Abstract
Prior studies have shown that delays in treatment are associated with increased mortality in patients with candidemia. The purpose of this study was to measure three separate time periods comprising the diagnosis and treatment of candidemia and to determine which one(s) is associated with hospital mortality. Patients with blood cultures positive for Candida spp. were identified. Subjects were excluded if no antifungal therapy was given or if there was preexisting antifungal therapy. Collected data included the time from blood culture collection to positivity (incubation period), the time from blood culture positivity to provider notification (provider notification period), and the time from provider notification to the first dose of antifungal given (antifungal initiation period). These times were assessed as predictors of inpatient mortality. A repeat analysis was done with adjustments for age, sex, race, underlying cancer, catheter removal, APACHE III score, acute renal failure, neutropenia, and non-Candida albicans species. A total of 106 episodes of candidemia were analyzed. The median incubation time was 32.1 h and was associated with mortality (univariate hazard ratio per hour, 1.025; P = 0.001). The median provider notification and antifungal initiation periods were 0.3 and 7.5 h, respectively, and were not associated with mortality. Adjusted analysis yielded similar results. For cancer patients with candidemia, the incubation period accounts for a significant amount of time, compared with the provider notification and antifungal initiation times, and is associated with in-hospital mortality. Strategies to shorten the incubation time, such as utilizing rapid molecularly based diagnostic methods, may help reduce in-hospital mortality.
Candidemia has been reported to be the fourth most common hospital-associated bloodstream infection in the United States (48). Estimates of its attributable mortality are substantial (14, 47). Among cancer patients, candidemia also contributes to overall mortality. According to one study, candidemia was thought to play either a primary or a secondary role in about two-thirds of all deaths in infected cancer patients (46).
For the general patient with candidemia, reported predictors of mortality include, but are not limited to, age, duration of hospitalization, severity-of-illness score, acute renal failure, intensive care unit (ICU) stay, retention of a central venous catheter, mechanical ventilation, non-Candida albicans Candida species, and persistence of the candidemia (5, 11, 21, 28). These variables are also risk factors in the cancer patient population, with some additional predictors: neutropenia, hematologic malignancy, visceral dissemination, poor performance status, and stage of disease (2, 29, 43).
In addition to all of these associations, two studies that were performed on general patients with candidemia have demonstrated that a delay in antifungal therapy is associated with an increase in mortality (13, 27). In both of these studies, delays were analyzed by measuring the time from blood culture collection to the initiation of antifungal therapy. However, during this overall time interval, several different events occur: the collected blood culture incubates until it becomes positive; the positive result is conveyed to the health care provider, who interprets its clinical significance; the health care provider decides whether to prescribe therapy, and if so, the drug has to be administered to the patient. Although the overall time interval has been demonstrated to be associated with mortality, it is not clear if a delay in one, two, or all of these components leads to the observed increased mortality, since no prior studies have examined these events separately. In addition, no prior studies have examined antifungal delay specifically for the cancer patient population. In this study, we sought to measure the duration of each event occurring from blood culture collection to the initiation of antifungal therapy in episodes of candidemia in cancer patients and to see which, if any, of these events were associated with mortality.
MATERIALS AND METHODS
Study population and design.
A historical cohort study was performed at Memorial Sloan-Kettering Cancer Center, a 432-bed tertiary cancer center located in New York City. Subjects of the study consisted of patients who had at least one blood culture positive for Candida species from 1 January 2005 to 31 December 2007. The outcome of interest was in-hospital death. If a patient had multiple blood cultures positive for Candida during a hospitalization, only the first blood culture was counted as an observation. Subjects were excluded if the patient died prior to the culture result, if no antifungal treatment was given, or if the patient was already on preexisting systemic antifungal therapy at the time of blood culture collection. The study was approved by our institutional review board.
Blood culture protocol.
Our institution employs the Becton Dickinson BACTEC 9240 instrumented blood culture system, which provides continuous automated fluorescent monitoring of drawn blood cultures for the growth of microorganisms. When a blood culture becomes positive, the microbiology staff performs initial microscopic assessment. Our hospital protocol requires that, in addition to entering these findings into the laboratory system, the staff notify the patient's health care provider of the results by phone. Beginning in August 2006, our institution began routine antifungal susceptibility testing of all Candida species isolated from blood culture.
Data collection.
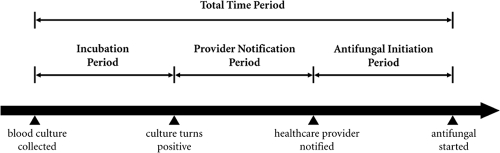
For each episode of candidemia, the following were recorded: date and time of blood culture collection, date and time of blood culture positivity, date and time of health care provider notification, and date and time of administration of the first antifungal agent. These data were obtained using computerized records from the microbiology laboratory, health care provider notification logs, and nursing records for medication administration. The following predefined time periods were then calculated: (i) incubation period (time needed for the blood culture to incubate and turn positive), (ii) provider notification period (time taken for the microbiologist to recognize yeast in the blood culture and to notify the health care provider), (iii) antifungal initiation period (time from provider notification to administration of the first dose of antifungal medication), and (iv) total time period (time from blood culture collection to antifungal initiation [or the sum of the three time periods listed above]).
In order to describe the distribution for each of the three individual time periods, histograms were constructed, and median times were calculated.
In addition, the following additional covariate data were collected via electronic medical records: age, sex, race (white versus nonwhite), type of underlying cancer (solid tumor versus hematologic malignancy), catheter removal, Acute Physiology and Chronic Health Evaluation (APACHE) III score (19) (at the time of blood culture collection), acute renal failure (as judged by the clinician), neutropenia (absolute neutrophil count of <500 cells/microliter), and Candida species (C. albicans versus a non-C. albicans species).
For the subset of candidemia episodes in which antifungal susceptibility results were available, therapy was judged to be inappropriate if the Candida isolate did not test susceptible to the first antifungal given, using Clinical and Laboratory Standards Institute (CLSI) definitions of antifungal susceptibility (8).
Analysis.
The variables described above were assessed as potential predictors of the inpatient mortality rate using Cox proportional hazards regression where the end point of interest was in-hospital death. Patients discharged alive were considered to be censored observations at the time of their discharge. First, all variables were analyzed separately in a univariate model. Variables with a univariate P value less than 0.25 were incorporated into a multivariate model. Variables considered for inclusion in the multivariate model included the three separate time periods (incubation, provider notification, antifungal initiation) and the other covariate data collected. Age, sex, and race were incorporated into the multivariate model regardless of their statistical significance in the univariate analysis. The total time period was not considered for multivariate analysis, because it represented the sum of the three separate time periods; incorporation of both total time and separate time periods would potentially lead to collinearity in the multivariate model. The appropriateness of therapy was also not considered for inclusion in the multivariate model, since it was determined only for a subset of patients. We used a P value cutoff of 0.05 for all other considerations of statistical significance. All analyses were performed using Stata, version 10.0 (2007; StataCorp, College Station, TX).
RESULTS
During the study period, there were 181 episodes of candidemia at our institution. Of these episodes, 67 observations were excluded based on study criteria. For another eight observations, information regarding the date and time of health care provider notification was unavailable, leaving a total of 106 observations for study.
Study population characteristics.
Table 1 shows the characteristics of the study sample. Approximately half of all patients were over the age of 60, and about half of the patients had an APACHE III score between 50 and 74. Most patients in our study population were white, did not have a hematologic malignancy, did not have acute renal failure, and were not neutropenic. In most cases, there was an existing central vascular catheter, which was removed. The appropriateness of therapy was assessed for 31 (29.2%) patients. Among these patients, there were no episodes of inappropriate therapy.
TABLE 1.
Study population characteristics
| Variablea | No. (%) of patients |
|---|---|
| Age (yr) | |
| 0-19 | 8 (7.55) |
| 20-39 | 17 (16.04) |
| 40-59 | 26 (24.53) |
| 60-79 | 44 (41.51) |
| 80+ | 11 (10.38) |
| Female sex | 52 (49.06) |
| Race | |
| White | 78 (73.58) |
| Black | 16 (15.09) |
| Other | 12 (11.32) |
| Hematologic malignancy | 17 (16.04) |
| Catheter removal | |
| No catheter | 13 (12.26) |
| No | 13 (12.26) |
| Yes | 80 (75.47) |
| APACHE III score | |
| 0-24 | 9 (8.49) |
| 25-49 | 24 (22.64) |
| 50-74 | 53 (50.00) |
| 75+ | 20 (18.87) |
| Acute renal failure | 27 (25.47) |
| Neutropenia (ANC, <500 cells/μl) | 6 (5.66) |
| Appropriate therapyb | |
| No | 0 (0.00) |
| Yes | 31 (29.25) |
| Evaluation not performed | 75 (70.75) |
| Total | 106 (100) |
APACHE, Acute Physiology and Chronic Health Evaluation; ANC, absolute neutrophil count.
The appropriateness of therapy was evaluated in 31 out of 106 cases (observations occurring on or after August 2006).
Table 2 shows the Candida species observed. The most frequently observed species was C. albicans (41.5%), followed by C. parapsilosis (19.8%) and C. glabrata (14.2%). Table 3 lists the first antifungal medication that was given. Echinocandins (59.4%) were given most frequently as initial therapy, followed by fluconazole (26.4%).
TABLE 2.
Candida species observed
| Organism | No. (%) of patients |
|---|---|
| Candida albicans | 44 (41.5) |
| Candida parapsilosis | 21 (19.8) |
| Candida glabrata | 15 (14.2) |
| Candida tropicalis | 14 (13.2) |
| Candida krusei | 3 (2.8) |
| Candida lusitaniae | 3 (2.8) |
| Candida guilliermondii | 2 (1.9) |
| Candida famata | 1 (0.9) |
| Candida kefyr | 1 (0.9) |
| Multiple Candida species | 2 (1.9) |
| Total | 106 (100) |
TABLE 3.
First antifungal drug given
| Drug | No. (%) of patients |
|---|---|
| Echinocandina | 63 (59.4) |
| Fluconazole | 28 (26.4) |
| Liposomal amphotericin B (AmBisome) | 14 (13.2) |
| Voriconazole | 1 (0.9) |
| Total | 106 (100) |
This term refers to either caspofungin or micafungin. Due to changes in the hospital formulary, episodes occurring before August 2005 were treated with caspofungin, and episodes occurring in August 2005 or afterwards were treated with micafungin.
Distribution of time periods.
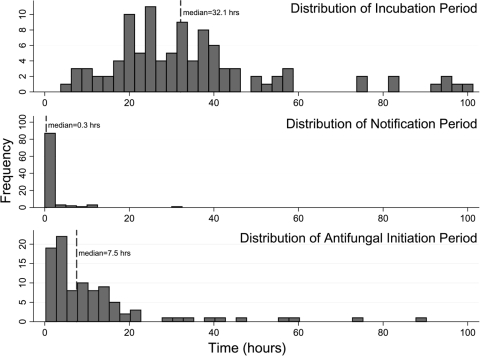
The time periods are graphically represented in Fig. 1. Figure 2 shows the distributions of the three defined time periods. The median total time was 43.2 h (95% confidence interval [95% CI], 39.0 to 49.0 h). The median incubation time was 32.1 h (95% CI, 27.9 to 34.8 h). In contrast, the median times for provider notification and antifungal initiation were much shorter: 0.3 h (95% CI, 0.27 to 0.41 h) and 7.5 h (95% CI, 4.2 to 9.8 h), respectively.
FIG. 1.
Timeline of events during an episode of candidemia. The following time periods are defined: (i) incubation period (time needed for blood culture to incubate and turn positive), (ii) provider notification period (time taken for microbiologist to recognize yeast in the blood culture and to notify the health care provider), (iii) antifungal initiation period (time from provider notification to administration of the first dose of antifungal medication), and (iv) total time period (time from blood culture collection to antifungal initiation [or the sum of the three time periods listed above]).
FIG. 2.
Distributions of the incubation, provider notification, and antifungal initiation periods. The median total time was 43.2 h (not shown in the figure). Confidence limits for the median estimates are given in Results.
Analysis of mortality.
Of the 106 episodes of candidemia, 25 (23.6%) of the subjects died during their hospitalization. Table 4 shows the results of the univariate analysis of in-hospital death. The total time period was significantly associated with mortality. An increase in the incubation period was significantly associated with mortality, but the provider notification and antifungal initiation periods were not. Increasing age and APACHE III score were also predictive of mortality. Sex, race, underlying cancer, central venous catheter removal, acute renal failure, neutropenia, and Candida species were not associated with mortality in this analysis. Since there were no observed cases of inappropriate therapy, univariate analysis was not performed on this variable.
TABLE 4.
Univariate Cox model of in-hospital mortality
| Variablea | Univariate HRb | P | 95% CI |
|---|---|---|---|
| Age (per yr) | 1.027 | 0.033 | 1.002-1.052 |
| Sex (female) | 1.257 | 0.578 | 0.562-2.812 |
| Race (nonwhite) | 0.695 | 0.447 | 0.273-1.773 |
| Underlying cancer (hematologic malignancy) | 0.619 | 0.397 | 0.204-1.879 |
| Central venous catheter removal | 0.549 | 0.170 | 0.233-1.292 |
| APACHE III score (per point) | 1.021 | 0.039 | 1.001-1.042 |
| Acute renal failure | 2.001 | 0.100 | 0.875-4.615 |
| Neutropenia (ANC, <500 cells/μl) | 0.566 | 0.457 | 0.126-2.535 |
| Organism (non-C. albicans) | 1.419 | 0.444 | 0.579-3.476 |
| Inappropriate therapy | −c | − | − |
| Total time (per h) | 1.013 | 0.023 | 1.002-1.025 |
| Incubation period (per h) | 1.025 | 0.001 | 1.011-1.040 |
| Provider notification period (per h) | 0.516 | 0.391 | 0.114-2.338 |
| Antifungal initiation period (per h) | 0.989 | 0.562 | 0.954-1.026 |
APACHE, Acute Physiology and Chronic Health Evaluation; ANC, absolute neutrophil count.
HR, hazard ratio.
−, hazard ratio estimates could not be calculated, because there were no cases of inappropriate therapy among the 31 episodes of candidemia evaluated.
Table 5 shows the results of the multivariate analysis for in-hospital death. Age, sex, race, central venous catheter removal, APACHE III, acute renal failure, and incubation period were included in the hazard model (demographic variables or variables with univariate P values of <0.25). Only the incubation period remained as a significant predictor of mortality. All other variables were not significant predictors in the multivariate model.
TABLE 5.
Multivariate Cox model of in-hospital mortality
| Variablea | Multivariate HRb | P | 95% CI |
|---|---|---|---|
| Age (per yr) | 1.021 | 0.165 | 0.992-1.05 |
| Sex (female) | 1.158 | 0.761 | 0.45-2.977 |
| Race (nonwhite) | 1.015 | 0.978 | 0.348-2.96 |
| Central venous catheter removal | 1.175 | 0.769 | 0.401-3.443 |
| APACHE III score (per point) | 1.006 | 0.632 | 0.981-1.033 |
| Acute renal failure | 1.400 | 0.573 | 0.435-4.504 |
| Incubation period (per h) | 1.026 | 0.005 | 1.008-1.045 |
APACHE, Acute Physiology and Chronic Health Evaluation.
HR, hazard ratio.
DISCUSSION
Prior studies by Morrell et al. (27) and Garey et al. (13) have shown that delaying antifungal therapy for the treatment of candidemia is associated with increased mortality. Both studies assessed this delay by measuring the time from blood culture collection until the start of antifungal treatment (defined in our study as the “total time period”). However, neither study was able to further assess the reason(s) for treatment delay. In their discussion, Garey et al. (13) list this as one of their study limitations. Our study examines the association with mortality more closely by separately examining the events that constitute this total time period. This has not been done previously.
Our results showed that the incubation period was predictive of hospital mortality for patients with candidemia. Based on the univariate hazard ratio estimate of 1.025, there would be a 1.025-fold increase in the hospital mortality rate for every additional hour of blood culture incubation. Based on this model, a 24-h delay in blood culture positivity would nearly double (1.823-fold increase) the risk of death. This result suggests that the association between the total time period and mortality is largely due to the time needed for blood cultures to incubate and turn positive.
In contrast to the incubation period, the provider notification and antifungal initiation periods were not significantly associated with mortality. However, one should not necessarily conclude that prompt action on the part of the microbiologist or health care professional is not needed or would truly have no effect. It is possible that a delay in any of the processes of care leading up to administration of antifungal therapy could lead to similar consequences of increased mortality. If our study had observed a greater range of values for the provider notification or antifungal initiation periods or if we had increased the number of observations, the study might have detected an association with mortality in all three time periods.
We performed an additional posthoc analysis by reanalyzing the final multivariate Cox model, using the total time period as a predictor instead of the incubation period. We found a somewhat weaker association (adjusted hazard ratio, 1.011; P = 0.109; 95% CI, 0.998 to 1.025) than in the original model, again suggesting that the incubation period is more clearly associated with hospital mortality than the other two periods.
At our institution, we found that the majority of the total time period is occupied by blood culture incubation, the length of which was found to be associated with mortality. Molecularly based approaches may represent an opportunity to reduce mortality by shortening the time to detection. Strategies specifically designed to detect Candida include a combined enzyme immunoassay (EIA) for detection of mannan antigen and antimannan antibodies (Platelia Candida-specific antigen and antibody assays; Bio-Rad Laboratories) (41), nucleic acid detection using PCR technology (23, 25-26, 44), and molecular beacons (34). Although it detects several types of fungi and is not specific to Candida, the beta-d-glucan (Fungitell) assay can also be useful in the early diagnosis of disease due to Candida (30).
Many of these methods appear to demonstrate good operating characteristics for detecting Candida and have much shorter turnaround times than conventional blood cultures. The TaqMan-based PCR, for example, was shown in a randomized clinical trial to be 90.9% sensitive and 100% specific for detecting target Candida species, and it took less than 6 h to complete (25).
Although these molecularly based methods show potential, additional supporting clinical data are needed to show that they can indeed provide clinical benefit in a real-time setting. In the absence of such evidence, the role of these tests is still poorly defined, and they remain at best a diagnostic adjunct. Furthermore, a number of practical issues would still need to be addressed prior to implementation. Ideally, such a test should be performed on site and with sufficient frequency to ensure rapid diagnosis, with results made available to health care providers in real time. Issues surrounding the cost of equipment and supplies, laboratory space, and trained personnel would have to be considered carefully prior to incorporation into a medical facility. PCR-based techniques have the added concern of false-positive results due to contamination (4). Deciding which patients to test is another important concern; a balance should exist between patient benefit, costs, and the positive predictive value of the test in a way that justifies sufficiently frequent testing.
Another way of bypassing the incubation time would be to provide prophylactic or preemptive antifungal therapy for individuals at high risk of having candidemia. Prior studies have attempted to identify patients at greatest risk for invasive candidiasis in the hopes of initiating an antifungal agent in a timely manner and improving clinical outcome. Some of the risk factors include total parenteral nutrition, major surgery, hemodialysis, Candida colonization, presence of a central venous catheter, and ICU admission (6, 37, 39). Unfortunately, the clinical profile of systemic candidiasis is very nonspecific. Prior attempts at developing rules for prophylactic or preemptive antifungal therapy for ICU adult patients have been unable to predict systemic candidiasis with good positive predictive value (22, 31, 32). The use of these rules could therefore lead to massive overtreating of patients, raising major concerns about toxicity, costs, and resistance. To date, clinical trials examining antifungal prophylaxis for critically ill adult patients have not demonstrated a clear benefit. Although some studies have shown a decrease in the incidence of invasive candidiasis among individuals receiving antifungal prophylaxis, most have not shown a benefit in mortality (10, 12, 35). Meta-analyses of these studies also demonstrate mixed results (9, 38, 42, 45). For all of these reasons, the strategy of initiating prophylactic or preemptive antifungal therapy remains controversial.
Several limitations and issues surrounding our study warrant discussion. A significant number of episodes of candidemia (67 observations) were excluded from the study. Since these episodes represent a nontrivial proportion of the body of candidemias seen at our institution, we will describe them briefly here. The most common reason for exclusion was preexisting antifungal use at the time of the positive blood culture result; this was the case for 55 of the 67 excluded patients. In the review of these cases, it was found that preexisting antifungal treatment was given for the following reasons: (i) antifungal prophylaxis for a hematopoietic stem cell transplant or chemotherapy for hematologic malignancies, (ii) therapy for Candida identified from a site other than blood, or (iii) empirical therapy for a patient who was febrile and neutropenic or critically ill. Because of our study population criteria, these patients were not studied. Therefore it is not clear whether our results should be considered generalizable to these patients. Furthermore, we also advise caution in generalizing these results to noncancer patients.
It is noteworthy that in our study of candidemia, longer incubation periods were associated with higher, rather than lower, mortality. This result differs from those of studies of bacterial bloodstream infections, which have shown that a shorter time to positivity is associated with increased mortality (18, 24, 36). Given these contrasting results, we considered potential confounding variables that may have affected our study.
First, we considered the possibility that different etiologies for candidemia, each with inherently different incubation periods, could account for the observed association between incubation time and mortality. For example, a patient with candidemia due to an infected tunneled venous catheter might have a shorter blood culture incubation time, since the infected catheter represents an endovascular source and may provide for a higher inoculum of Candida during blood culture collection. This is supported by work by Ben-Ami et al. (3), who demonstrated that a blood culture time to positivity less than or equal to 30 h for Candida was predictive of a catheter-related infection, whereas a time to positivity greater than 30 h suggested a noncatheter source. If patients with catheter infections had a lower mortality rate than those with noncatheter sources, this fact could explain the seemingly paradoxical increase in mortality seen with longer incubation times. For example, patients with catheter infections might have lower mortality because of catheter removal, as opposed to patients with nonremovable sources of infection.
In order to further examine the concern of differing etiologies for candidemia, we determined whether persistently positive blood cultures had any effect on mortality. We found that the number of positive blood cultures for each patient ranged from 1 to 11 but that there was no detectable difference in mortality. This result is not shown in our final results, since having a persistently positive blood culture as a variable would probably create a survival bias by requiring that the patient live long enough to have subsequent blood cultures drawn in the first place.
We also attempted to account for the general health of the patients in our analysis by adjusting for age and APACHE III score in our multivariate analysis; we found no notable changes in our results. Presumably, adjusting for general health would serve to minimize the possibility of bias due to differences in mortality among patients with differing etiologies for infection.
As a side note, the finding that the APACHE III score was a significant predictor of mortality in the univariate analysis but not in the multivariate analysis differs from the results of previous studies of candidemic patients, many of which showed that the severity of illness is a strong independent predictor of mortality (2, 11, 13, 15, 20, 27, 33). Although most prior studies examining the severity of illness in candidemic patients were performed using APACHE II rather than APACHE III, we do not believe our results were different because of differences in derivation between the two scores. APACHE III has been used in several prior studies of candidemia and has been shown to be predictive of death (1, 2). The lack of significance of APACHE III in the multivariate analysis of our study could be due to insufficient power. A similar result occurred in the study of candidemia conducted by Nolla-Salas and colleagues, in which the APACHE II score was a significant univariate predictor of mortality but was not significant in the combined model (28).
Next, we considered the possibility that the association between incubation time and death was confounded by the volume of blood taken. It is known that drawing a higher volume of blood during culture collection can increase microorganism recovery and shorten the time to positivity (17, 40). This could be a concern for patients for whom venipuncture is difficult. Since our institution does not typically measure the volume of blood drawn in each blood culture, we were unable to adjust for this possible confounder. However, since 87.7% of our study patients had central venous catheters at the time of candidemia, we suspect that blood collection was not difficult for most patients and that the volume of blood taken was generally uniform across subjects.
Finally, we considered the possibility that the association between incubation time and death could be explained by differences in Candida species. Although blood culture detection times seem to be similar for most Candida species, the time to positivity appears to be significantly longer for C. glabrata (3, 16). Since C. glabrata infections typically occur in sicker patients (7, 46), individuals for whom incubation times were longer may have experienced higher mortality rates because they were patients with C. glabrata infections. However, our univariate analysis did not show non-C. albicans species to be significantly associated with mortality. We further explored this possibility by recoding Candida species into C. glabrata and non-C. glabrata species as a posthoc analysis; again, we found no association with death in our study population (results not shown).
In summary, we found that the incubation time needed to detect candidemia by blood culture represents a significant proportion of the time from blood culture collection to institution of antifungal therapy in our cancer patient population. The length of this interval is an independent risk factor for mortality, with an estimated 1.025-fold increase in hospital mortality for every additional hour of incubation time. Strategies such as the use of non-culture-based molecular methods to shorten or bypass the incubation time may help to reduce in-hospital mortality.
Acknowledgments
We thank Kathy Gilhuley for assistance with microbiologic data collection.
Footnotes
Published ahead of print on 2 November 2009.
REFERENCES
- 1.Abi-Said, D., O. Uzun, and E. Anaissie. 1996. Use of severity score indexes for stratification of prognosis in hospitalized cancer patients with candidemia. Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J43. American Society for Microbiology, Washington, DC.
- 2.Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ami, R., M. Weinberger, R. Orni-Wasserlauff, D. Schwartz, A. Itzhaki, T. Lazarovitch, E. Bash, Y. Aharoni, I. Moroz, and M. Giladi. 2008. Time to blood culture positivity as a marker for catheter-related candidemia. J. Clin. Microbiol. 46:2222-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, J. 2008. Is real-time polymerase chain reaction ready for real use in detecting candidemia? Clin. Infect. Dis. 46:897-898. [DOI] [PubMed] [Google Scholar]
- 5.Blot, S., K. Vandewoude, E. Hoste, J. Poelaert, and F. Colardyn. 2001. Outcome in critically ill patients with candidal fungaemia: Candida albicans vs. Candida glabrata. J. Hosp. Infect. 47:308-313. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, and R. P. Wenzel. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin. Infect. Dis. 33:177-186. [DOI] [PubMed] [Google Scholar]
- 7.Bodey, G. P., M. Mardani, H. A. Hanna, M. Boktour, J. Abbas, E. Girgawy, R. Y. Hachem, D. P. Kontoyiannis, and I. I. Raad. 2002. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 112:380-385. [DOI] [PubMed] [Google Scholar]
- 8.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; third informational supplement, CLSI document M27-S3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Cruciani, M., F. de Lalla, and C. Mengoli. 2005. Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intensive Care Med. 31:1479-1487. [DOI] [PubMed] [Google Scholar]
- 10.Eggimann, P., P. Francioli, J. Bille, R. Schneider, M. M. Wu, G. Chapuis, R. Chiolero, A. Pannatier, J. Schilling, S. Geroulanos, M. P. Glauser, and T. Calandra. 1999. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit. Care Med. 27:1066-1072. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, V. J., M. Jones, J. Dunkel, S. Storfer, G. Medoff, and W. C. Dunagan. 1992. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 12.Garbino, J., D. P. Lew, J.-A. Romand, S. Hugonnet, R. Auckenthaler, and D. Pittet. 2002. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 28:1708-1717. [DOI] [PubMed] [Google Scholar]
- 13.Garey, K. W., M. Rege, M. P. Pai, D. E. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 14.Gudlaugsson, O., S. Gillespie, K. Lee, J. Vande Berg, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 15.Holley, A., J. Dulhunty, S. Blot, J. Lipman, S. Lobo, C. Dancer, J. Rello, and G. Dimopoulos. 2009. Temporal trends, risk factors and outcomes in albicans and non-albicans candidaemia: an international epidemiological study in four multidisciplinary intensive care units. Int. J. Antimicrob. Agents 33:554.e1-554.e7. [DOI] [PubMed] [Google Scholar]
- 16.Horvath, L. L., B. J. George, C. K. Murray, L. S. Harrison, and D. R. Hospenthal. 2004. Direct comparison of the BACTEC 9240 and BacT/ALERT 3D automated blood culture systems for Candida growth detection. J. Clin. Microbiol. 42:115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilstrup, D. M., and J. A. Washington. 1983. The importance of volume of blood cultured in the detection of bacteremia and fungemia. Diagn. Microbiol. Infect. Dis. 1:107-110. [DOI] [PubMed] [Google Scholar]
- 18.Khatib, R., K. Riederer, S. Saeed, L. B. Johnson, M. G. Fakih, M. Sharma, M. S. Tabriz, and A. Khosrovaneh. 2005. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin. Infect. Dis. 41:594-598. [DOI] [PubMed] [Google Scholar]
- 19.Knaus, W. A., D. P. Wagner, E. A. Draper, J. E. Zimmerman, M. Bergner, P. G. Bastos, C. A. Sirio, D. J. Murphy, T. Lotring, A. Damiano, et al. 1991. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619-1636. [DOI] [PubMed] [Google Scholar]
- 20.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 21.Labelle, A. J., S. T. Micek, N. Roubinian, and M. H. Kollef. 2008. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit. Care Med. 36:2967-2972. [DOI] [PubMed] [Google Scholar]
- 22.León, C., S. Ruiz-Santana, P. Saavedra, B. Almirante, J. Nolla-Salas, F. Alvarez-Lerma, J. Garnacho-Montero, and M. A. León. 2006. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit. Care Med. 34:730-737. [DOI] [PubMed] [Google Scholar]
- 23.Maaroufi, Y., J. M. De Bruyne, V. Duchateau, A. Georgala, and F. Crokaert. 2004. Early detection and identification of commonly encountered Candida species from simulated blood cultures by using a real-time PCR-based assay. J. Mol. Diagn. 6:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra, A. R., M. B. Edmond, B. A. Forbes, R. P. Wenzel, and G. M. Bearman. 2006. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J. Clin. Microbiol. 44:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMullan, R., L. Metwally, P. V. Coyle, S. Hedderwick, B. McCloskey, H. J. O'Neill, C. C. Patterson, G. Thompson, C. H. Webb, and R. J. Hay. 2008. A prospective clinical trial of a real-time polymerase chain reaction assay for the diagnosis of candidemia in nonneutropenic, critically ill adults. Clin. Infect. Dis. 46:890-896. [DOI] [PubMed] [Google Scholar]
- 26.Moreira-Oliveira, M. S., Y. Mikami, M. Miyaji, T. Imai, A. Z. Schreiber, and M. L. Moretti. 2005. Diagnosis of candidemia by polymerase chain reaction and blood culture: prospective study in a high-risk population and identification of variables associated with development of candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:721-726. [DOI] [PubMed] [Google Scholar]
- 27.Morrell, M., V. J. Fraser, and M. H. Kollef. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolla-Salas, J., A. Sitges-Serra, C. Leon-Gil, J. Martinez-Gonzalez, M. A. Leon-Regidor, P. Ibanez-Lucia, and J. M. Torres-Rodriguez. 1997. Candidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICU. Intensive Care Med. 23:23-30. [DOI] [PubMed] [Google Scholar]
- 29.Nucci, M., M. I. Silveira, N. Spector, F. Silveira, E. Velasco, T. Akiti, G. Barreiros, A. Derossi, A. L. Colombo, and W. Pulcheri. 1998. Risk factors for death among cancer patients with fungemia. Clin. Infect. Dis. 27:107-111. [DOI] [PubMed] [Google Scholar]
- 30.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. Vazquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 31.Ostrosky-Zeichner, L., C. Sable, J. Sobel, B. D. Alexander, G. Donowitz, V. Kan, C. A. Kauffman, D. Kett, R. A. Larsen, V. Morrison, M. Nucci, P. G. Pappas, M. E. Bradley, S. Major, L. Zimmer, D. Wallace, W. E. Dismukes, and J. H. Rex. 2007. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur. J. Clin. Microbiol. Infect. Dis. 26:271-276. [DOI] [PubMed] [Google Scholar]
- 32.Paphitou, N. I., L. Ostrosky-Zeichner, and J. H. Rex. 2005. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med. Mycol. 43:235-243. [DOI] [PubMed] [Google Scholar]
- 33.Pappas, P. G., J. H. Rex, J. Lee, R. J. Hamill, R. A. Larsen, W. Powderly, C. A. Kauffman, N. Hyslop, J. E. Mangino, S. Chapman, H. W. Horowitz, J. E. Edwards, and W. E. Dismukes. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634-643. [DOI] [PubMed] [Google Scholar]
- 34.Park, S., M. Wong, S. A. Marras, E. W. Cross, T. E. Kiehn, V. Chaturvedi, S. Tyagi, and D. S. Perlin. 2000. Rapid identification of Candida dubliniensis using a species-specific molecular beacon. J. Clin. Microbiol. 38:2829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelz, R. K., C. W. Hendrix, S. M. Swoboda, M. Diener-West, W. G. Merz, J. Hammond, and P. A. Lipsett. 2001. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann. Surg. 233:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peralta, G., M. P. Roiz, M. B. Sanchez, J. C. Garrido, B. Ceballos, M. J. Rodriguez-Lera, F. Mateos, and I. De Benito. 2007. Time-to-positivity in patients with Escherichia coli bacteraemia. Clin. Microbiol. Infect. 13:1077-1082. [DOI] [PubMed] [Google Scholar]
- 37.Pittet, D., M. Monod, P. M. Suter, E. Frenk, and R. Auckenthaler. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 220:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Playford, E. G., A. C. Webster, T. C. Sorrell, and J. C. Craig. 2006. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J. Antimicrob. Chemother. 57:628-638. [DOI] [PubMed] [Google Scholar]
- 39.Puzniak, L., S. Teutsch, W. Powderly, and L. Polish. 2004. Has the epidemiology of nosocomial candidemia changed? Infect. Control Hosp. Epidemiol. 25:628-633. [DOI] [PubMed] [Google Scholar]
- 40.Schelonka, R. L., M. K. Chai, B. A. Yoder, D. Hensley, R. M. Brockett, and D. P. Ascher. 1996. Volume of blood required to detect common neonatal pathogens. J. Pediatr. 129:275-278. [DOI] [PubMed] [Google Scholar]
- 41.Sendid, B., T. Jouault, R. Coudriau, D. Camus, F. Odds, M. Tabouret, and D. Poulain. 2004. Increased sensitivity of mannanemia detection tests by joint detection of alpha- and beta-linked oligomannosides during experimental and human systemic candidiasis. J. Clin. Microbiol. 42:164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shorr, A. F., K. Chung, W. L. Jackson, P. E. Waterman, and M. H. Kollef. 2005. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit. Care Med. 33:1928-1936. [DOI] [PubMed] [Google Scholar]
- 43.Uzun, O., and E. J. Anaissie. 2000. Predictors of outcome in cancer patients with candidemia. Ann. Oncol. 11:1517-1521. [DOI] [PubMed] [Google Scholar]
- 44.van Deventer, A. J., W. H. Goessens, A. van Belkum, H. J. van Vliet, E. W. van Etten, and H. A. Verbrugh. 1995. Improved detection of Candida albicans by PCR in blood of neutropenic mice with systemic candidiasis. J. Clin. Microbiol. 33:625-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vardakas, K. Z., G. Samonis, A. Michalopoulos, E. S. Soteriades, and M. E. Falagas. 2006. Antifungal prophylaxis with azoles in high-risk, surgical intensive care unit patients: a meta-analysis of randomized, placebo-controlled trials. Crit. Care Med. 34:1216-1224. [DOI] [PubMed] [Google Scholar]
- 46.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 47.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 48.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]