Abstract
Heme oxygenase-1 (HO-1) is a key cytoprotective enzyme and an established marker of oxidative stress. Increased HO-1 expression has been found in the resident macrophages in the alveolar spaces of smokers. The lipid peroxidation product 4-hydroxynonenal (HNE) is also increased in the bronchial and alveolar epithelium in response to cigarette smoke. This suggests a link between a chronic environmental stress, HNE formation, and HO-1 induction. HNE is both an agent of oxidative stress in vivo and a potent cell signaling molecule. We hypothesize that HNE acts as an endogenously produced pulmonary signaling molecule that elicits an adaptive response culminating in the induction of HO-1. Here we demonstrate that HNE increases HO-1 mRNA, protein, and activity in pulmonary epithelial cells and identify ERK as a key pathway involved. Treatment with HNE increased ERK phosphorylation, c-Fos protein, JNK phosphorylation, c-Jun phosphorylation, and AP-1 binding. Whereas inhibiting the ERK pathway with the MEK inhibitor PD98059 significantly decreased HNE-mediated ERK phosphorylation, c-Fos protein induction, AP-1 binding, and HO-1 protein induction, inhibition of the ERK pathway had no effect on HNE-induced HO-1 mRNA. This suggests that ERK is involved in the increase in HO-1 through regulation of translation rather than transcription.
Keywords: 4-Hydroxynonenal, Oxidative stress, HO-1, MAPK signaling, Free radicals
Heme oxygenase-1 (HO-1) is an important cytoprotective enzyme and widely accepted marker of oxidative stress. HO-1 catalyzes the first and rate-limiting step in the catabolism of heme, which generates equimolar amounts of biliverdin, ferrous iron, and carbon monoxide [1–3]. Although heme is the major substrate of HO-1, a variety of non-heme-containing agents are also strong inducers of HO-1. HO-1 has been shown to respond to a number of proinflammatory cytokines, including TNF-α, IL-1, and IL-11 [4]. HO-1 is also induced by a variety of agents that cause oxidative stress and/or in response to environmental stress [5–8]. For example, increased HO-1 expression has recently been found in the alveolar spaces in the resident macrophages of smokers [9]. Once induced, HO-1 provides protection against multiple types of tissue injury [10–13]; specific to the lung, overexpression of HO-1 in pulmonary epithelial cells has been shown to protect against oxygen toxicity [14]. In contrast, HO-1 deficiency in mice (HO-1−/−) increases susceptibility to inflammatory lung injury [15], as does HO-1 deficiency in humans [16]. Although the precise mechanisms by which HO-1 exerts its cytoprotective effects are not known, there is mounting evidence that carbon monoxide plays a key role in this process. For thorough reviews of the recent literature see [17,18].
Another characteristic of oxidative stress is the formation of the lipid peroxidation product 4-hydroxy-2-nonenal (HNE). HNE has been shown to increase HO-1 in the cell [19,20], but the mechanism(s) has not been clearly defined. HNE is an α,β-unsaturated aldehyde that is formed from the reaction of oxygen species with arachidonate in cellular membranes during many forms of environmental stress, including exposure to cigarette smoke [9,21,22]. HNE is known to impact the cell in many ways, which include inactivation of enzymes, depletion of intracellular glutathione, and inhibition of DNA and protein synthesis [23–25]. Recent data suggest that HNE may also modulate biological responses by triggering intracellular signal transduction pathways [26–28].
The mitogen-activated protein kinases (MAPK) are among the signal transduction pathways activated by HNE. As such, the MAPK pathways may play a significant role in mediating the many cellular functions that are affected by HNE. The MAPK are activated by dual phosphorylation on specific tyrosine and threonine residues [29]. The MAPK include the extracellular signal-regulated kinases 1 and 2 (ERK1/2), p38 MAP kinases, c-Jun N-terminal kinases (JNK), and the big MAPK. The MAPK are activated in response to various stressors, including oxidative stress. They phosphorylate and activate key transcription factors, thereby regulating the transcription of many genes. For a comprehensive review of MAPK-redox signaling see [29]. Members of the MAPK family are critical regulators of the transcription factor complex AP-1 (activator protein-1). AP-1 consists of proteins of the Jun, Fos, and ATF families that can form homo- and heterodimers. Activation of AP-1 involves expression of AP-1-driven genes that include c-Jun. c-Jun may also form part of the stress response (StRE) and electrophile response elements (EpRE), also key regulators of redox-sensitive genes [30,31].
HO-1 gene and protein expression is also modulated by MAPK activation. Seminal studies support a role for these kinases as mediators of HO-1 induction in a broad range of tissues and cell types. Studies have shown that HO-1 is induced via the ERK pathway by LPS + IFN-γ [32], through p38 MAPK in murine macrophages in response to IL-1β, and in human lung epithelial cells by TGF-β1 [33,34]. However, care must be taken not to generalize, as the signaling pathways that are activated are both agonist-and species-specific.
In this study, we set out to determine if HNE induces HO-1 in lung pulmonary epithelial cells, and, if so, the pathway or pathways involved. As HNE and HO-1 are increased in the bronchial and alveolar epithelium in response to chronic environmental insults such as cigarette smoke, mechanistically, this raises the possibility that such insults increase HNE, which in turn induces HO-1. We tested this hypothesis in vitro, by treating lung epithelial cells with HNE, assaying the effects on HO-1 induction, and then determining which of the MAPK pathways are involved.
Materials and methods
Materials
HNE was purchased from Cayman Chemical (Ann Arbor, MI, USA). SB202190 (SB), PD98059 (PD), SP600125 (SP), and the JNK inhibitor peptide (JNKi) were purchased from Calbiochem. TRIzol reagent was purchased from Life Technologies (Grand Island, NY, USA). The antiserum to the small Maf proteins was a gift from Volker Blank (McGill University, Montréal, QC, Canada). All other antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All chemicals were at least of analytical grade and were purchased from Sigma–Aldrich unless otherwise noted.
Cell culture and treatments
L2 cells are a rat alveolar epithelial type II-like cell line that was purchased from the ATCC. Cultures were maintained in F12-K nutrient mixture supplemented with 1% penicillin/streptomycin and 10% BSA in a humidified incubator at 37°C with 5% CO2. Cells were grown to near confluence and then treated as outlined below.
HNE was dissolved in ethanol; the inhibitors SB, PD, and SP were dissolved in dimethyl sulfoxide (DMSO); and the JNKi was dissolved in water. The final concentrations of ethanol and DMSO were 0.05 and 0.1%, respectively. Treatments were performed when the cells reached 90–95% confluence. For inhibitor studies cells were pretreated with 25 µM SB, 50 µM PD, 20 µM SP, 20 µM JNKi, or the dilution buffer (containing DMSO) for 30 min and then incubated with 20 µM HNE for increasing lengths of time as outlined under Results. After treatments, cells were washed once with 1 × phosphate-buffered saline (PBS) and harvested with a cell scraper in 1 × PBS. Cells were centrifuged at 500 g for 5 min, trace PBS was removed, and the pellets were either processed immediately or stored at −80°C.
Western blotting analysis
Western blotting was done as described previously [35,36]. Briefly, cell lysates were prepared using the MPER reagent (Pierce, Rockford, IL, USA) following the manufacturer’s instructions. Between 20 and 50 µg of protein was electrophoresed under denaturing conditions on a 10% Tris–glycine acrylamide gel (Invitrogen), transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore), and then blocked in nonfat dry milk (NFDM) at room temperature for 1 h. Membranes were then incubated overnight at 4°C with the appropriate primary antibody in 5% NFDM in Tris-buffered saline with 0.05% Tween 20 (T-TBS). After rigorous washes with T-TBS, the membranes were incubated with the appropriate HRP-coupled secondary antibody at room temperature for 2 h. After repeated rigorous washes with T-TBS membranes were incubated with ECL Plus reagent (Amersham). Blots were exposed to ECL Hyperfilm (Amersham), and quantitation was performed by densitometric analysis of the exposed films using SigmaScan software.
RNA quantification
The content of HO-1 message was determined using real-time PCR on a Cepheid SmartCycler 1.2 with Gapdh message content as an active control, using the threshold cycle (CT) method. Briefly, total RNA was isolated using TRIzol reagent (Invitrogen), following the manufacturer’s recommendations, and then treated with DNA-free (Ambion) at 37°C for 30 min to remove any contaminating DNA. The remaining RNA was quantitated spectrophotometrically at 260 nm. Reverse transcriptase was performed using TaqMan random hexamers (Applied Biosystems). OligoPerfect was used to design gene-specific primers for rat HO-1 (sense, L2 5′-GAAGAAGATTGCACAGAAGG-3′, and antisense, 5′-GAAGGCGGTCTTAGCCTCTT-3′) and Gapdh (sense, 5′-ACCCCAATGTATCCGTTGT-3′, and antisense, 5′-TACTCCTTGGAGGCCATGT-3′), which were used to amplify a segment of reverse-transcribed mRNA using SYBR Green PCR MasterMix (Applied Biosystems). The annealing temperature was the same for each pair of gene-specific primers, and the amplicon sizes were similar. Each mRNA species was measured four times.
HO-1 activity assay
HO-1 activity was measured as described by Visner et al. [37]. L2 cells were grown to confluence in 100-mm tissue culture dishes. After treatment, cells were rinsed twice with 1 × PBS, scraped with a rubber policeman, and pelleted at 2000g for 10 min. The resulting pellet was resuspended in 0.1 mol/L KPO4 and 2 mmol/L MgCl2 and frozen (−80°C) and thawed three times to break the cell membrane. The samples were sonicated on ice, and an aliquot was reserved for protein determination. Proteins were measured using a modification of the Bradford assay. The remaining sample was centrifuged at 12,000 g at 4°C for 20 min, and the supernatant was added to the reaction mixture containing 3 mg rat liver cytosol, 20 µM hemin, 2 mM glucose 6-phosphate, 0.2 units glucose-6-phosphate dehydrogenase, and 0.8 mM β-NADPH and incubated at 37°C for 60 min in the dark. One milliliter of chloroform was added to the extract, and the change in optical density at 464–530 nm was measured. The concentration of bilirubin produced in 60 min was calculated using the extinction coefficient, 40 mM−1 cm−1 for bilirubin per milligram of protein.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared using the NE-PER kit (Pierce) following the manufacturer’s instructions. Protein concentrations were determined with Protein Assay Dye (Bio-Rad), using a modification of the Bradford method. Nuclear extracts were either quick frozen in liquid nitrogen and reserved at −80°C or used immediately. EMSAs were done as previously described [36,38,39]. Briefly, AP-1 and EpRE probes were made from AP-1 oligonucleotide (5′CGCTTGATGAGTCAGCCGGAA-3′) or EpRE oligonucleotide (5′-TCAACTAGAGTCACAGTGACTTGGCAAAAT-3′) hybridized to its complementary DNA sequence. Nuclear protein (2 µg) was preincubated with 5× binding buffer for 15 min at 37°C, followed by incubation with double-stranded 32P-labeled AP-1 or EpRE probe for 20 min at room temperature. For supershift experiments, this was followed by an additional incubation with 1 µg of the appropriate antibody for 5–45 min at room temperature. Samples were electrophoresed at 150 V for 3 h. Gels were dried and imaged via electronic autoradiography on a Packard Instant Imager (Canberra Co.).
Statistical analysis
Statistical analysis was done using a Student t test or a one-way ANOVA where appropriate with a Tukey’s test post hoc. Differences were considered statistically significant at p < 0.05.
Results
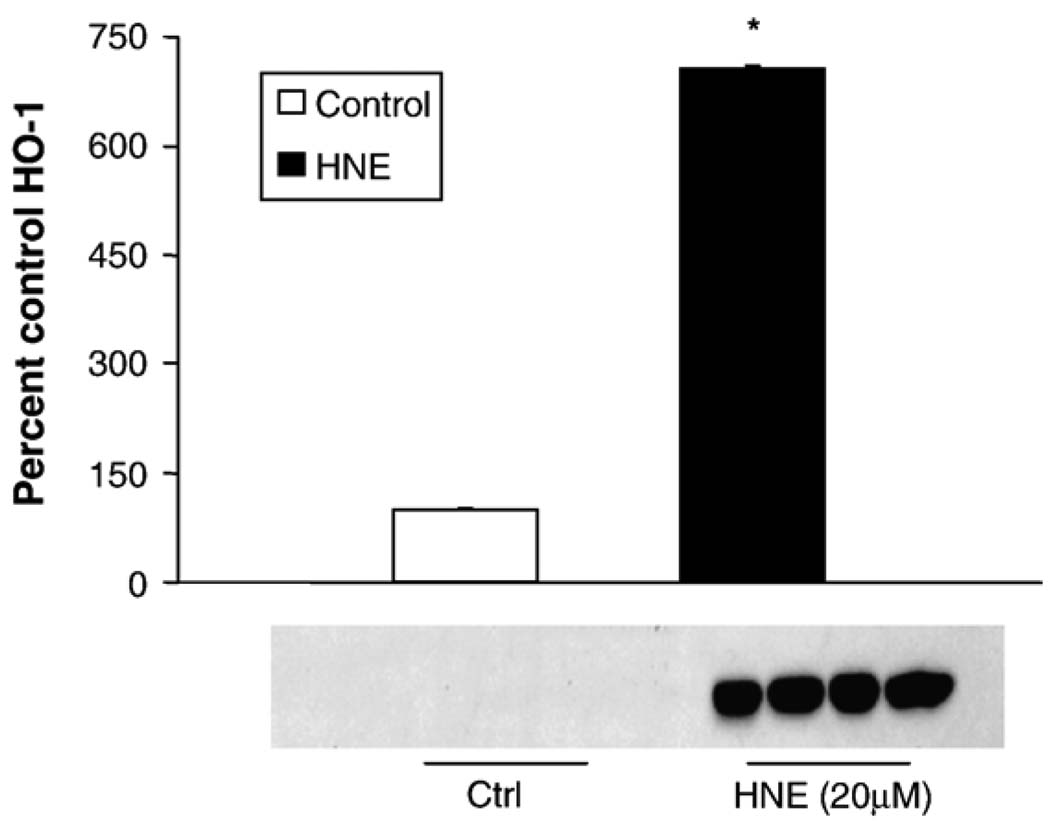
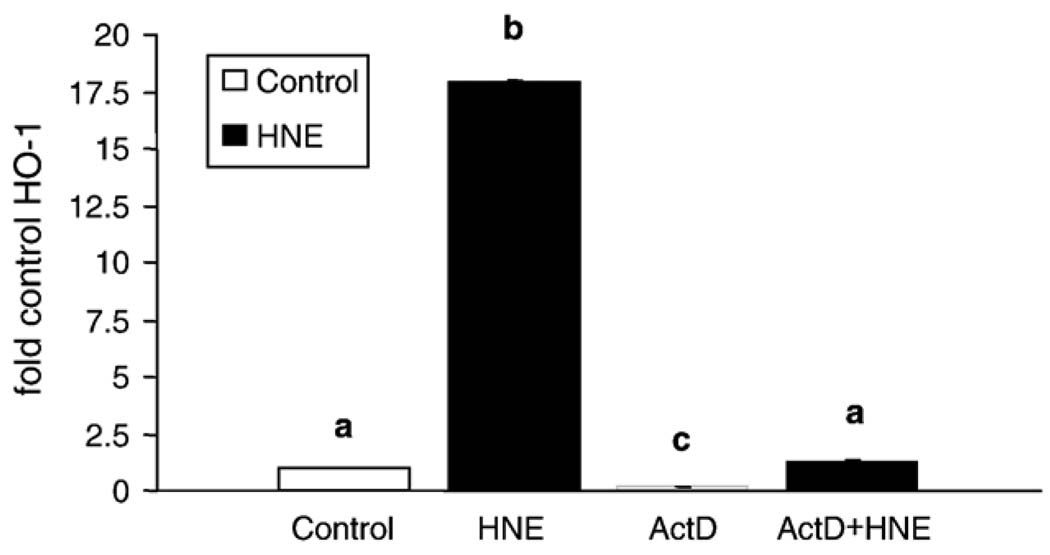
As HNE-mediated induction of HO-1 protein has been reported in alveolar macrophages [40], but not in other lung cell types, we first sought to determine if HNE induced HO-1 in pulmonary epithelial cells. L2 cells treated with 20 µM HNE for 5 h exhibited an ~ 7-fold increase in HO-1 protein versus controls (Fig. 1). HNE was dissolved in ethanol, yielding a final concentration of ethanol in the HNE-treated samples of 0.05%. This concentration of ethanol does not generally affect cell viability; however, as ethanol is a stress that can alter cell signaling, gene expression, and protein synthesis, initial experiments included both an untreated control and a 0.05% ethanol control. There was no difference in HO-1 protein in the untreated cells and the cells exposed to 0.05% ethanol (data not shown), with HO-1 being almost undetectable in both. As the vehicle did not affect the endpoint, only the vehicle control was used in subsequent experiments. To determine the mechanism of the HNE-mediated increase in HO-1 (i.e., transcriptional versus translational control) the change in HO-1 mRNA with HNE treatment was measured. Results from replicate real-time PCR assays showed that HNE increased HO-1 mRNA ~ 20- fold by 2 h postincubation (Fig. 2). This increase was blocked by the mRNA synthesis inhibitor actinomycin D, suggesting transcriptional control of HO-1 protein.
Fig. 1.
HNE induces HO-1 protein in pulmonary epithelial cells. Rat L2 cells were grown to 95–100% confluence and then treated with 20 µM HNE for 5 h. The experiment was repeated three times, and a representative blot is shown. For quantification gels were overexposed and relative densitometry was performed. Data are expressed as a percentage of control with control HO-1 arbitrarily being set at 100%. There was a rapid and robust (700%+) increase in HO-1 protein in cells treated with HNE ( p < 0.001).
Fig. 2.
HNE increases HO-1 mRNA in pulmonary epithelial cells. L2 cells were pretreated with and without actinomycin D for 30 min and then treated with or without 20 µM HNE for 2 h. Each mRNA species was measured four times. Data are expressed as fold increase in HO-1 mRNA versus control. There was an ~ 20-fold increase in HO-1 mRNA in only 2 h ( p < 0.001). Actinomycin D, an inhibitor of transcription, completely blocked the HNE-mediated increase in HO-1 mRNA ( p < 0.001). Different lowercase letters (a, b, c) denote a statistically significant difference in HO-1 mRNA.
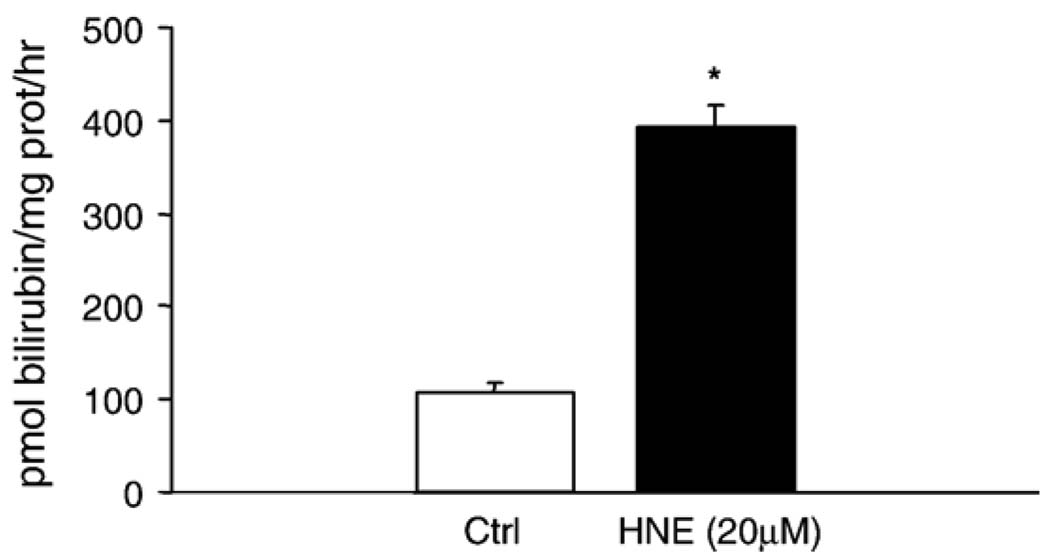
As HO-1 requires posttranslational modification for activity [41], HO-1 activity was also measured. An approximately threefold increase in HO-1 activity was observed 6 h postincubation (Fig. 3). This confirmed that the increase in HO-1 mRNA and protein is accompanied by a functional change in HO-1 in the cell.
Fig. 3.
HNE increases HO-1 activity in pulmonary epithelial cells. L2 cells were treated with 20 µM HNE for 8 h. Activity was measured four times in each treatment group. Data are expressed as pmol bilirubin formed/mg protein/h. HNE caused a significant ( p < 0.01) increase in HO-1 activity versus control.
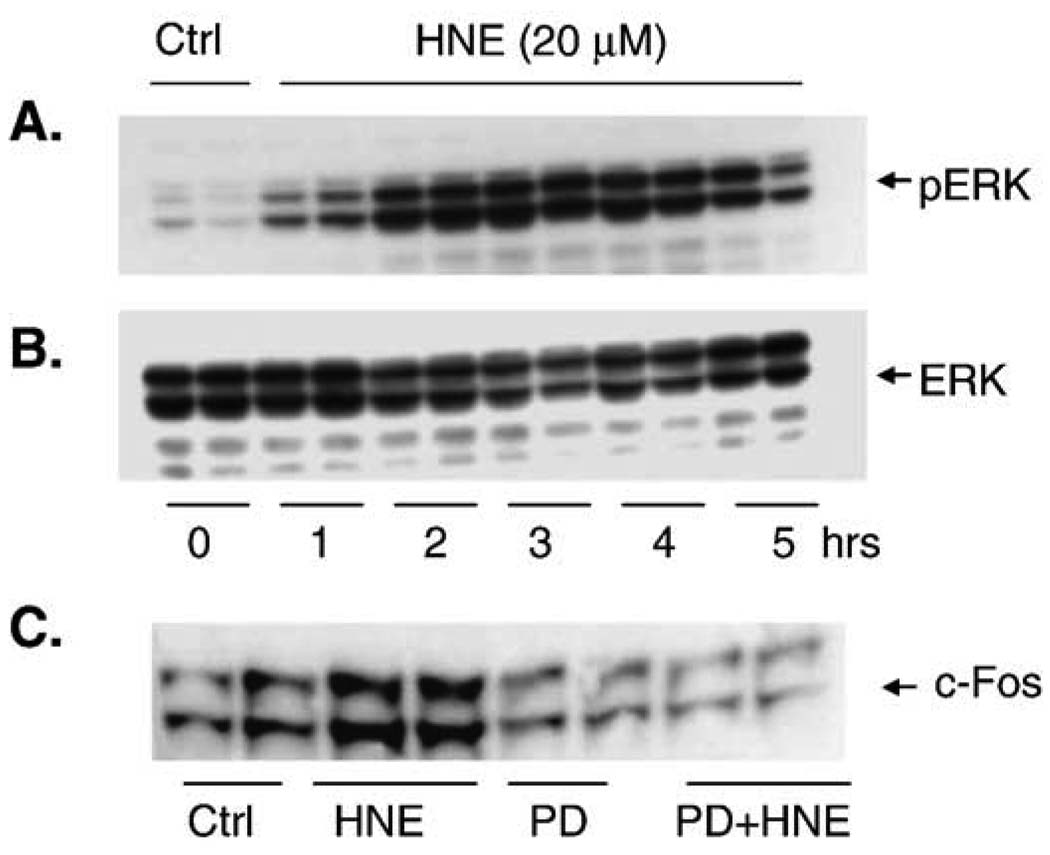
Having established that HNE increases HO-1 mRNA, protein, and activity, we sought to determine if MAPK activation is required for HO-1 induction in pulmonary epithelial cells. MAPK activation by HNE was assayed by Western blot with phosphospecific antibodies for each of the respective MAPK (JNK, ERK, and p38). A 5-h time course compared the phosphorylation and activation of each of the three major MAPK under identical experimental conditions. Phosphorylation of all three MAPK increased during this time, with ERK MAPK showing the most profound increase (Fig. 4). Downstream indicators of ERK activation such as c-Fos (Fig. 4) and p-Elk were also increased (data not shown). c-Fos protein was markedly increased 1 h after HNE exposure, suggesting that ERK pathway activation occurred even earlier. Addition of the ERK pathway inhibitor PD98059 blocked the increase in c-Fos and confirmed that the increase in c-Fos protein was via activation of the ERK pathway and not by either mRNA or c-Fos protein stabilization.
Fig. 4.
HNE activates the ERK pathway and increases c-Fos protein. (A) Cells were treated with 20 µM HNE for 0 –5 h, producing a significant increase in ERK phosphorylation. (B) The same blot was stripped and reprobed with a pan-ERK antibody to show equal loading. (C) Cells were treated with and without the ERK pathway inhibitor PD98059 (50 µM) for 30 min and then exposed to 20 µM HNE. HNE increased c-Fos protein, and this increase was blocked by inhibiting the ERK pathway with PD98059.
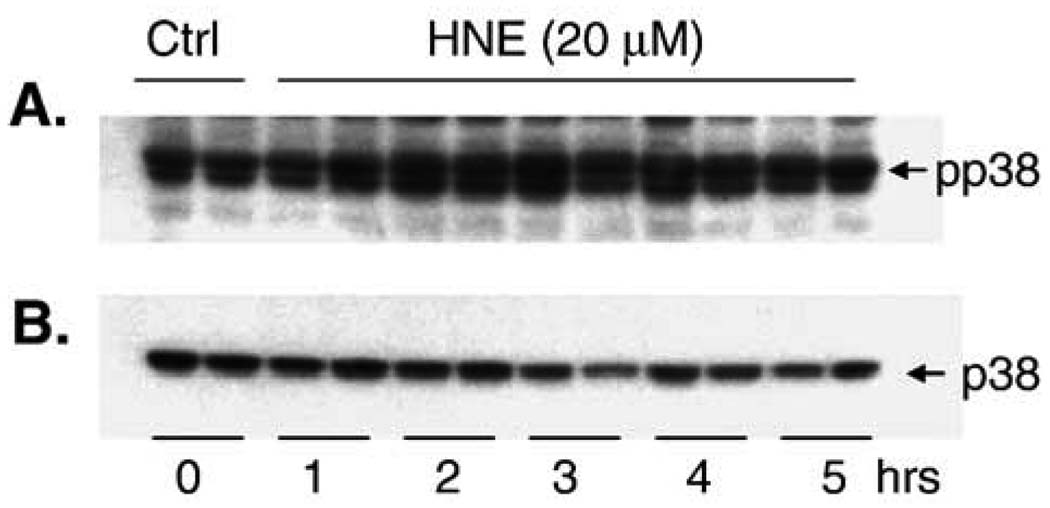
In contrast to the modest increase in pJNK that was observed, there was a dramatic increase in the phosphorylation of c-Jun (Fig. 5). JNK phosphorylation is often rapid and transient, whereas phospho-c-Jun can persist in the cell for some time depending on the experimental conditions (K. Iles, unpublished data). This may explain the apparent discrepancy between the magnitudes of JNK and c-Jun phosphorylation. Alternatively, c-Jun may also be phosphorylated by an HNE-driven JNK-independent mechanism [42]. Phosphorylation of p38 in response to HNE was also significantly increased (Fig. 6); there was no increase in the phosphorylation of a primary downstream target of p38, ATF-2 (data not shown).
Fig. 5.
HNE activates the JNK pathway and increases c-Jun phosphorylation. (A) Cells were treated with 20 µM HNE for 0 – 5 h and a marked increase in JNK phosphorylation was observed. (B) The same blot was stripped and reprobed with a pan-JNK antibody to show equal loading. (C) Phosphorylated c-Jun also increased significantly when the cells were treated with HNE.
Fig. 6.
HNE increases the phosphorylation of p38. (A) Cells were treated with 20 µM HNE or 0.05% EtOH (control) for 0 –5 h and a marked increase in p38 phosphorylation was observed. (B) The same blot was stripped and reprobed with a pan-p38 antibody to show equal loading. No change in the phosphorylation of ATF-2 (a target protein of p38 phosphorylation) was observed (data not shown).
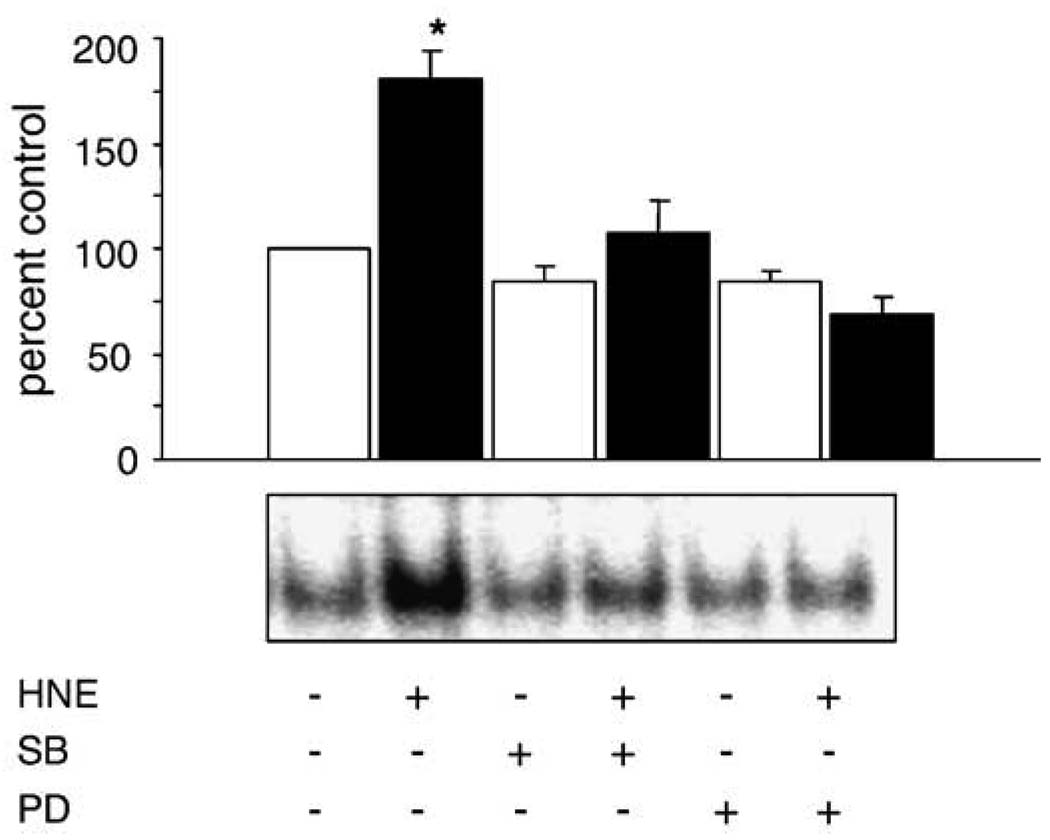
A common downstream consequence of MAPK activation is increased transcription of AP-1-mediated genes. Next we sought to determine the effect of treatment with HNE on AP-1 binding. HNE caused a significant increase in AP-1 binding ( p < 0.05%) that could be blocked by the addition of MAPK pathway inhibitors (Fig. 7). The HNE-mediated increase in AP-1 binding was partially blocked by the p38 pathway inhibitor SB202190 and completely blocked by the ERK pathway inhibitor PD98059. Supershift assays confirmed that c-Jun is present in the HNE-induced AP-1 binding complex (data not shown).
Fig. 7.
HNE-mediated increase in AP-1 binding is via the ERK and p38 pathways. Cells were pretreated for 30 min with and without PD98059 (50 µM) and SB202190 (25 µM) to inhibit the ERK and p38 pathways, respectively. Data are expressed as a percentage of control AP-1 binding, with the control being arbitrarily set at 100%. The different experimental treatment groups were repeated at least four times and a representative EMSA is shown. HNE caused a significant increase in AP-1 binding that was blocked by both SB and PD.
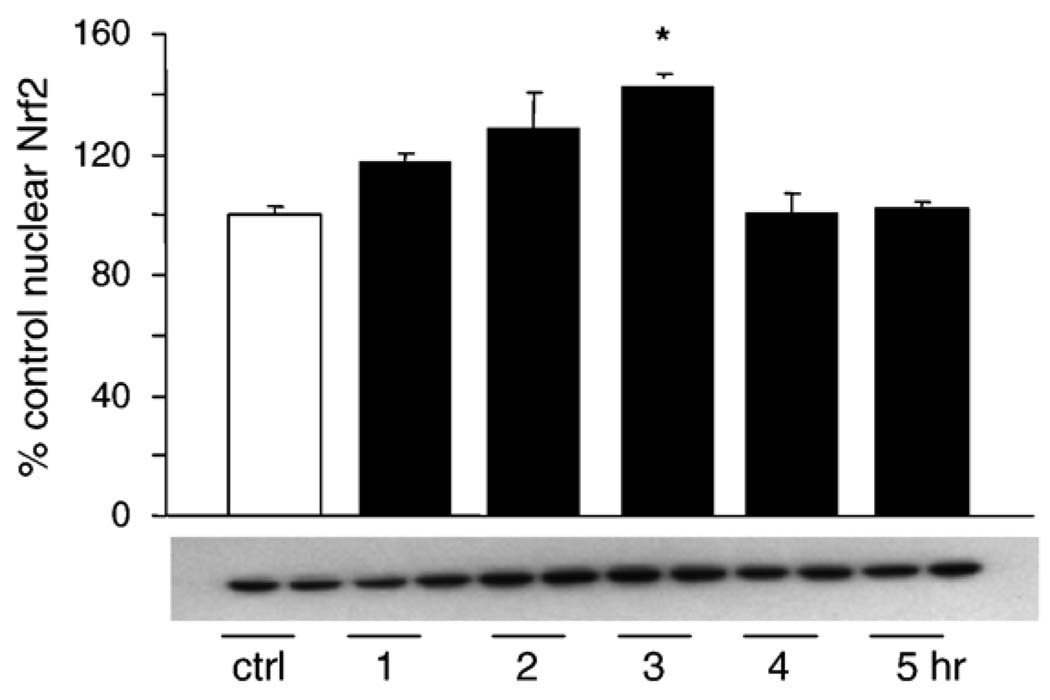
Next we investigated other potential mechanisms for HNE-mediated MAPK activation leading to increased HO-1 protein expression. It has been shown that activation of the ERK pathway may be required for Nrf2 nuclear translocation [43]. Nrf2 is a transcription factor that is part of the StRE and EpRE binding complexes, which may also be involved in HO-1 gene regulation [31,44,45]. No change in total EpRE binding was observed after treatment with HNE (data not shown). Even though HNE did not increase total EpRE binding, EpRE-regulated transcription can also be increased by alterations in the composition of the EpRE binding complex [39]. L2 cells were treated with 20 µM HNE from 1 to 5 h, nuclear extracts were generated as described under Materials and methods, and the nuclear Nrf2 content was assayed by Western blotting. HNE caused a small but rapid increase in nuclear Nrf2 that was seen as early as 1 h postincubation and reached significance 3 h postincubation (Fig. 9). There was a rapid turn-on/turn-off of this signal, with nuclear Nrf2 levels returning to baseline by 4 h postincubation.
Fig. 9.
HNE causes Nrf-2 nuclear translocation after ERK pathway activation. Cells were treated with 20 µM 4HNE for 1 – 5 h. Nuclear lysates were generated using the NE-PER kit as outlined under Materials and methods. The experiment was repeated two times, and a representative blot is shown. Data are expressed as a percentage of control (untreated at time 0) with control Nrf-2 arbitrarily being set at 100%. At 3 h there was a significant increase in Nrf-2 in the nucleus in response to HNE, which had returned to baseline levels by 4 h postincubation.
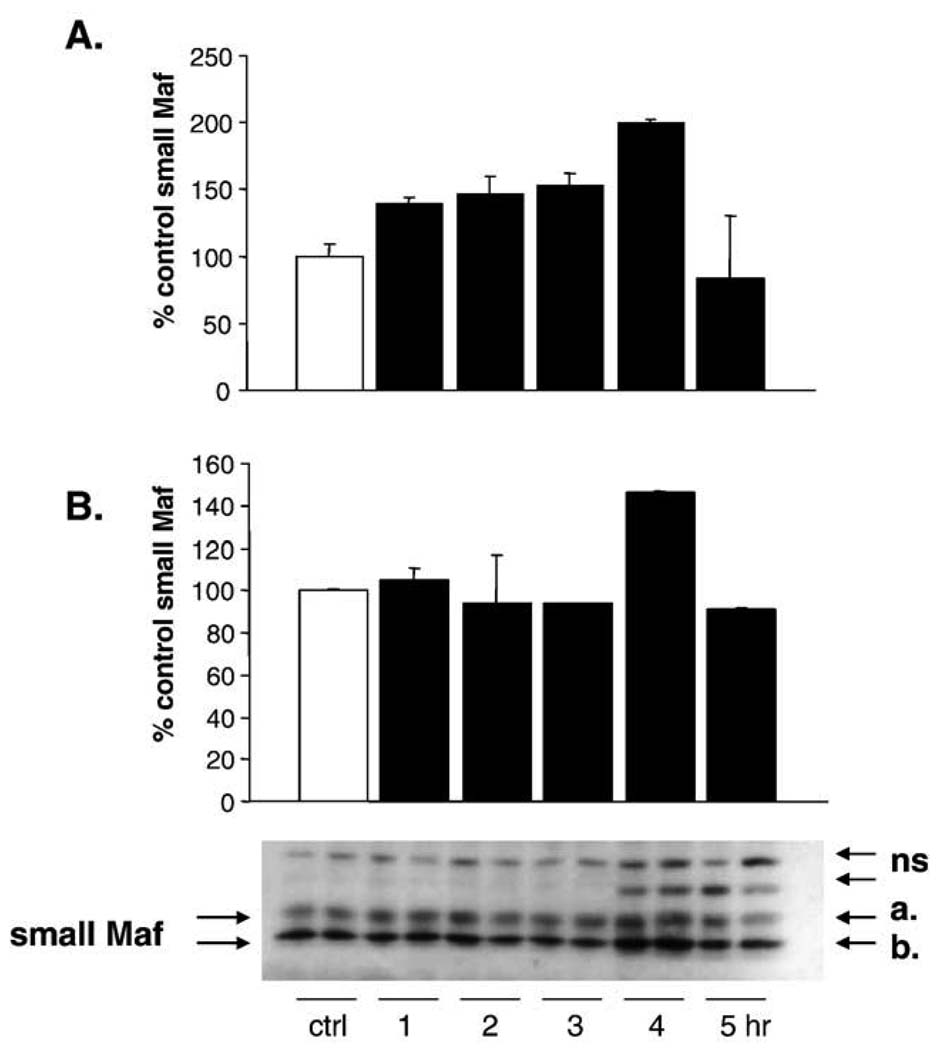
MafG has also been reported to be part of the StRE and EpRE binding complexes [31,39,46,47]. In some reports it has been identified as an inhibitor [46] and in other reports as a positive regulator of transcription [47]. MafG content was assayed by Western blotting in L2 cells as described above (Fig. 10). The MafG antibody used was designed to bind to MafG, but does cross-react with the other small Maf proteins. As the antibody produced multiple bands, the small Maf proteins were identified based on molecular weight. Two bands corresponding to the correct molecular weight were seen, and the intensity of these two bands was determined using an Alpha-Innotech. The intensity of the top band gradually increased, peaked at 4 h postincubation, and then returned to baseline. The bottom band increased abruptly at 4 h and also returned rapidly to baseline levels. This increase in the small Maf proteins follows the increase in Nrf2 in the nucleus by 1 h, and they may be functioning as inhibitors to turn off a Nrf-2-containing positive regulatory binding complex. Alternatively, there may be a brief window of overlap in the nucleus when they function coordinately to increase HO-1 transcription.
Fig. 10.
HNE increases the nuclear content of the small Maf proteins. Cells were treated with 20 µM 4HNE for 1 – 5 h. Nuclear lysates were generated using the NE-PER kit as outlined under Materials and methods. The experiment was repeated, and a representative blot is shown. Data are expressed as a percent of control with control Maf arbitrarily being set at 100%. The antibody detects all three of the small Maf proteins. As multiple bands were obtained, the small Mafs were identified based on molecular weight markers. At 4 h there was a significant increase in small Maf proteins in the nucleus in response to HNE. This decreased to baseline by 5 h postincubation.
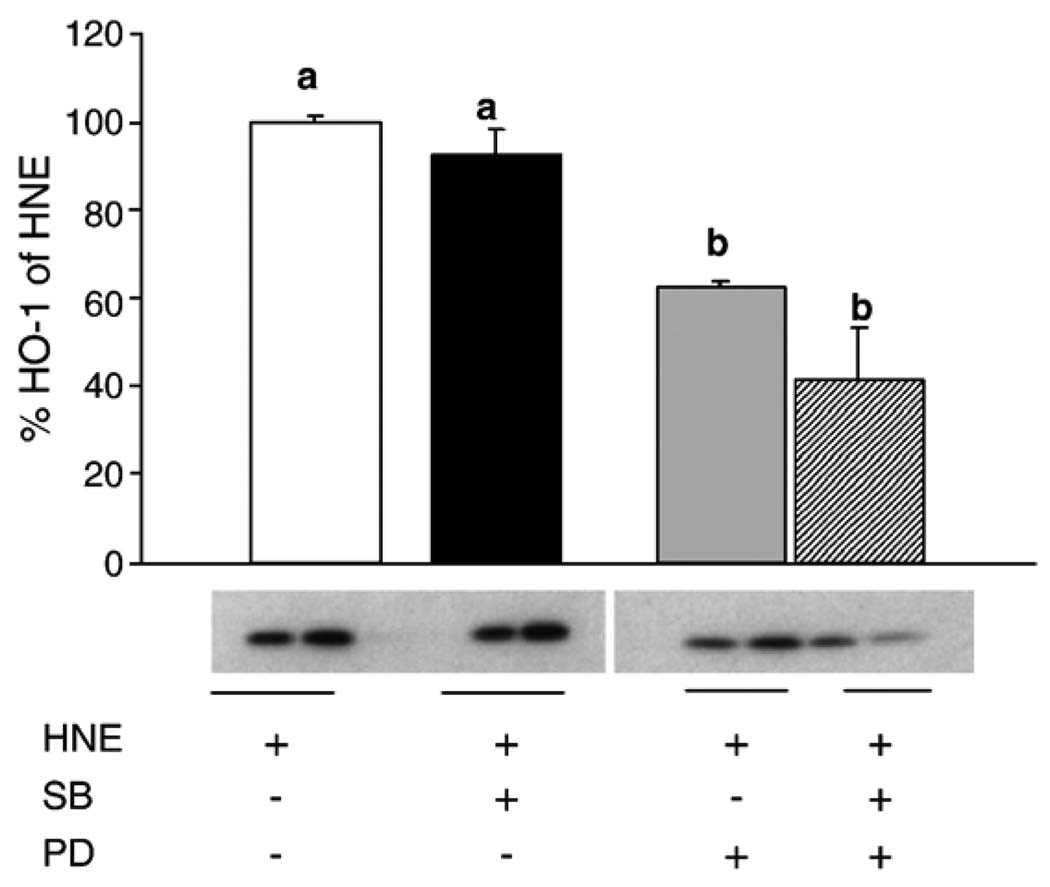
It is possible that HNE-mediated increases in AP-1 binding via activation of the MAPK pathways are unrelated to the subsequent downstream increases in HO-1 mRNA and protein. To address this possibility, L2 cells were pretreated with the ERK pathway inhibitor PD98059, the p38 pathway inhibitor SB202190, or both, and then treated with 20 µM HNE for 5 h. HO-1 protein was then assayed by Western blotting (Fig. 8). Pretreatment with the p38 pathway inhibitor SB202190 had no significant effect on HO-1 protein levels. In contrast, pretreatment with the ERK pathway inhibitor PD98059 partially blocked HO-1 protein induction. Pretreatment with both inhibitors does not cause a statistically significant decrease versus pretreatment with either inhibitor alone. Inhibitor studies were repeated with SB, PD, and JNKi, and HO-1 mRNA was assayed. Surprisingly, none of these inhibitors blocked the HNE-induced increase in HO-1 mRNA (data not shown). Collectively these data suggest that the HNE-mediated increase in p38 phosphorylation is unrelated to the downstream increases in HO-1 protein and activity. Furthermore, as inhibition of the ERK pathway with PD98059 completely inhibited the HNE-induced increase in AP-1 binding but had no effect upon HNE-induced HO-1 mRNA expression, it is likely that AP-1 activation is unrelated to HO-1 induction by HNE. Mechanistically, this confirms that even though the ERK pathway activation is required for induction of HO-1 protein, it is not involved in transcription.
Fig. 8.
HNE-mediated increase in HO-1 requires activation of the ERK pathway. Cells were pretreated for 30 min with PD98059 (50 µM) or SB202190 (25 µM) followed by treatment with 20 µM HNE for 5 h. The experiment was repeated three times, and a representative blot is shown. Data are expressed as a percentage of HNE-treated controls, with HNE treatment being arbitrarily set at 100%. Inhibiting the ERK pathway significantly decreased HO-1 protein. Surprisingly, blocking the p38 pathway had no effect on HO-1 protein.
Inhibitor studies were also conducted with the JNK inhibitors SP600125 and JNKi, a peptide JNK inhibitor. Although SP600125 is not specific for JNK, it is a potent inhibitor (Ki = 40 nM for both JNK1 and JNK 2) [48]. Although SP600125 did not block the HNE-mediated increase in HO-1 protein, it also failed to block the HNE-mediated increase in c-Jun phosphorylation (data not shown). Thus, there was a question of whether this agent was effective in L2 cells. Regardless, there were two reasons for not pursuing further investigation with SP600125. First, a rigorous study of the specificity of 28 commercially available inhibitors has identified multiple cellular effects of SP600125 and has brought its specificity into question [49]. Nonspecific effects of SP600125 have also been reported by others [50,51]. Second, a commercially available peptide inhibitor of JNK (JNKi) with greater specificity was available. Nonetheless, pretreatment with JNKi also did not block the HNE-mediated increase in HO-1 protein or c-Jun phosphorylation. Collectively, these data support the conclusion that HNE-induced c-Jun phosphorylation occurs via a JNK-independent mechanism such as through activation of the COP9 signalosome.
Discussion
The link between chronic environmental insults such as cigarette smoke and HNE formation and HO-1 induction in the lung is still being elucidated. In this report we show for the first time that HNE induces HO-1 mRNA, protein, and activity in pulmonary epithelial cells. HNE is not only a marker of oxidative stress that is induced by environmental exposure to “pollutants” and in diseases and disorders of the lung with an inflammatory component; it is also a mediator of both injury and adaptive responses [22,24,52–55]. The induction of HO-1 by HNE was both rapid and dramatic. HO-1 induction via most characterized agonists does not peak until 8 or even 12 h postincubation [6,56–58], and often no change was seen at the time points assayed in this study. The rapid induction of HO-1 mRNA also distinguishes our findings from those of other reports in the literature. Interestingly, one notable exception was the rapid response to TGF-β reported by Ning et al. in pulmonary epithelial cells [34]. In this study, HO-1 mRNA increased at 1 h and protein at 3 h postincubation. This raises the possibility that the mechanism of HO-1 induction by HNE in pulmonary epithelial cells is different from other systems. As the lung is the “first line of defense,” it is not surprising that pulmonary epithelial cells may have evolved mechanisms to rapidly respond to cellular insults.
It is well established in the literature that HNE activates some, or even all, of the MAPK in other cell systems [38,59–62]. We hypothesized that HNE-mediated activation of MAPK signaling pathways culminated in phosphorylation and/or nuclear translocation of transcription factors critical to the induction of HO-1 mRNA synthesis and was thus the mechanism for increased HO-1 protein and activity. Each of the three main MAPK (ERK, JNK, and p38) was phosphorylated in response to exposure to HNE, but when the p38 pathway was inhibited by SB202190, it did not block the HNE-mediated increase in HO-1 protein. This is consistent with p38 pathway activation not being required for induction of HO-1 mRNA and protein in this system. In contrast, we have seen that HNE-mediated induction of GGT occurs via ERK and p38 in the same cell type [63]. This is consistent with downstream differences in the regulation of these two genes.
The increase in JNK phosphorylation after HNE exposure was consistent with the JNK pathway being required for the HNE-mediated increase in HO-1 protein. HNE increased JNK and c-Jun phosphorylation, and c-Jun was present in the HNE-activated AP-1 binding complex. However, neither chemical nor peptide inhibitors of JNK had any effect on HNE-mediated c-Jun phosphorylation. It should also be noted an alternate mechanism of c-Jun phosphorylation by the COP9 signalosome has been reported. This has been shown using in vitro kinase activity assays [42] and may explain the JNK-independent c-Jun phosphorylation observed in response to HNE in this study.
The remaining data confirm that activation of the ERK MAPK pathway is required for HNE-mediated induction of HO-1. Not only did HNE increase ERK phosphorylation and c-Fos protein, but c-Fos was also found in the HNE-activated AP-1 binding complex. Furthermore, AP-1 binding was blocked by the MEK inhibitor PD, indicating a requirement for ERK activation for the HNE-mediated increase in AP-1 binding. However, the most critical observation is that the HNE-mediated increase in HO-1 protein was decreased by inhibiting the ERK pathway. As already noted, it did not completely block the increase, suggesting that other mechanisms are also involved.
Studies with the transcriptional inhibitor actinomycin D provided clues to the mechanism of HO-1 induction by HNE. The HNE-mediated increase in HO-1 mRNA was completely blocked by actinomycin D, suggesting that transcription is required for the up-regulation of HO-1 by HNE in pulmonary epithelial cells. Given that the MEK inhibitor PD98059 blocked HNE-mediated induction of HO-1 protein, we expected that it would also block the HNE-mediated increase in HO-1 mRNA. Surprisingly, pretreatment with the MEK inhibitor had no effect on HO-1 message. Together these data are consistent with the conclusion that the increase in HO-1 message is via a MAPK-independent mechanism. However, as the increase in HO-1 protein was dependent on ERK activation this suggests that there may be at least two complementary mechanisms producing the profound increase in HO-1 protein that is observed with HNE: an ERK-independent activation of HO-1 transcription and an ERK-dependent effect on translation of HO-1 mRNA.
The surprising but not unprecedented finding of JNK-independent c-Jun phosphorylation in these cells seems to be a novel mechanism of HNE activation of gene expression. c-Jun can form part of the AP-1, StRE [31], and EpRE binding complexes [64] and further, StRE- and EpRE-mediated induction of HO-1 has been shown previously in rat cell lines [31,64,65]. Although no change in total EpRE binding was observed in response to HNE, the EpRE and/or StRE enhancer elements could still be involved in the increase in HO-1 mRNA via transcription factor complex remodeling [39], specifically through activated c-Jun joining the complex and potentially displacing an inhibitor [66].
It has also been shown that Nrf-2 nuclear translocation can be dependent on ERK phosphorylation [43], but other protein kinases may act in place of ERK in Nrf-2 nuclear translocation. The Western data (Fig. 9 and Fig. 10) are consistent with the conclusion that HNE causes Nrf2 nuclear translocation which is later followed by increased small Maf proteins in the nucleus. As the studies with the MEK inhibitor showed that increased HO-1 mRNA did not require ERK activation, the nuclear translocation of Nrf-2 and subsequent activation of EpRE-mediated transcription can account for the increased HO-1 mRNA only if this is occurring through an ERK-independent mechanism.
There are conflicting reports that the small Maf proteins can be both positive and negative regulators of transcription. In our system, Nrf-2 nuclear translocation and HO-1 protein induction occurred sequentially. This was followed by a rapid drop in nuclear Nrf-2, which coincided with a spike in the nuclear content of the small Maf proteins. Although it is possible that a small, critical window of overlap of these two proteins occurred in the nucleus, it is more plausible that the small Maf proteins are acting as inhibitors of HO-1 in these cells.
In summary, we have found that HNE induces HO-1 mRNA and protein in rat pulmonary epithelial cells and that ERK activation is critical to this process. The data are also consistent with the increases in HO-1 mRNA occurring through a c-Jun phosphorylation/MAPK-independent mechanism. However, there is still a key role for MAPK-mediated signaling in this process as the increase in HO-1 protein can be partially blocked by inhibiting the ERK pathway. There is further evidence that the increase in HO-1 protein is not mediated through AP-1 because PD completely inhibited HNE-induced AP-1 binding but had no effect upon the HNE-induced increase in HO-1 mRNA. Finally, whereas previous studies have suggested that HO-1 induction is an adaptive response to oxidative stress, the increase in HO-1 in lung epithelium induced by HNE may be a mechanism for that increase as HNE is produced by the oxidative stress caused by environmental or inflammatory challenges.
Acknowledgments
This work was supported by NIEHS ES05511 to H.J.F., by a Parker B. Francis Fellowship to K.E.I., and by a grant from the Canadian Institutes of Health Research to V.B. The authors thank Drs. Rui-Ming Liu and Jinah Choi for many helpful comments and suggestions.
Abbreviations
- AP-1
activator protein-1
- EMSA
electrophoretic mobility shift assay
- EpRE
electrophile response element
- ERK
extracellular signal-regulated kinase
- HNE
4-hydroxynonenal
- HO-1
heme oxygenase-1
- HRP
horseradish peroxidase
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- NFDM
nonfat dry milk
- PD
PD98059
- SB
SB202190
- SP
SP600125
- StRE
stress response element
- T-TBS
Tris-buffered saline, with 0.05% Tween 20
References
- 1.Tenhunen R, Marver HS, Schmidt R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J. Lab. Clin. Med. 1970;76:410–421. [PubMed] [Google Scholar]
- 2.Yoshida T, Kikuchi G. Sequence of the reaction of heme catabolism catalyzed by the microsomal heme oxygenase system. FEBS Lett. 1974;48:256–261. doi: 10.1016/0014-5793(74)80481-3. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Noguchi M, Kikuchi G. A new intermediate of heme degradation catalyzed by the heme oxygenase system. J. Biochem. (Tokyo) 1980;88:557–563. doi: 10.1093/oxfordjournals.jbchem.a133003. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez P, Guillen MI, Gomar F, Alcaraz MJ. Expression of heme oxygenase-1 and regulation by cytokines in human osteoarthritic chondrocytes. Biochem. Pharmacol. 2003;66:2049–2052. doi: 10.1016/s0006-2952(03)00543-4. [DOI] [PubMed] [Google Scholar]
- 5.Grasso S, Scifo C, Cardile V, Gulino R, Renis M. Adaptive responses to the stress induced by hyperthermia or hydrogen peroxide in human fibroblasts. Exp. Biol. Med. (Maywood) 2003;228:491–498. doi: 10.1177/15353702-0322805-12. [DOI] [PubMed] [Google Scholar]
- 6.Hartsfield CL, Alam J, Choi AM. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. FASEB J. 1998;12:1675–1682. doi: 10.1096/fasebj.12.15.1675. [DOI] [PubMed] [Google Scholar]
- 7.Inoue S, Suzuki M, Nagashima Y, Suzuki S, Hashiba T, Tsuburai T, Ikehara K, Matsuse T, Ishigatsubo Y. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum. Gene Ther. 2001;12:967–979. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 8.Oguro T, Hayashi M, Numazawa S, Asakawa K, Yoshida T. Heme oxygenase-1 gene expression by a glutathione depletor, phorone, mediated through AP-1 activation in rats. Biochem. Biophys. Res. Commun. 2003;221:259–265. doi: 10.1006/bbrc.1996.0583. [DOI] [PubMed] [Google Scholar]
- 9.Maestrelli P, El Messlemani AH, De Fina O, Nowicki Y, Aetta M, App C, Abbri LM. Increased expression of heme oxygenase (HO)-1 in alveolar spaces and HO-2 in alveolar walls of smokers. Am. J. Respir. Crit. Care Med. 2001;164:1508–1513. doi: 10.1164/ajrccm.164.8.2011083. [DOI] [PubMed] [Google Scholar]
- 10.Dennery PA, Visner G, Weng YH, Nguyen X, Lu F, Zander D, Yang G. Resistance to hyperoxia with heme oxygenase-1 disruption: role of iron. Free Radic. Biol. Med. 2003;34:124–133. doi: 10.1016/s0891-5849(02)01295-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu F, Zander DS, Visner GA. Increased expression of heme oxygenase-1 in human lung transplantation. J. Heart Lung Transplant. 2002;21:1120–1126. doi: 10.1016/s1053-2498(02)00423-0. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Tashiro S, Sun J, Doi H, Satomi S, Igarashi K. Cadmium induces nuclear export of Bach1, a transcriptional repressor of heme oxygenase-1 gene. J. Biol. Chem. 2003;278:49246–49253. doi: 10.1074/jbc.M306764200. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Zhu X, Zhang G, Ling T. Protective effect of hemoglobin-induced heme oxygenase-1 on injured lungs caused by limb ischemia-reperfusion in rats. Chin. J. Traumatol. 2002;5:86–91. [PubMed] [Google Scholar]
- 14.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum. Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 17.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell. Biochem. 2002;234– 235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am. J. Physiol. Renal Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 19.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 20.Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am. J. Physiol. Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton RF, Li L, Eschenbacher WL, Szweda I, Holian A. Potential involvement of 4-hydroxynonenal in the response of human lung cells to ozone. Am. J. Physiol. 1998;274:L8–L16. doi: 10.1152/ajplung.1998.274.1.L8. [DOI] [PubMed] [Google Scholar]
- 22.Robison TW, Forman HJ, Thomas MJ. Release of aldehydes from rat alveolar macrophages exposed in vitro to low concentrations of nitrogen dioxide. Biochim. Biophys. Acta. 1995;1256:334–340. doi: 10.1016/0005-2760(95)00041-a. [DOI] [PubMed] [Google Scholar]
- 23.Tjalkens RB, Cook LW, Petersen DR. Formation and export of the glutathione conjugate of 4-hydroxy-2, 3-E-nonenal (4-HNE) in hepatoma cells. Arch. Biochem. Biophys. 1999;361:113–119. doi: 10.1006/abbi.1998.0946. [DOI] [PubMed] [Google Scholar]
- 24.Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 25.Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid. Redox Signaling. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- 26.Rinaldi M, Barrera G, Aquino A, Spinsanti P, Pizzimenti S, Farace MG, Dianzani MU, Fazio VM. 4-Hydroxynonenal-induced MEL cell differentiation involves PKC activity translocation. Biochem. Biophys. Res. Commun. 2000;272:75–80. doi: 10.1006/bbrc.2000.2691. [DOI] [PubMed] [Google Scholar]
- 27.Calonghi N, Boga C, Cappadone C, Pagnotta E, Bertucci C, Fiori J, Masotti L. Cytotoxic and cytostatic effects induced by 4- hydroxynonenal in human osteosarcoma cells. Biochem. Biophys. Res. Commun. 2002;293:1502–1507. doi: 10.1016/S0006-291X(02)00397-2. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, Awasthi S, Awasthi YC. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J. Biol. Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- 29.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 30.Friling RS, Bergelson S, Daniel V. Two adjacent AP-1-like binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Proc. Natl. Acad. Sci. USA. 1992;89:668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam J, Killeen E, Gong P, Naquin R, Hu B, Stewart D, Ingelfinger JR, Nath KA. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am. J. Physiol. Renal Physiol. 2003;284:F743–F752. doi: 10.1152/ajprenal.00376.2002. [DOI] [PubMed] [Google Scholar]
- 32.Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Lee TJ. Nitric oxide and prostaglandin E2 participate in lipopolysaccharide/interferon-gamma-induced heme oxygenase 1 and prevent RAW264.7 macrophages from UV-irradiation-induced cell death. J. Cell. Biochem. 2002;86:331–339. doi: 10.1002/jcb.10230. [DOI] [PubMed] [Google Scholar]
- 33.Lee TS, Chau LY. Heme oxygenase-1 mediates the antiinflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 34.Ning W, Song R, Li C, Park E, Mohsenin A, Choi AM, Choi M. TGF-beta1 stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L1094–L1102. doi: 10.1152/ajplung.00151.2002. [DOI] [PubMed] [Google Scholar]
- 35.Iles KE, Nagy LE. Chronic ethanol feeding increases the quantity of G alpha S-protein in rat liver plasma membranes. Hepatology. 1995;21:1154–1160. [PubMed] [Google Scholar]
- 36.Iles KE, Dickinson DA, Watanabe N, Iwamoto T, Forman HJ. AP-1 activation through endogenous H2O2 generation by alveolar macrophages. Free Radic. Biol. Med. 2002;32:1304–1313. doi: 10.1016/s0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 37.Visner GA, Lu F, Zhou H, Liu J, Kazemfar K, Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–916. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson DA, Iles KE, Watanbe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-Hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 39.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate– cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Hamilton RFJ, Kirichenko A, Holian A. 4-Hydroxynonenal-induced cell death in murine alveolar macrophages. Toxicol. Appl. Pharmacol. 1996;139:135–143. doi: 10.1006/taap.1996.0152. [DOI] [PubMed] [Google Scholar]
- 41.Salinas M, Wang J, Rosa de Sagarra M, Martin D, Rojo A, Martin-Perez J, Ortiz de Montellano P, Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004;578:90–94. doi: 10.1016/j.febslet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 42.Naumann M, Bech-Otschir D, Huang X, Ferrell K, Dubiel W. COP9 signalosome-directed c-Jun activation/stabilization is independent of JNK. J. Biol. Chem. 1999;274:35297–35300. doi: 10.1074/jbc.274.50.35297. [DOI] [PubMed] [Google Scholar]
- 43.Zipper LM, Mulcahy RT. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 2003;73:124–134. doi: 10.1093/toxsci/kfg083. [DOI] [PubMed] [Google Scholar]
- 44.Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem. Biophys. Res. Commun. 1999;261:661–668. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- 45.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AMK, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf-2-interacting protein. J. Biol. Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka K, Igarashi K, Itoh K, Fujiwara KT, Noda M, Yamamoto M, Nishizawa M. Small Maf proteins heterodimerize with Fos and may act as competitive repressors of the NF-E2 transcription factor. Mol. Cell. Biochem. 1995;15:2180–2190. doi: 10.1128/mcb.15.4.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong P, Hu B, Stewart D, Ellerbe M, Figueroa YG, Blank V, Beckman BS, Alam J. Cobalt induces heme oxygenase-1 expression by a hypoxia-inducible factor-independent mechanism in Chinese hamster ovary cells: regulation by Nrf2 and MafG transcription factors. J. Biol. Chem. 2001;276:27018–27025. doi: 10.1074/jbc.M103658200. [DOI] [PubMed] [Google Scholar]
- 48.Bogoyevitch M, Boehm I, Oakley A, Ketterman A, Barr R. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim. Biophys. Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J. 2005;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaishnav D, Jambal P, Reusch JE, Pugazhenthi S. SP600125, an inhibitor of c-jun N-terminal kinase, activates CREB by a p38 MAPK-mediated pathway. Biochem. Biophys. Res. Commun. 2003;307:855–860. doi: 10.1016/s0006-291x(03)01287-7. [DOI] [PubMed] [Google Scholar]
- 51.Minutoli L, Altavilla D, Marini H, Passaniti M, Bitto A, Seminara P, Venuti FS, Famulari C, Macri A, Versaci A, Squadrito F. Protective effects of SP600125 a new inhibitor of c-jun N-terminal kinase (JNK) and extracellular-regulated kinase (ERK1/2) in an experimental model of cerulein-induced pancreatitis. Life Sci. 2004;75:2853–2866. doi: 10.1016/j.lfs.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 52.Rahman I, Van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 53.Oudijk EJ, Lammers JW, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2003;46:5s–13s. doi: 10.1183/09031936.03.00004603a. [DOI] [PubMed] [Google Scholar]
- 54.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 55.Dickinson DA, Moellering DR, Iles KE, Patel RP, Levonen AL, Wigley A, Darley-Usmar VM, Forman HJ. Cytoprotection against oxidative stress and the regulation of glutathione synthesis. Biol. Chem. 2003;384:527–537. doi: 10.1515/BC.2003.061. [DOI] [PubMed] [Google Scholar]
- 56.Oberle S, Abate A, Grosser N, Vreman HJ, Dennery PA, Schneider HT, Stalleicken D, Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of pentaerythrityl trinitrate. Biochem. Biophys. Res. Commun. 2002;290:1539–1544. doi: 10.1006/bbrc.2002.6379. [DOI] [PubMed] [Google Scholar]
- 57.Hill-Kapturczak N, Thamilselvan V, Liu F, Nick HS, Agarwal A. Mechanism of heme oxygenase-1 gene induction by curcumin in human renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2001;281:F851–F859. doi: 10.1152/ajprenal.2001.281.5.F851. [DOI] [PubMed] [Google Scholar]
- 58.Hill-Kapturczak N, Sikorski E, Voakes C, Garcia J, Nick HS, Agarwal A. An internal enhancer regulates heme- and cadmium-mediated induction of human heme oxygenase-1. Am. J. Physiol. Renal Physiol. 2003;285:F515–F523. doi: 10.1152/ajprenal.00137.2003. [DOI] [PubMed] [Google Scholar]
- 59.Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J. Biol. Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- 60.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 61.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola P, Poli G, Warg G, Gentilini P, Dianzani MU. HNE interacts with JNK isoforms in human hepatic stellate cells. J. Clin. Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O. H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp. Neurol. 2003;180:144–155. doi: 10.1016/s0014-4886(02)00059-6. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Dickinson D, Liu R, Forman H. 4-Hydroxynonenal increases g-glutamyl transpeptidase gene expression through mitogen- activated protein kinase pathways. Free Radic. Biol. Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Peyton K, Ensenat D, Wang H, Schafer A, Alam J. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle: role in cell survival. J. Biol. Chem. 2005;280:872–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 65.He C, Gong P, Hu B, Stewart D, Choi M, Choi A, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2- interacting protein: implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 66.Jaiswal A. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]