Abstract
Clinical translation of scientific discoveries is often the long-term goal of academic medical research. However, this goal is not always realized due to the complicated path between bench research and clinical use. In this review, we outline the fundamental steps required for first-in-human testing of a new imaging device, and use the FLARE™ (Fluorescence-Assisted Resection and Exploration) near-infrared fluorescence optical imaging platform as an example.
I. Introduction
Translation of scientific discoveries from bench to bedside is the long-term goal for the majority of medical research. However, the path from bench to bedside is often confusing and difficult to navigate. Although the process of translation can differ dramatically depending upon the type of discovery, there is a common goal: to fulfill a clinical need. The aim of this review is to explain the clinical translation process for new medical imaging devices and to provide an example of translation using the FLARE™ (Fluorescence-Assisted Resection and Exploration) near-infrared (NIR) fluorescence optical imaging platform [1].
Imaging technology currently available in the clinic includes magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasound (US), computed tomography (CT), plain film x-rays, and x-ray fluoroscopy [2]. Although these imaging modalities enable visualization of a variety of internal structures and assessment of diseased tissues, many clinical imaging needs remain unmet. One such unmet clinical need is image-guided surgery. Currently, most surgery is performed without real-time image-guidance. There are a wide variety of reasons why image-guidance is not currently in use, but perhaps the most compelling reason is that optimized imaging systems and corresponding contrast agents for image-guided surgery are not readily available. Current intraoperative imaging techniques include MRI, CT, x-ray fluoroscopy, and US [3]. Although MRI can provide high soft-tissue contrast, its use in the operating room is limited due to its size, high magnetic field strength, and significant image acquisition time. CT and x-ray fluoroscopy provide excellent bone to soft tissue contrast, but expose patient and caregivers to ionizing radiation [3]. MRI and CT systems are also prohibitively expensive for use as routine tools in the operating room. US has gained popularity for image-guided surgery due to its small size, low cost, and excellent safety profile. However, US imaging during surgery is limited by the need for direct contact with the tissue through a matching medium, limited field of view, and limited types of contrast agents [2], [3].
Optical imaging has the potential to fulfill the need for real-time, non-contact imaging during surgery. The FLARE™ intraoperative near-infrared fluorescence imaging system is the product of numerous discussions between surgeons and engineers to create a system specifically designed for image-guided surgery using exogenous contrast agents.
II. Clinical Translation of Imaging Technology is Based on Risk
A. Scope of this Review
This review outlines the general process for clinical translation at any medical institution but applies only to imaging devices that require no exogenous contrast agent or those that utilize a contrast agent already approved for other indications by the US Food and Drug Administration (FDA). The clinical translation of imaging devices that require novel contrast agents, or require FDA-approved agents to be used in unusual ways, is beyond the scope of our review. This is because the contrast agent will require an investigational new drug (IND) application to be approved by the FDA, and the combination of device and drug may fall under “combination” regulations, as specified in Title 21 of the Code of Federal Regulations (CFR) Part 3.2, Subpart (e) (i.e., 21 CFR 3.2(e)), which require both an investigational device exemption (IDE) and an IND.
B. Initial Imaging System Development
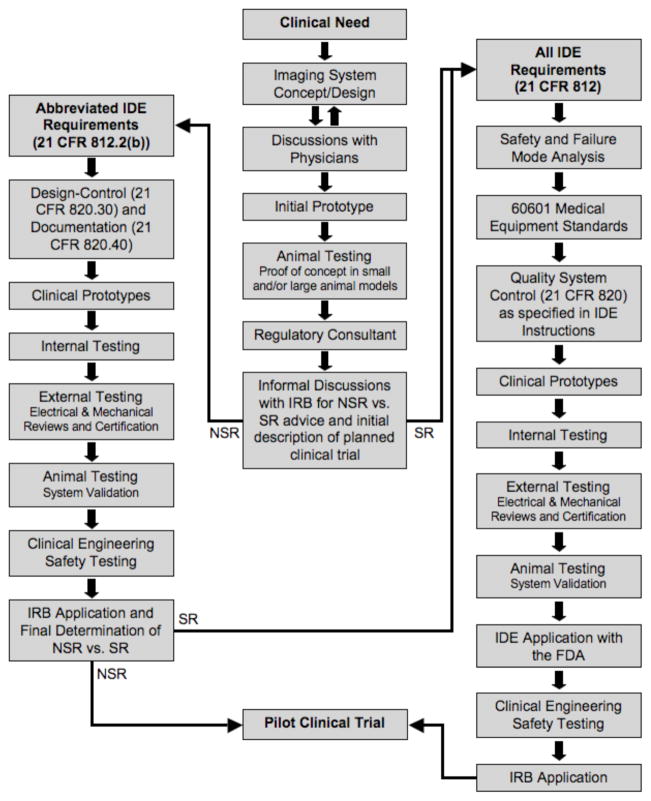
Imaging system development must begin with a real and unequivocal clinical need. Technology that is not expected to have a prolonged impact on patient care should be abandoned because the costs and risks associated with clinical translation are significant. The key to successful translation is iterative discussions with the physicians who will use the system (Fig. 1). Following these discussions, an initial prototype system should be constructed and tested in appropriate animal models to show proof of concept. Translation of any device to the clinic requires approval by the institutional review board (IRB) of the hospital where the device will be tested (discussed below). Because of this, a regulatory consultant is an essential member of the development team and should be engaged as early as possible in the translation process.
Fig. 1.
Flow chart for first-in-human testing of a new device for medical imaging, starting with clinical need and ending with a pilot clinical trial. SR vs. NSR status is a key decision point early in the process, but a binding determination by the IRB can only occur after a full application is filed (the “risk conundrum”).
C. The Risk Conundrum
For first-in-human testing of medical devices, the FDA recognizes two general statuses: significant risk (SR) and non-significant risk (NSR) [4]. Confusion often arises, because, for marketing and distribution of medical devices, the FDA recognizes three classes of devices (I, II, and III), with class determining the stringency (and cost) of the approval process.
IRBs are mandated to protect the safety of human subjects, and have the sole authority to determine whether a medical device is SR or NSR prior to first-in-human testing. This determination is critical to the translation process since a NSR device does not require submission and approval of an IDE application with the FDA prior to submission of the IRB application. The inherent conundrum, though, is highlighted in Fig. 1. The IRB does not have to render a final decision on SR vs. NSR status until a full IRB application is filed. However, the SR process is so much more difficult and costly than the NSR process that it should be pursued only when necessary. Thus, we recommend informal discussions with the IRB regarding SR vs. NSR status as soon as initial animal testing has been completed and the desired clinical trial has been conceived. Of special note, these informal discussions with the IRB are non-binding, and, after review of the full IRB application, the IRB may determine the device to be SR. In this case, an IDE will be required, which typically takes months to prepare and receive approval.
D. Non-Significant Risk Devices
NSR devices need to be designed and built to an abbreviated subset of IDE requirements as outlined in 21 CFR 812.2(b) [5]. Quality System Regulation Design Controls (21 CFR 820.30 [6]) and Documentation (21 CFR 820.40 [6]) detailing how the system was built and tested are essential, and should be completed as the clinical prototypes are being built. Following construction, extensive testing is necessary. Internal testing should be performed to ensure device safety. In addition, qualified consultants should conduct independent mechanical and electrical safety testing and provide safety approval documentation. A prototype identical to the clinical prototype should be used for final animal testing and system validation. Any new device intended for use in patient care must also be tested for safety by the clinical engineering department of the hospital prior to its clinical use. After these tests are completed, an IRB application can be submitted.
E. Significant Risk Devices
SR medical devices must be designed to meet all IDE requirements (21 CFR 812 [5]) and will be subject to extensive safety and failure mode analysis. They must also be engineered to meet relevant subsections of the Association for the Advancement of Medical Instrumentation (AAMI)/International Electrotechnical Commission (IEC) standard #60601 [7]. Full Quality System Control documentation (21 CFR 820 [6]) as specified in the IDE instructions is required prior to clinical translation. This documentation should be written as the clinical prototypes are being built. Similar to NSR devices, extensive testing is necessary to ensure device safety, including internal and external testing by qualified consultants, as well as clinical prototype testing with an equivalent system on animal models.
After completion of appropriate documentation and testing of the clinical prototype, an IDE application must be submitted to the FDA. Of note, some academic institutions require that the principal investigator be personally responsible for the IDE (i.e., “hold” the IDE). This has serious liability implications and should be considered thoroughly before pursuing. If the FDA approves the IDE application, testing can be completed by the hospital’s clinical engineering department, followed by submission of a full application to the IRB. If IRB approval is granted, the clinical trial may begin.
In summary, the major pathways for first-in-human testing of imaging devices are very similar, with the differences between NSR and SR being less regulatory control, as well as less stringent record keeping and reporting requirements for NSR devices, thus permitting faster translation to the clinic.
III. Translation of the FLARE™ Imaging System
A. The FLARE™ Optical Imaging Platform
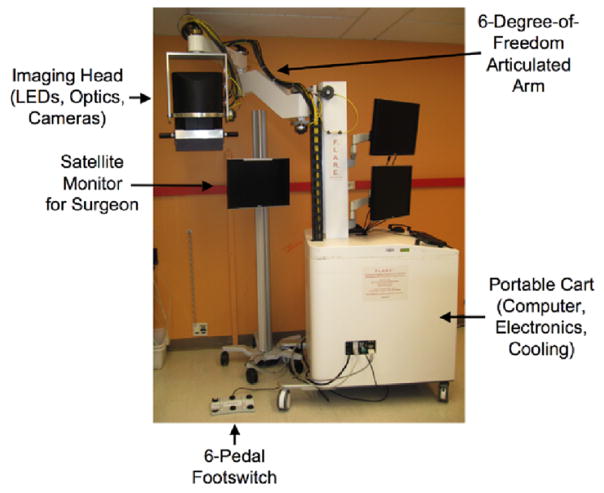
Near-infrared (NIR) light in the range of 700 to 900 nm can penetrate relatively deeply into living tissue due to decreased absorbance, scatter, and autofluorescence at these wavelengths [8], [9]. NIR light is an ideal tool for surgical guidance because fluorophores and systems for their visualization can be designed to guide surgeons to structures that should be resected, such as tumors, and to illuminate structures that should be avoided, such as nerves and blood vessels. One such system, the FLARE™ intraoperative NIR fluorescence imaging system has been designed as a general-purpose image-guided surgery system (Fig. 2) [10]. The FLARE™ system permits real-time, simultaneous acquisition from a color video camera (to display surgical anatomy), and two independent NIR fluorescence cameras. The custom software also permits pseudo-coloring of the normally grayscale NIR fluorescence images, and overlays them onto the image from the color video camera, to provide a complete map of tissue on the surgical field that needs to be resected and tissue that needs to be avoided. Moreover, the FLARE™ system has no moving parts, a long working distance (18”), portability, and uses LED light rather than lasers for excitation [1], [11], [12].
Fig. 2.
The FLARE™ Near-Infrared Fluorescence Imaging System: The articulated arm has a reach of 50” laterally and 70” vertically, which permits positioning of the imaging head over the surgical field. The footswitch and satellite monitor are positioned according to surgeon preference.
B. Clinical Translation of the FLARE™ System
The FLARE™ system was designed, constructed, and translated to the clinic with the end goal of producing an approvable device. The system was designed as a “platform” for grafting of advanced optical imaging techniques and to stimulate exogenous contrast agent development. These contrast agents will help guide surgeons during surgery by highlighting tissues that need to be resected or avoided. A regulatory consultant was employed as part of the team to assist with the complicated process of clinical translation.
Substantial emphasis was placed on design-control documentation, which was developed in parallel with the clinical prototype. The clinical prototype underwent rigorous internal testing and was also externally tested by electrical and mechanical safety consultants. Following completion of both internal and external system testing, clinical engineering at Beth Israel Deaconess Medical Center (BIDMC) confirmed electrical safety for use in the operating room. The IRB at the BIDMC determined that the FLARE™ imaging system qualified as a NSR device. On this basis, IRB approval was applied for and granted for a study using the FLARE™ imaging system with breast cancer patients.
C. Pilot Clinical Trial
In a pilot clinical study using indocyanine green (ICG) adsorbed to human serum albumin (HSA) [13] as the lymphatic tracer (ICG:HSA), the FLARE™ system was tested in women undergoing sentinel lymph node (SLN) mapping for breast cancer [10]. All subjects received the standard of care with radiocolloid and lymphoscintigraphy. Because the FLARE™ system was not used in this pilot trial to guide surgery, it was deemed NSR. This is an important point, because if the standard of care with radiocolloid were not used, the IRB determination would almost certainly have been SR. Nevertheless, the potential advantages to using the FLARE™ system and ICG:HSA for SLN mapping over radiotracers and blue dyes are that it does not require ionizing radiation, provides real-time visualization of the SLN in the context of surgical anatomy, and does not alter the look of the surgical field.
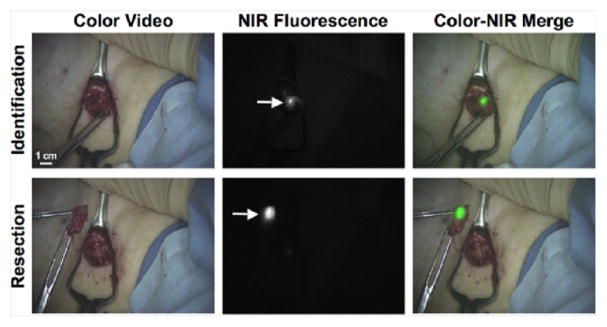
The system was tested in six patients to determine its safety profile and whether its use was practical for the breast surgeon [10]. Lymphatic flow was often visible prior to the first incision. The fluorescent signal from the ICG:HSA was recorded, while the radiotracer, i.e., standard of care, was used to guide the course of treatment for each patient. The ICG:HSA accumulated in the SLN and was brightly fluorescent, allowing the nodes to be resected under real-time NIR fluorescence guidance (Fig. 3).
Fig. 3.
Example images from the pilot clinical trial of the FLARE™ imaging system during SLN mapping for breast cancer. The FLARE™ system collected color video and 800 nm NIR fluorescence images simultaneously. The NIR fluorescence image was pseudo-colored and merged with the color image in real-time, permitting the surgeon to resect the SLN (arrow) under fluorescence-guidance.
IV. Conclusions
Clinical translation is a regularly discussed goal of research, but frequently unattainable. Part of the reason for this is the difficulty in navigating the steps from bench research to a pilot clinical study. In this review, the fundamental steps that enable clinical translation of an imaging device at any medical institution have been outlined and discussed. The clinical translation of the FLARE™ intraoperative NIR fluorescence imaging system at BIDMC was reviewed as an example of NSR device translation. The FLARE™ system was designed as a platform for contrast agent and imaging technology development. However, in order to qualify as an NSR device, all patients received the standard of care and FLARE™ was not used to make clinical decisions. This first-in-human study with the FLARE™ system enabled translation of this technology to the clinic as a first step in the pathway towards a tool for surgical decision-making.
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health under grants R01-CA-115296 and R01-EB-005805.
We thank Jeffrey Thumm (Duke River Engineering, Norfolk, MA) and Gordon D. Row (Yankee Modern Engineering, Groton, MA) for electrical engineering and mechanical engineering certifications, respectively.
References
- 1.Gioux S, Kianzad V, Ciocan R, Gupta S, Oketokoun R, Frangioni JV. Molecular Imaging. 2009. High power, computer-controlled, LED-based light sources for fluorescence imaging and image-guided surgery. vol. In Press. [PMC free article] [PubMed] [Google Scholar]
- 2.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–21. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters TM. Image-guidance for surgical procedures. Phys Med Biol. 2006;51:R505–40. doi: 10.1088/0031-9155/51/14/R01. [DOI] [PubMed] [Google Scholar]
- 4.“U.S. Food and Drug Administration: Significant Risk and Nonsignificant Risk Medical Device Studies,” FDA Information Sheets October 1, 1995, pp. http://www.fda.gov/cdrh/d861.html, 1995.
- 5.“U.S. Code of Federal Regulations: Title 21 Part 812,” pp. http://www.access.gpo.gov/nara/cfr/waisidx_06/21cfr812_06.html
- 6.“U.S. Code of Federal Regulations: Title 21 Part 820,” pp. http://www.access.gpo.gov/nara/cfr/waisidx_06/21cfr820_06.html
- 7.“Medical electrical equipment, Part 1: General requirements for basic safety and essential performance,” vol. 60601: AAMI/American National Standard, 2005, pp. 1–396.
- 8.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Klohs J, Wunder A, Licha K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res Cardiol, vol. 2008;103:144–51. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 10.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, Ngo L, Khamene A, Azar F, Frangioni JV. The FLARE™ intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0594-2. vol. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–62. doi: 10.1177/153303460300200607. [DOI] [PubMed] [Google Scholar]
- 12.Gioux S, De Grand AM, Lee DS, Yazdanfar S, Idoine JD, Lomnes SJ, Frangioni JV. Improved optical sub-system for intraoperative near-infrared fluorescence imaging. Proceedings of SPIE. 2005;6009:60090C-1–60090C-10. [Google Scholar]
- 13.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–81. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]