Abstract
Thyroid hormone receptor β2 (TRβ2) controls the patterning of cone opsin photopigments that mediate colour vision. We raised an antiserum against TRβ2 to study cone photoreceptor development by western blot and immunostaining analyses. TRβ2-positive cells first appeared between embryonic day 10 (E10) and E12. Numbers increased until near birth, correlating with generation of the cone population. At birth, signals decreased until postnatal day 10 (P10), then declined to very low levels in adulthood. TRβ2-positive cells were initially dispersed but became aligned at the edge of the outer neuroblastic layer by E15. Postnatally, these cells migrated inwardly until P10, then outwardly to the edge of the outer nuclear layer, the location of mature cones. TRβ2 represents a functionally unique marker for cone development.
Keywords: thyroid hormone receptor β2, nuclear receptor, cone photoreceptor, colour visual system, retina
Introduction
Colour vision in most mammals, including mice, is dichromatic, being mediated by M and S opsin photopigments for sensitivity to medium-long ("green") and short ("blue") wavelengths of light, respectively [1,2]. Opsins are expressed in cone photoreceptors which are generated before birth in the mouse [3]. Cones begin to express S opsin near birth and M opsin at P8 [4,5]. There is a paucity of markers for immature cones before expression of opsins.
Thyroid hormone receptor β2 (TRβ2), a ligand-regulated transcription factor, is critical for opsin patterning [6,7]. TRβ2-deficient (Thrb2−/−) mice lack M opsin and all cones instead express S opsin, indicating that TRβ2 controls M opsin induction and the differential distribution of M and S opsins. Colour visual deficits have been noted in rare cases of human resistance to thyroid hormone, which is associated with TRβ mutations [8,9]. The Thrb gene encodes TRβ2 and TRβ1, a more widely expressed isoform that differs from TRβ2 in its N-terminus [10,11].
TRβ2 expression in retina was first demonstrated at the mRNA level in the chick embryo [6] and subsequently in the mouse [7]. Detection of TRβ2 by in situ hybridization [6,12,13] or use of a transgenic reporter [11] is limited in terms of sensitivity and cellular resolution. We have generated an antiserum to study TRβ2 as a marker for cone development and migration.
Methods
Antiserum
A cDNA encoding mouse TRβ2 N-terminus (108 amino acids) was cloned into pQE30 (Qiagen). The His6-tagged protein was expressed in bacteria, purified using Ni-chelate chromatography, then sent to Berkeley Antibody Company (BABCO) for raising antiserum (b2N) in rabbits.
Immunohistochemistry
Eyes from C57BL/6J mice were fixed in 4% paraformaldehye for 3 h at 4°C, immersed in 30% sucrose and embedded in OCT for preparation of 10 µm cryosections. Immunostaining was performed using a Vectastain ABC Elite Kit with biotinylated goat anti-rabbit second antibody (Vector Laboratories) according to manufacturer’s instructions. The b2N antiserum (1:2,500) was incubated overnight at room temperature. TRβ2-positive cells were counted around the entire retina on 10 µm sections near the vertical midline of the eye, on 3 – 4 sections from 3 – 5 embryos per age. Mice carrying the S opsin-lacZ-Thrb intron transgene have been described [11]. Animal experiments were performed in accordance with approved protocols at NIDDK / NIH.
Western blot analysis and cell transfections
Transfected cells were sonicated in 20 mM Tris-Cl, pH 7.5, 200 mM NaCl, 0.5% Nondet-P40, 0.5 mM EDTA, 10 mM DTT and PMSF. Lysates were centrifuged at 3,000 rpm for 5 min and the supernatant used for western blot analysis. Pituitary (n = 3, pooled) and neuroretina dissected from eyes (n ≥ 3, pooled) were homogenized in lysis buffer and incubated on ice for 30 min. To separate nuclear and non-nuclear fractions, tissues were homogenized in 10 mM Tris-Cl, pH 8, 10 mM KCL, 0.1 mM EDTA, 1 mM DTT and PMSF to yield a supernatant as the non-nuclear fraction and a pellet used to extract the nuclear fraction as described [14]. 20 µg samples were analyzed by 10% SDS-polyacrylamide gel electrophoresis in Tris-Glycine buffer with electrotransfer to nitrocellulose. The membrane was probed with b2N antiserum (1:2,500) or actin monoclonal antibody (Chemicon, 1:5,000) in PBS containing 5% non-fat milk and 0.2% Tween-20 overnight at 4°C. Second antibodies were HRP-goat rabbit or mouse IgG (Zymed) (1:10,000). Signals were detected with Amersham ECL Plus Western Blotting kit (GE Healthcare). Kodak Biomax film was exposed for 1 min or for 10 min for long exposure. TR cDNAs [10] were cloned into pSG5 for in vitro translation using TNT-Coupled Reticulocyte Lysates (Promega). NIH3T3 cells were transfected with 2 µg of pSG5 expressing TRβ2 or TRβ1 using FuGENE HD Reagent (Roche). After 48 h, cells were fixed in 2% PFA for 5 min and immunostained with b2N. Second antibody was Alexa Fluor 488 goat anti-rabbit antibody and the co-stain was Alexa Fluor 546 phalloidin.
Results
The antiserum against TRβ2 detected over-expressed TRβ2 but not TRβ1 in transfected NIH3T3 cells (Fig. 1A, B). Signals were primarily nuclear with low signals in the cytoplasm. In western blot analysis, the antiserum detected a TRβ2 band with a size of ~58 kDa but not TRβ1 or TRα1 when expressed in transfected cells or translated in vitro (Fig. 1C). The antiserum detected endogenous TRβ2 in mouse tissue extracts (Fig. 1D). TRβ2 is much more abundant in the anterior pituitary than retina at optimal ages for expression in each tissue (pituitary, post-weaning; retina, late embryo) [7,11]. In lysates of pituitary at P25 and eye at E17.5, TRβ2 was detected in the nuclear but not non-nuclear fraction. The TRβ2 band was absent in tissues from Thrb2−/− mice.
Fig. 1. Specificity of antiserum against TRβ2.
A, The 108 amino acid long N terminus of TRβ2 used to generate b2N antiserum. TRβ2 and TRβ1 differ in the N terminus and are identical in DNA binding (DBD) and ligand binding (LBD) domains. Numbers indicate amino acid coordinates of receptor domains.
B, Detection of TRβ2 but not TRβ1 expressed from pSG5 in NIH3T3 cells. TRβ2 immunofluorescence is mainly nuclear (green); cell co-stain is phalloidin (red).
C, Western blot analysis: (left 3 lanes) extracts from NIH3T3 cells transfected with empty pSG5, or pSG5 expressing TRβ2 or TRβ1; (right 4 lanes) in vitro translations of TRβ2, TRβ1, TRα1 or empty pSG5 (mock) in reticulocyte lysates. Arrowhead, specific TRβ2 band detected with b2N (estimated size ~58 kDa compared to size predicted from cDNA sequence of 52 kDa); actin, control antibody used on same blot.
D, Western blot analysis of endogenous TRβ2 in mouse pituitary and eye extracts at optimal ages for TRβ2 expression. TRβ2 (arrowhead) is detected in the nuclear but not non-nuclear fractions. The TRβ2 band is absent in Thrb2−/− samples.
E, TRβ2 profile in retinal development. Right, prolonged exposure detects a faint TRβ2 band that is absent in Thrb2−/− mice at P80.
Western blot analysis of retinal rather than eye lysates enriched the TRβ2 band and reduced non-specific background bands (Fig. 1E). Developmental analysis of retinal extracts detected peak TRβ2 signals between E15 and E18 and lower levels from birth to P5. After P10, levels declined to very low levels in adults. Prolonged exposure times detected a faint TRβ2 band at P80 in wild type but not Thrb2−/− mice. The embryonic peak and postnatal decline in TRβ2 levels is consistent with the general trend observed for TRβ2 mRNA [6,7,12].
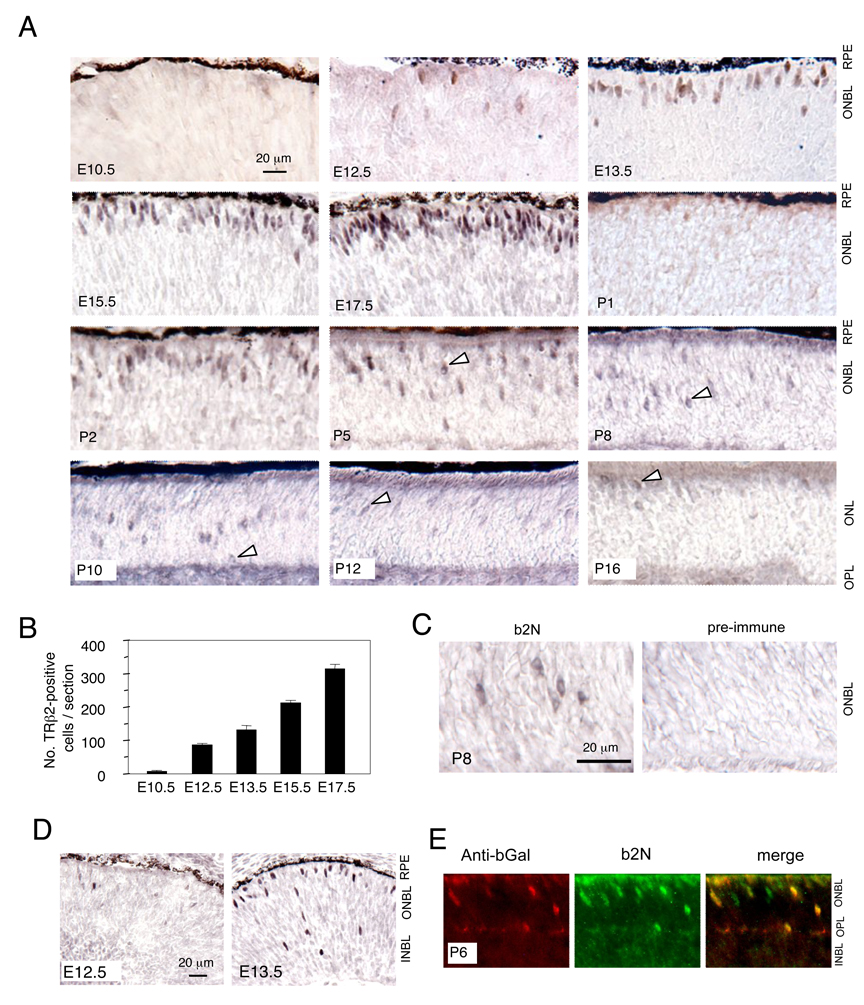
Immunostaining detected sparse, faintly-positive cells in wild type retina at E10.5 (Fig. 2A). Signals strengthened by E12.5. TRβ2-positive cell numbers increased progressively until near birth, with a peak at E17 – E18, characteristic of the profile of cone generation in mice [3](Fig. 2B). Total numbers were relatively low, consistent with the small population of cones in mice, in which cones represent only 3% and rods 97% of photoreceptors [15]. At birth, signals per cell decreased sharply but strengthened again at P2 – P10. Signals declined again after P10 and only faintly-positive cells were detected at or after P16. The abrupt dip in TRβ2 signals at P1 detected by immunostaining contrasted with the more gradual postnatal decrease detected by western blot analysis (Fig. 1E). A possible explanation is that although TRβ2 protein remains present at P1, it is transiently sequestered in protein complexes or cell compartments as the cell develops morphologically such that immunohistochemistry is less sensitive. Pre-immune serum gave no specific signal at any age (Fig. 2C).
Fig. 2. Detection of TRβ2 in mouse retinal sections.
A, TRβ2-positive cells first appear at E10.5 – E12.5 and numbers increase until near birth. Postnatally, TRβ2-positive cells migrate inwardly through the ONBL, then outwardly to the outer edge of the ONL. Arrowheads, examples of perinuclear or cytoplasmic signal in cells at postnatal ages. Scale bar, 20 µm, same at all ages.
B, Counts of TRβ2-positive cells on retinal sections during embryonic development. Means ± sem; n = 3 – 5 embryos per age.
C, Pre-immune serum gives no specific signal. Note, that at P8, TRβ2 signal is in a perinuclear or cytoplasmic rather than nuclear pattern as detected in embryonic retina.
D, Low power view of embryonic retina showing dispersed location of the first appearing TRβ2-positive cells.
E, Cones from mice carrying a cone-specific lacZ transgene are positive for endogenous TRβ2 (green) and for β-galactosidase (red). The transgene is driven by an S opsin promoter and retinal enhancer of the Thrb gene. Doubly-fluorescent cells are yellow.
Abbreviations, INBL, inner neuroblastic layer, ONBL, outer neuroblastic layer, ONL, outer nuclear layer, OPL, outer plexiform layer, RPE, retinal pigmented epithelium.
After P5, TRβ2 signal was often enriched in the perinuclear or adjacent cytoplasmic zone of the cell body unlike in embryonic cells in which the signal was more localized in the nucleus (Fig. 2A, C). The perinuclear signal may be a result of differences in response to fixation at postnatal stages. However, the consistent perinuclear signal at these later ages may reflect a developmental change in the sub-cellular localization of TRβ2 as cones adopt a more mature morphology, perhaps suggesting compartmentalization as a means of control of TRβ2 activity.
TRβ2-positive cells initially showed a dispersed localization in the outer neuroblastic layer (ONBL) with a few cells also located in inner retinal layers, suggesting that recently-generated cones were not attached to a particular location (Fig. 2D). From E13.5 until birth, almost all TRβ2-positive cells became aligned near the outer edge of the ONBL, suggesting that immature cones were constrained from movement despite the ongoing proliferation and migration of other cell types in the retinal neuroblastic layers during this period.
From birth until P10, TRβ2-positive cells migrated inwardly across the nascent outer nuclear layer (ONL). After P10, these cells re-migrated outwardly to the outermost region of the ONL, the location of mature cone cell bodies [15]. The cone identity of TRβ2-positive cells was demonstrated using mice carrying a cone-specific transgenic lacZ reporter driven by an S opsin promoter [11]. TRβ2-positive cells co-expressed β-galactosidase (Fig. 2E).
Discussion
TRβ2 represents an early cone marker according to several criteria: i) The accumulation of TRβ2-positive cells in utero correlates with the period of generation of the cone population, as indicated previously by [3H]-thymidine labeling in birth-dating studies [3]. ii) The relatively small number of TRβ2-positive cells is characteristic of the small cone population in mice [15]. iii) The postnatal migration pattern of TRβ2-positive cells follows that of maturing cones [16]. iv) TRβ2 co-localizes in cells that express a cone marker transgene (Fig. 2E). The data indicate that TRβ2 identifies cones for much of the early phase of their life history. RXRγ, a possible heterodimerization partner for TRβ2, also influences S opsin distribution in mice [17] and it is also expressed in cones, as well as inner neuroblastic and ganglion cells [18]. More widely-expressed cone markers include CRX, which is also in rods [19,20] and neuron specific enolase which is also in bipolar, horizontal and ganglion cells [16].
TRβ2 expression follows two phases: First, TRβ2 is induced soon after cones are generated in utero. This early peak of TRβ2 expression may be necessary to prime cones for the later expression of opsins. TRβ2 is also a candidate hallmark that distinguishes newly-generated cones and rods. Although cones and rods are functionally distinct types of photoreceptors, precursors of both cell types share considerable developmental plasticity [21] such that the identification of early, specific markers for rods and cones is of practical interest. In the second, postnatal phase of expression, after cone generation is complete [3], lower levels of TRβ2 persist as cones migrate to their final location in the ONL. It is during this phase that M opsin is induced and M and S opsins become differentially distributed, events that require TRβ2 together with rising levels of thyroid hormone [22–24].
The postnatal shift in sub-cellular distribution of TRβ2 signal towards a perinuclear or cytoplasmic location as opposed to the nuclear location at earlier stages may have functional implications. Given that TRβ2 acts as a ligand-regulated transcription factor, this could effectively reduce the nuclear activity of TRβ2, paradoxically at the time when thyroid hormone induces M opsin expression. If so, one speculation is that the correct response of the postnatal cone to thyroid hormone requires some reduction of sensitivity. Another speculation is that in addition to its transcriptional activity, TRβ2 may possess some non-nuclear signaling function.
TRβ2-positive cells display a double wave of migration during development. At E12 – E13, the first cells detected are somewhat scattered in the neuroblastic layers. Soon thereafter, TRβ2-positive cells localize at the outer edge of the ONBL, suggesting that immature cones are tethered despite the ongoing, large-scale proliferation of progenitors and migration of other retinal cell types during this period. Postnatally, cones migrate inwardly through the nascent ONL, which has been proposed to be a result of displacement by the expanding rod population [16]. After P10, cones re-migrate outwardly to their mature location at the outer edge of the ONL, which may involve attractive cues [16]. These attractive cues and the earlier signals that tether immature cones in the ONBL in the embryo remain to be identified.
In the late embryo, TRβ2-positive cells form a layer 2 – 4 cells thick at the edge of the ONBL whereas in the adult, cone cell bodies are sparser in the ONL [15]. This reduced density may be explained potentially by cell death, a natural process in retinal differentiation [25] and by a process of dilution as the eye grows and other cell populations expand. Cones are one of the first retinal populations to be generated such that the later expansion of rods would reduce cone density in the mature ONL.
Conclusion
The results indicate that TRβ2 identifies cones from their initial generation in the embryo to postnatal maturation stages. Expression peaks with the completion of cone generation before birth, then declines progressively during postnatal phases of migration and maturation.
Acknowledgments
This work was supported in part by a Hirschl Award and the Intramural Program at NIDDK.
References
- 1.Mollon JD. Color vision: opsins and options. Proc Natl Acad Sci USA. 1999;96(9):4743–4745. doi: 10.1073/pnas.96.9.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24(2):299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- 3.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188(2):263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Macke JP, Merbs SL, et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9(3):429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- 5.Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol. 1993;331(4):564–577. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- 6.Sjöberg M, Vennström B, Forrest D. Thyroid hormone receptors in chick retinal development: differential expression of mRNAs for α and N-terminal variant β receptors. Development. 1992;114:39–47. doi: 10.1242/dev.114.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Ng L, Hurley JB, Dierks B, et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27(1):94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- 8.Newell FW, Diddie KR. Typical monochromacy, congenital deafness, and resistance to intracellular action of thyroid hormone. Klin Monatsbl Augenheilkd. 1977;171(5):731–734. [PubMed] [Google Scholar]
- 9.Lindstedt G, Lundberg PA, Sjogren B, Ernest I, Sundquist O. Thyroid hormone resistance in a 35-year old man with recurrent goitre. Scand J Clin Lab Invest. 1982;42(7):585–593. [PubMed] [Google Scholar]
- 10.Wood WM, Ocran KW, Gordon DF, Ridgway EC. Isolation and characterization of mouse complementary DNAs encoding α and β thyroid hormone receptors from thyrotrope cells: the mouse pituitary-specific β2 isoform differs at the amino terminus from the corresponding species from rat pituitary tumor cells. Mol. Endocrinol. 1991;5:1049–1061. doi: 10.1210/mend-5-8-1049. [DOI] [PubMed] [Google Scholar]
- 11.Jones I, Ng L, Liu H, Forrest D. An intron control region differentially regulates expression of thyroid hormone receptor β2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol. 2007;21(5):1108–1119. doi: 10.1210/me.2007-0037. [DOI] [PubMed] [Google Scholar]
- 12.Applebury ML, Farhangfar F, Glosmann M, et al. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Dev Dyn. 2007;236(5):1203–1212. doi: 10.1002/dvdy.21155. [DOI] [PubMed] [Google Scholar]
- 13.Trimarchi JM, Harpavat S, Billings NA, Cepko CL. Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Dev Biol. 2008;8:101. doi: 10.1186/1471-213X-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber E, Matthias P, Müller M, Schaffner W. Rapid detection of octamer binding proteins with mini-extracts prepared from a small number of cells. Nuc. Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188(2):245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- 16.Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. J Comp Neurol. 1997;388(1):47–63. [PubMed] [Google Scholar]
- 17.Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46(8):2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- 18.Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42(6):1312–1318. [PubMed] [Google Scholar]
- 19.Chen S, Wang QL, Nie Z, et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19(5):1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23(4):466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- 21.Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci U S A. 2007;104(5):1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103(16):6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu A, Ng L, Ma M, et al. Retarded developmental expression and patterning of retinal cone opsins in hypothyroid mice. Endocrinology. doi: 10.1210/en.2008-1092. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pessoa CN, Santiago LA, Santiago DA, et al. Thyroid Hormone Action Is Required for Normal Cone Opsin Expression during Mouse Retinal Development. Invest Ophthalmol Vis Sci. 2008;49(5):2039–2045. doi: 10.1167/iovs.07-0908. [DOI] [PubMed] [Google Scholar]
- 25.Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229(3):362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]