.
Oseltamivir carboxylate and T-705 were evaluated for inhibitory effects on influenza virus neuraminidase activity in a 96-well microplate assay (Table 1). Oseltamivir carboxylate was most inhibitory to the H3N2 neuraminidase and least inhibitory to the H5N1 neuraminidase. This pattern of inhibition of H1N1, H3N2, and H5N1 viral neuraminidases has been described previously (18, 30). The potent inhibition of A/NWS neuraminidase activity is indicative that the compound is highly virus inhibitory (demonstrated in animal studies described below) in spite of the weak activity in MDCK cell culture assays (described above). T-705 was not inhibitory to viral neuraminidase at ≤100 μM, which was expected.
In vivo toxicities of oseltamivir and T-705 used alone and in combination.
Uninfected mice were treated with the compounds alone at up to 100 mg/kg/day for 7 days or in combination using this dose of each inhibitor. No adverse effects were seen in terms of body weight loss or appearance of the animals (data not shown). T-705 has been used at higher doses than 100 mg/kg/day without adverse effects (23).
Treatment of influenza A/NWS/33 (H1N1) infection in mice.
Table 2 presents survival results during the treatment of the H1N1 virus infection. T-705 alone was not effective in preventing death at 20 mg/kg/day. Oseltamivir alone was highly protective at 1 mg/kg/day and 42% protective at 0.3 mg/kg/day. Oseltamivir doses of 0.1 and 0.03 mg/kg/day were not significantly protective, although a 25% survival benefit was achieved. The 0.1-mg/kg/day dose of oseltamivir caused a significant increase in the time to death. The combination of T-705 at 20 mg/kg/day with 1 mg/kg/day of oseltamivir was effective, but due to the high activity of oseltamivir alone, no conclusions can be made about the added benefit of the combination on survival. However, 20 mg/kg/day of T-705 combined with weaker doses of oseltamivir (0.3 and 0.1 mg/kg/day) afforded protection significantly greater than that of oseltamivir alone (P < 0.01). The 0.03-mg/kg/day dose of oseltamivir combined with T-705 did not significantly improve survival over oseltamivir alone. The treatment delayed the time to death, however. Significantly lower risks of death were seen for all groups (0.06 to 0.34 hazard ratios) except for the group treated with T-705 alone. Three-dimensional analyses of the number of survivors/total number of mice per group and hazard ratio data indicated volumes of synergy of 141 and 177, respectively, which were indicative of strong synergy. Three-dimensional plots of the results are shown in Fig. S1 in the supplemental material.
TABLE 2.
Effects of combinations of T-705 and oseltamivir on an influenza A/NWS/33 (H1N1) virus infection in mice with treatments started 24 h after infectiona
| Compound (mg/kg/day) | No. of survivors/ total no. of mice per group | Mean day of deathb ± SD | Hazard ratio |
|---|---|---|---|
| T-705 (20) | 1/12 | 10.4 ± 3.2 | 0.52 |
| Os (1) | 11/12*** | 11.0 | 0.08*** |
| Os (0.3) | 5/12* | 9.1 ± 2.1 | 0.28** |
| Os (0.1) | 3/12 | 10.7 ± 2.8* | 0.34* |
| Os (0.03) | 3/12 | 9.0 ± 1.3 | 0.45 |
| T-705 (20) + Os (1) | 12/12*** | 0.06*** | |
| T-705 (20) + Os (0.3) | 12/12*** φ | 0.06*** | |
| T-705 (20) + Os (0.1) | 11/12*** φ | 11.0 | 0.07*** |
| T-705 (20) + Os (0.03) | 4/12 | 11.0 ± 3.0* | 0.25** |
| Placebo | 1/20 | 8.8 ± 1.8 | 1.00 |
Oral treatments were given twice a day for 5 days starting 24 h after infection. Os, oseltamivir; *, P < 0.05, **, P < 0.01, and ***, P < 0.001 compared to placebo group; φ, P < 0.01 compared to either compound used alone.
Results for day of death are shown for mice that died prior to day 21.
Comparison of efficacy of Tamiflu capsules to pure oseltamivir phosphate.
The majority of animal studies presented here were conducted using oseltamivir derived from Tamiflu capsules, our primary source of the drug. The use of this material may not be optimal because the capsules contain filler material that may affect the activity of oseltamivir and/or T-705. The procedure described above to prepare the compound for treatment of mice (weighing out the entire 75-mg capsule each time) was designed to minimize errors due to uneven distribution of oseltamivir in the capsule. An assumption was made that each capsule contained 75 mg.
We hypothesized that treatment of mice with Tamiflu capsules would give results comparable to those for treatment with pure oseltamivir. To test this hypothesis, treatment of an influenza A/NWS H1N1 virus infection was conducted using a study design very similar to that of the experiment whose results are shown in Table 2, the results of which are presented in Table 3. There was no statistically significant difference between the efficacy of capsules and that of pure drug in any treatment group. No inhibition of the combination of oseltamivir plus T-705 was seen between Tamiflu and pure oseltamivir. It appeared that a slightly better effect was achieved by treatment with the capsules than with pure oseltamivir phosphate, based upon the number of survivors. Further research into this issue was conducted in consultation with a company representative. We were informed that the 75-mg dose in Tamiflu capsules represents the free base. Accounting for the molecular-weight difference between oseltamivir and oseltamivir phosphate means that the capsules actually contain 98.5 mg of oseltamivir phosphate. Thus, the experiment whose results are reported in Table 3 used oseltamivir phosphate from Tamiflu at 1.31 times the dose of pure oseltamivir phosphate. This helps explain the differences in the results.
TABLE 3.
Efficacy of oseltamivir from Tamiflu capsules compared to that of pure oseltamivir (as oseltamivir phosphate) in anti-influenza virus activity and their activities when combined with T-705 with treatments started 24 h after infectiona
| Compound (mg/kg/day) | No. of survivors/ total no. of mice per group | Mean day of deathb ± SD | Hazard ratio |
|---|---|---|---|
| T-705 (20) | 1/10 | 12.2 ± 2.2*** | 0.32** |
| Tam (1) | 10/10*** | 0.08*** | |
| Tam (0.3) | 9/10*** | 12.0 | 0.10*** |
| Tam (0.1) | 1/10 | 10.0 ± 1.9 | 0.55 |
| Os (1) | 10/10*** | 0.08*** | |
| Os (0.3) | 5/10** | 13.6 ± 2.7** | 0.16*** |
| Os (0.1) | 0/10 | 9.5 ± 1.0 | 0.93 |
| T-705 (20) + Tam (0.3) | 10/10*** | 0.08*** | |
| T-705 (20) + Tam (0.1) | 8/10*** φ | 11.0 ± 2.8 | 0.12*** |
| T-705 (20) + Os (0.3) | 10/10*** | 0.08*** | |
| T-705 (20) + Os (0.1) | 4/10* | 12.7 ± 2.3** | 0.19*** |
| Placebo | 1/20 | 9.2 ± 1.5 | 1.00 |
Oral treatments were given twice a day for 5 days starting 24 h after infection. Os, oseltamivir; Tam, Tamiflu; *, P < 0.05, **, P < 0.01, and ***, P < 0.001 compared to placebo group; φ, P < 0.01 compared to either compound used alone.
Results for day of death are shown for mice that died prior to day 21.
Besides validating that Tamiflu capsules can successfully be used for treating the infections and assurance that it performs at least as well as pure oseltamivir (if not better), this study also served to confirm the results shown in Table 2. T-705 at 20 mg/kg/day combined with oseltamivir at 0.1 mg/kg/day produced higher survival rates than either compound used alone. The monotherapy doses produced slightly different numbers of survivors between the two studies, however. This is attributed to biological variation from experiment to experiment. Hazard ratio analysis for the experiment indicated that all treated groups were significantly different from the placebo group. Three-dimensional analysis of the data for Tamiflu plus T-705 gave volume-of-synergy values of 70 for the number of survivors/total number of mice per group data and 35 for the hazard ratio results, indicating moderate and weak synergy, respectively. The same analysis for oseltamivir phosphate plus T-705 showed a volume-of-synergy value of 75 for the number of survivors/total number of mice per group data and 40 for the hazard ratio data, demonstrating moderate and minor synergy, respectively. Three-dimensional plots of these results are not shown but are similar to those presented in Fig. S1 in the supplemental material (except for the synergy being of lesser magnitude).
Treatment of influenza A/Victoria/3/75 (H3N2) infection in mice.
The results of treatment of the lethal H3N2 infection with T-705 and oseltamivir alone and in combination are reported in Table 4. T-705 was 70 to 80% protective at 50 and 100 mg/kg/day. Oseltamivir was 60% protective at 50 mg/kg/day. The 25-mg/kg/day dose of each compound was not significantly protective. Drug combinations generally produced more survivors than each compound separately, but most of the increases could not be validated statistically. However, the 25-mg/kg/day dose of T-705 combined with the 25-mg/kg/day dose of oseltamivir protected 90% of mice, compared to 10 to 11% survival for either group alone, which was significant. Hazard ratio analysis indicated significantly lower risks of death for all groups (0.06 to 0.15) except for monotherapy with T-705 (25 mg/kg/day) and oseltamivir (25 mg/kg/day). Three-dimensional analysis of the data gave volume-of-synergy values of 153 for the number of survivors/total number of mice per group data and 284 for the hazard ratio results, indicating strong synergy. Three-dimensional plots of the results are shown in Fig. S2 in the supplemental material.
TABLE 4.
Effects of combinations of T-705 and oseltamivir on an influenza A/Victoria/3/75 (H3N2) virus infection in mice with treatments started 24 h after infectiona
| Compound (mg/kg/day) | No. of survivors/ total no. of mice per group | Mean day of deathb ± SD | Hazard ratio |
|---|---|---|---|
| T-705 (100) | 7/9* | 5.5 ± 0.7 | 0.15* |
| T-705 (50) | 7/10* | 8.3 ± 1.2 | 0.13* |
| T-705 (25) | 1/10 | 7.9 ± 1.5 | 0.80 |
| Os (50) | 6/10* | 8.8 ± 1.5 | 0.15* |
| Os (25) | 1/9 | 7.5 ± 1.3 | 1.01 |
| T-705 (100) + Os (50) | 10/10* | 0.06* | |
| T-705 (100) + Os (25) | 10/10* | 0.06* | |
| T-705 (50) + Os (50) | 9/10* | 8.0 | 0.08* |
| T-705 (50) + Os (25) | 9/10* | 8.0 | 0.08* |
| T-705 (25) + Os (50) | 10/10* | 0.06* | |
| T-705 (25) + Os (25) | 9/10* φ | 8.0 | 0.08* |
| Placebo | 0/20 | 8.2 ± 1.1 | 1.00 |
Oral treatments were given twice a day for 7 days starting 24 h after infection. Os, oseltamivir; *, P < 0.001, compared to placebo group; φ, P < 0.01 compared to either compound used alone.
Results for day of death are shown for mice that died prior to day 21.
Treatment of influenza A/Duck/MN/1525/81 (H5N1) infections in mice.
The results of treatment of the lethal H5N1 infection with T-705 and oseltamivir alone and in combination are reported in Table 5. In this experiment, the first treatment was given 24 h after virus exposure, as was done for the H1N1 and H3N2 virus infections. T-705 was 30 to 70% protective at 25 to 100 mg/kg/day. Oseltamivir gave no protection at these same doses. The two highest doses of T-705 in combination with the three doses of oseltamivir caused significant increases in the mean time to death. Treatment with 100 mg/kg/day of T-705 in combination with oseltamivir provided increased numbers of survivors compared to the numbers with monotherapy. Treatment with 50 mg/kg/day of T-705 in combination with oseltamivir provided protection similar to that with T-705 alone. Treatment with 25 mg/kg/day of T-705 in combination with oseltamivir at 25 and 50 mg/kg/day provided increased numbers of survivors compared to the numbers with monotherapy. All of the combinations exhibited significantly lower risks of death (0.14 to 0.29), as did T-705 (50 mg/kg/day) alone. Three-dimensional analysis of the data gave volume-of-synergy values of 170 for the number of survivors/total number of mice per group data and 52 for the hazard ratio results, demonstrating strong and weak synergy, respectively. Three-dimensional plots of the results are shown in Fig. S3 in the supplemental material.
TABLE 5.
Effect of combinations of T-705 and oseltamivir on an influenza A/Duck/MN/1525/81 (H5N1) virus infection in mice with treatments started 24 h after infectiona
| Compound (mg/kg/day) | No. of survivors/ total no. of mice per group | Mean day of deathb ± SD | Hazard ratio |
|---|---|---|---|
| T-705 (100) | 4/10** | 7.8 ± 1.0** | 0.10*** |
| T-705 (50) | 7/10*** | 8.7 ± 0.6** | 0.06*** |
| T-705 (25) | 3/10* | 8.6 ± 5.3 | 0.26 |
| Os (100) | 0/10 | 7.8 ± 0.4*** | 0.10*** |
| Os (50) | 0/10 | 8.4 ± 1.6*** | 0.14** |
| Os (25) | 0/10 | 7.7 ± 0.9** | 0.16** |
| T-705 (100) + Os (100) | 9/10*** | 11.0 | 0.06*** |
| T-705 (100) + Os (50) | 7/10*** | 8.3 ± 4.0 | 0.11** |
| T-705 (100) + Os (25) | 8/10*** | 9.0 ± 5.7 | 0.09*** |
| T-705 (50) + Os (100) | 7/10*** | 11.3 ± 4.0*** | 0.06*** |
| T-705 (50) + Os (50) | 5/10** | 10.2 ± 3.9*** | 0.07*** |
| T-705 (50) + Os (25) | 8/10*** | 8.0 ± 0.0* | 0.07*** |
| T-705 (25) + Os (100) | 3/10* | 10.1 ± 2.0*** | 0.07*** |
| T-705 (25) + Os (50) | 5/10** | 9.0 ± 2.2*** | 0.07*** |
| T-705 (25) + Os (25) | 7/10*** | 9.0 ± 1.7* | 0.07*** |
| Placebo | 0/20 | 6.6 ± 0.7 | 1.00 |
Oral treatments were given twice a day for 7 days starting 24 h after infection. Os, oseltamivir; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results for day of death are shown for mice that died prior to day 21.
Because of the lack of efficacy of oseltamivir in the A/Duck model when treatments were initiated 24 h after infection, a second experiment was performed where treatments began 2 h prior to infection (Table 6). T-705 was 100% protective at doses of 40 and 80 mg/kg/day, with no protective activity seen at 20 mg/kg/day. However, a significant increase in the time to death was noted at the 20-mg/kg/day dose. Oseltamivir gave no significant protection at doses of 10, 20, and 40 mg/kg/day, but all three doses significantly increased the time to death. Because the mice that were treated with T-705 alone at 40 and 80 mg/kg/day survived, the addition of oseltamivir could not alter the outcome unless there was antagonism. In this case, the combinations provided 90 to 100% protection. Combining the ineffective 20-mg/kg/day dose of T-705 with the three ineffective doses of oseltamivir resulted in 60 to 80% protection. This increase in protection was significant compared to the survival rates with either compound used alone. The hazard ratios for all treatment groups were significant compared to those for the placebo group. Analysis of the data by the three-dimensional method gave volume-of-synergy values of 180 for the number of survivors/total number of mice per group data and 38 for the hazard ratio data, indicating strong and minor synergy, respectively. Three-dimensional plots of the results are shown in Fig. S4 in the supplemental material.
TABLE 6.
Effect of combinations of T-705 and oseltamivir on an influenza A/Duck/MN/1525/81 (H5N1) virus infection in mice with treatments started 2 h before infectiona
| Compound (mg/kg/day) | No. of survivors/ total no. of mice per group | Mean day of deathb ± SD | Hazard ratio |
|---|---|---|---|
| T-705 (80) | 10/10** | 0.06** | |
| T-705 (40) | 10/10** | 0.06** | |
| T-705 (20) | 0/10 | 8.7 ± 2.1** | 0.10** |
| Os (40) | 2/10 | 7.4 ± 0.9* | 0.12** |
| Os (20) | 0/10 | 8.8 ± 0.8** | 0.06** |
| Os (10) | 0/10 | 8.3 ± 1.2** | 0.09** |
| T-705 (80) + Os (40) | 10/10** | 0.06** | |
| T-705 (80) + Os (20) | 10/10** | 0.06** | |
| T-705 (80) + Os (10) | 9/10** | 12.0 | 0.06** |
| T-705 (40) + Os (40) | 9/10** | 8.0 | 0.06** |
| T-705 (40) + Os (20) | 10/10** | 0.06** | |
| T-705 (40) + Os (10) | 10/10** | 0.06** | |
| T-705 (20) + Os (40) | 8/10** φ | 9.5 ± 2.1* | 0.06** |
| T-705 (20) + Os (20) | 6/10** φ | 13.0 ± 3.4* | 0.06** |
| T-705 (20) + Os (10) | 8/10**φφ | 8.5 ± 0.7* | 0.06** |
| Placebo | 0/20 | 6.0 ± 0.7 | 1.00 |
Oral treatments were given twice a day for 7 days starting 2 h before infection. Os, oseltamivir; *, P < 0.01, and **, P < 0.001 compared to placebo group; φ, P < 0.05, and φφ, P < 0.001 compared to either compound used alone.
Results for day of death are shown for mice that died prior to day 21.
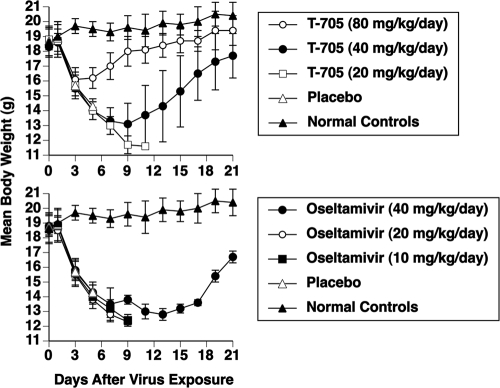
Figure 1 displays body weights during monotherapy of the infection (the same experiment for which results are shown in Table 6). Body weights fell in parallel with those of the placebo group for 3 days in the T-705 (80 mg/kg/day) group and for 7 days in the T-705 (40 mg/kg/day) group. After that time, the animals began to recover. The difference in body weight curves between the 40- and 80-mg/kg/day groups from days 3 to 21 of the infection was highly significant (P < 0.001). Body weights in the T-705 (20 mg/kg/day) group continued to fall until the mice died from the infection. In the oseltamivir groups, body weights declined for 7 to 9 days, with only 2 mice in the 40-mg/kg/day group surviving the infection and showing signs of recovery starting on day 15.
FIG. 1.
Effects of monotherapy with T-705 and oseltamivir on mean body weights (± standard deviation) during an influenza A/Duck/MN/1525/81 (H5N1) virus infection in mice. Oral treatments were given twice a day for 7 days starting 2 h before virus exposure. Survival data accompanying the figures are presented in Table 6.
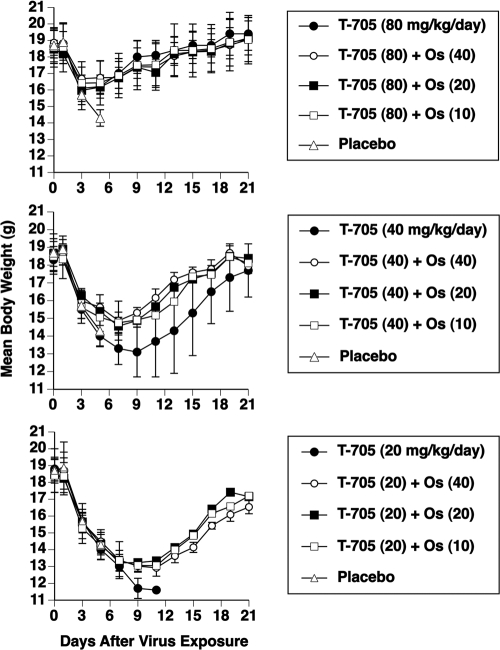
Figure 2 shows body weights during combination treatment of the infection for which results are shown in Table 6. At the 80-mg/kg/day dose of T-705, there was no significant improvement in body weight afforded by combining it with oseltamivir. At the 40-mg/kg/day dose of T-705, the addition of oseltamivir at the three doses resulted in significant improvements in body weight compared to those seen with T-705 alone (P < 0.001 for all three comparisons) from days 3 to 21 of the infection. Since all of the mice treated with 20 mg/kg/day of T-705 were dead by day 11, a statistical comparison of that group with the combination treatment was not possible. However, body weight in the group treated only with T-705 dropped to a lower level prior to death than did body weight in the combination groups, indicating superiority for the drug combination groups.
FIG. 2.
Effects of combination treatment with T-705 and oseltamivir (Os) on mean body weights (± standard deviation) during an influenza A/Duck/MN/1525/81 (H5N1) infection in mice. Oral treatments were given twice a day for 7 days starting 2 h before virus exposure. Survival data accompanying the figures are presented in Table 6. Doses in mg/kg/day are shown in parentheses.
DISCUSSION
In these studies, different dosage combinations of oseltamivir and T-705 produced improvements in survival and body weight compared to those seen with either compound used alone. At the lower doses of T-705, the combinations containing oseltamivir produced statistically significant improvements in survival and in body weight. Analysis of the survival data demonstrated that certain low-dose combinations of T-705 and oseltamivir produced synergistic interactions. At higher doses, where greater protection was provided by treatment with oseltamivir or T-705 alone, increased survival was also demonstrated. The differences were not significant due to limitations in numbers of animals and because survival approached the limit of protection (100%). In the second H5N1 experiment, where a high degree of protection was afforded, the significant improvement in body weight was indicative that the combination was superior to T-705 alone (Fig. 2), since improved body weight correlates with milder disease.
Differences were noted in the effectiveness of treatment of H1N1, H3N2, and H5N1 virus infections with oseltamivir and T-705. The A/NWS/33 (H1N1) virus infection responded most favorably to treatment with oseltamivir. A 5-day course of treatment with 1 mg/kg/day was sufficient to protect nearly all of the mice from death. In contrast, 50 mg/kg/day was only partially protective against A/Victoria/3/75 (H3N2). We had to extend treatment to 7 days to even get efficacy against the H3N2 virus. A 5-day course of oseltamivir treatment of this infection was ineffective (data not shown). Oseltamivir did not have significant protective activity against the H5N1 virus infection when treatments were initiated either 2 h before or 24 h after infection. T-705 in the range of 20 to 25 mg/kg/day was not effective or only weakly active against the three viruses. However, T-705 was more effective against the H5N1 infection when treatments started at −2 h than at +24 h.
The reasons why oseltamivir and T-705 were less effective against the A/Duck (H5N1) virus than against the other viruses in mice are not understood. The results of the in vitro studies indicate that the H5N1 viral neuraminidase was inhibited less by oseltamivir carboxylate than the H3N2 and H1N1 neuraminidases, but the differences among them were small. This might make a difference in terms of drug efficacy. Viral neuraminidase inhibition was more relevant than cell culture assays for demonstrating efficacy in the case of the A/NWS (H1N1) virus, since poor cell culture activity of oseltamivir carboxylate was observed against this virus. Although T-705 is less potent than oseltamivir in vitro, it was more effective than oseltamivir against the H5N1 virus infections in mice. T-705 was slightly less potent against the H5N1 virus than against the other viruses in cell culture, but this may not necessarily explain why the A/Duck virus infection is more difficult to treat in mice.
One reason that the A/Duck virus may be more difficult to treat than the other viruses in vivo is its very rapid replication in mouse lungs (data not shown). This translates into an early time to death (compare mean days of death of placebo groups in Tables2 to 6). Compounds must be given early to combat such a rapidly evolving infection. Decreasing the A/Duck virus challenge dose does not appreciably lengthen the time to death but does cause some animals to survive the infection altogether (our unpublished data). Virulence factors (such as certain cytokines and chemokines) that could differ in their expression with infection with different virus strains may also be important in disease progression. Research in this area is in progress in our laboratory.
Another observation from the treatment of the A/Duck infection was the poor dose-responsive effect of T-705 seen in some studies, such as that whose results are shown in Table 5 where the 100-mg/kg/day dose gave less survival benefit than the 50-mg/kg/day dose. In other studies, depending upon timing and dose, a better dose-responsive effect was achieved. It may be that some mice are more prone to die from infection than others (creating intragroup variability), but this would be difficult to assess.
The variability in daily body weights during the H5N1 infection was greater in the 40-mg/kg/day T-705 group than in either the 80- or 20-mg/kg/day group (Fig. 1 and 2). We attribute this to the efficacy of the compound at this particular dose. The 80-mg/kg/day dose controlled the infection better than the 40-mg/kg/day dose, and the 20-mg/kg/day dose was largely ineffective. Some of the mice treated with 40 mg/kg/day of T-705 were close to dying from the infection (and consequently had a lower body weight) but managed to survive the infection. Other animals fared better and, thus, had higher body weights. It was this range of protection at the 40-mg/kg/day dose that resulted in more variability in body weights.
In these experiments, we did not investigate the effects of treatment on lung virus titer production. This would have added considerable numbers of animals to the already large studies. We have previously reported the effects of drug combinations (amantadine, ribavirin, and oseltamivir) on A/Duck (H5N1) virus titers (26). In these experiments, the combinations reduced lung virus production, but there were not necessarily differences seen in virus titers for the combinations versus monotherapy, in spite of a survival benefit with the combinations. The overall effect of treatment to reduce lung virus titers was small. Single time points (such as day 6 after infection) for assessing inhibition of lung virus production do not seem to reflect the benefit derived from the full course of treatment. Increases in numbers of survivors and improvements in body weight are more indicative of treatment efficacy.
Combination treatments may be necessary due to widespread emergence of drug-resistant viruses. Because of the high frequency of amantadine- (or rimantadine-) resistant viruses in nature, this may limit the use of either of these compounds as a combination agent. In support of this statement, treatment of amantadine-resistant virus infections with amantadine plus oseltamivir or ribavirin provided no improvement compared to using oseltamivir or ribavirin alone (15, 26). Although the combination of oseltamivir and ribavirin is effective, ribavirin has some undesirable toxic side effects. T-705 appears to be a safer compound to administer than ribavirin. As T-705 advances through clinical development, it may become a viable option for the treatment of seasonal (H1N1 and H3N2), pandemic (the recent 2009 emerging virus), and highly pathogenic (H5N1) influenza virus infections, either used alone or in combination with oseltamivir.
Supplementary Material
Acknowledgments
This work was supported by contracts NO1-AI-15435, NO1-AI-30048, and NO1-AI-30063 (awarded to Southern Research Institute) from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The contents of this article do not necessarily reflect the position or policy of the government, and no official endorsement should be inferred.
The investigators adhered to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (20a), and used facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Footnotes
Published ahead of print on 9 November 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Besselaar, T. G., D. Naidoo, A. Buys, V. Gregory, J. McAnerney, J. M. Manamela, L. Blumberg, and B. D. Schoub. 2008. Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg. Infect. Dis. 14:1809-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2009. Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:467-470. [PubMed] [Google Scholar]
- 3.Cheung, C. L., J. M. Rayner, G. J. Smith, P. Wang, T. S. Naipospos, J. Zhang, K. Y. Yuen, R. G. Webster, J. S. Peiris, Y. Guan, and H. Chen. 2006. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 193:1626-1629. [DOI] [PubMed] [Google Scholar]
- 4.Deyde, V. M., X. Xu, R. A. Bright, M. Shaw, C. B. Smith, Y. Zhang, Y. Shu, L. V. Gubareva. N. J. Cox, and A. I. Klimov. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 196:249-257. [DOI] [PubMed] [Google Scholar]
- 5.Dharan, N. J., L. V. Gubareva, J. J. Meyer, M. Okomo-Adhiambo, R. C. McClinton, S. A. Marshall, K. St. George, S. Epperson, L. Brammer, A. I. Klimov, J. S. Bresee, and A. M. Fry for the Oseltamivir-Resistance Working Group. 2009. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301:1034-1041. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson, B., E. Helgstrand, N. G. Johansson, A. Larsson, A. Misiorny, J. O. Norén, L. Philipson, K. Stenberg, G. Stening, S. Stridh, and B. Oberg. 1977. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 11:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fereidouni, S. R., T. C. Harder, and E. Starick. 2008. Rapid pathotyping of recent H5N1 highly pathogenic avian influenza viruses and of H5 viruses with low pathogenicity by RT-PCR and restriction enzyme cleavage pattern (RECP). J. Virol. Methods 154:14-19. [DOI] [PubMed] [Google Scholar]
- 8.Furuta, Y., K. Takahashi, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, Y. Watanabe, H. Narita, and K. Shiraki. 2002. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 46:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuta, Y., K. Takahashi, M. Kuno-Maekawa, H. Sangawa, S. Uehara, K. Kozaki, N. Nomura, H. Egawa, and K. Shiraki. 2005. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 49:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galabov, A. S., L. Simeonova, and G. Gegova. 2006. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type 1 (H3N2) influenza virus in mice. Antivir. Chem. Chemother. 17:251-258. [DOI] [PubMed] [Google Scholar]
- 11.Hata, M., M. Tsuzuki, Y. Goto, N. Kumagai, M. Harada, M. Hashimoto, S. Tanaka, K. Sakae, T. Kimura, H. Minagawa, and Y. Miyazaki. 2007. High frequency of amantadine-resistant influenza A (H3N2) viruses in the 2005-2006 season and rapid detection of amantadine-resistant influenza A (H3N2) viruses by MAMA-PCR. Jpn. J. Infect. Dis. 60:202-204. [PubMed] [Google Scholar]
- 12.Ilyushina, N. A., N. V. Bovin, R. G. Webster, and E. A. Govorkova. 2006. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 70:121-131. [DOI] [PubMed] [Google Scholar]
- 13.Ilyushina, N. A., E. A. Govorkova, and R. G. Webster. 2005. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology 341:102-106. [DOI] [PubMed] [Google Scholar]
- 14.Ilyushina, N. A., A. Hay, N. Yilmaz, A. C. Boon, R. G. Webster, and E. A. Govorkova. 2008. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob. Agents Chemother. 52:3889-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilyushina, N. A., E. Hoffmann, R. Salomon, R. G. Webster, and E. A. Govorkova. 2007. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antiviral Ther. 12:363-370. [PubMed] [Google Scholar]
- 16.Leneva, I. A., N. Roberts, E. A. Govorkova, O. G. Goloubeva, and R. G. Webster. 2000. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza virus. Antiviral Res. 48:101-115. [DOI] [PubMed] [Google Scholar]
- 17.Masihi, K. N., B. Schweiger, T. Finsterbusch, and H. Hengel. 2007. Low dose oral combination chemoprophylaxis with oseltamivir and amantadine for influenza A virus infections in mice. J. Chemother. 19:295-303. [DOI] [PubMed] [Google Scholar]
- 18.McKimm-Breschkin, J., T. Trivedi, A. Hampson, A. Hay, A. Klimov, M. Tashiro, F. Hayden, and M. Zambon. 2003. Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob. Agents Chemother. 47:2264-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer, A., A. Lackenby, O. Hungnes, B. Lina, S. van-der-Werf, B. Schweiger, M. Opp, J. Paget, J. van-de-Kassteele, A. Hay, and M. Zambon on behalf of the European Influenza Surveillance Scheme. 2009. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg. Infect. Dis. 15:552-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossad, S. B. 2009. The resurgence of swine-origin influenza A (H1N1). Cleve. Clin. J. Med. 76:337-343. [DOI] [PubMed] [Google Scholar]
- 20a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 21.Prichard, M. N., and C. Shipman, Jr. 1990. A three dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181-206. [DOI] [PubMed] [Google Scholar]
- 22.Sheu, T. G., V. M. Deyde, M. Okomo-Adhiambo, R. J. Garten, X. Xu, R. A. Bright, E. N. Butler, T. R. Wallis, A. I. Klimov, and L. V. Gubareva. 2008. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 52:3284-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidwell, R. W., D. L. Barnard, C. W. Day, D. F. Smee, K. W. Bailey, M.-H. Wong, J. D. Morrey, and Y. Furuta. 2007. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 51:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smee, D. F., K. W. Bailey, A. C. Morrison, and R. W. Sidwell. 2002. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy 48:88-93. [DOI] [PubMed] [Google Scholar]
- 25.Smee, D. F., J. H. Huffman, A. C. Morrison, D. L. Barnard, and R. W. Sidwell. 2001. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activity. Antimicrob. Agents Chemother. 45:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smee, D. F., B. L. Hurst, M.-H. Wong, K. W. Bailey, and J. D. Morrey. 2009. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob. Agents Chemother. 53:2120-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smee, D. F., M.-H. Wong, K. W. Bailey, and R. W. Sidwell. 2006. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir. Chem. Chemother. 17:185-192. [DOI] [PubMed] [Google Scholar]
- 28.Streeter, D. G., J. T. Witkowski, G. P. Khare, R. W. Sidwell, R. J. Bauer, R. K. Robins, and L. N. Simon. 1973. Mechanism of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad spectrum antiviral agent. Proc. Natl. Acad. Sci. U. S. A. 70:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi, K., Y. Furuta, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, and K. Shiraki. 2003. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir. Chem. Chemother. 14:235-241. [DOI] [PubMed] [Google Scholar]
- 30.Wetherall, N. T., T. Trivedi, J. Zeller, C. Hodges-Savola, M. McKimm-Breschkin, J. L. McKimm-Breschkin, M. Zambon, and F. G. Hayden. 2003. Evaluation of neuraminidase enzyme assays using different substrates to measure susceptibility of influenza virus clinical isolates to neuraminidase inhibitors: report of the neuraminidase inhibitor susceptibility network. J. Clin. Microbiol. 41:742-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.