Abstract
Objective
To determine differences in the rates of growth, endocrine and calcium related abnormalities in the various thalassemia syndromes in North America with current therapy.
Methods
Medical history, physical examinations and blood and urine collections were obtained from patients with all thalassemia syndromes age 6 years and older in the Thalassemia Clinical Research Network.
Results
361 subjects, 49% male, mean age 23.2 years (range 6.1 to 75 years) were studied. Approximately 25% of children and adults, regardless of the thalassemia syndrome, had short stature. Overall growth in children was mildly affected. Final height was close to midparental height (z = -0.73 ± 1.24). Patients with beta thalassemia major (TM) had higher rates of hypogonadism, multiple endocrinopathies, worse hyperglycemia, subclinical hypoparathyroidism and hypercalciuria. Hypogonadism remained the most frequent endocrinopathy and was frequently under-treated. 12.8% of the subjects had 25 vitamin D concentrations less than 27nmol/L and 82% less than 75nmol/L, regardless of the thalassemia syndrome. Adolescents had lower 25 vitamin D levels than children and adults.
Conclusions
Compared to patients with other thalassemia syndromes, those with beta TM suffer from higher rates of multiple endocrinopathies, abnormal calcium metabolism and hypercalciuria. Vitamin D abnormalities are high among adolescents.
Introduction
Poor growth and multiple endocrinopathies, including hypogonadotropic hypogonadism, growth hormone deficiency and diabetes, are known complications in beta thalassemia major (TM), and are considered the result of iron overload (De Sanctis et al, 1994; Jensen et al, 1997; Roth et al, 1997). Iron chelation therapy with deferoxamine (DFO), introduced in the mid 1970's, combined with a transfusion regimen that maintains near normal pre-transfusion hemoglobin concentrations, initiated in the 1980's, has dramatically changed the course of beta TM and prolonged survival (Brittenham et al, 1994; Calleja et al, 1998; Cunningham et al, 2004). Even with iron chelation, the rate of endocrinopathies remains high among patients with beta TM, with hypogonadism reported in approximately 40-60% of the patients, short stature in 30% and diabetes in 5-14 % (Caruso-Nicoletti et al, 2004; De Sanctis et al, 2004; Gamberini et al, 2004; Al-Rimawi et al, 2006). Little is known, however, about the current prevalence of various endocrinopathies and growth disturbances among patients with other thalassemia syndromes with today's therapy. One would expect better outcomes in patients with mild to moderate disease who do not require regular transfusions, such as beta thalassemia intermedia (TI) and Hemoglobin H disease (HbH), compared to those transfused regularly, including Beta TM, E-beta thalassemia (E-beta), homozygous alpha thalassemia (Hz α) and the co-inheritance of HbH and hemoglobin Constant Spring (HbH/CS).
The Thalassemia Clinical Research Network (TCRN) consists of five thalassemia centers in North America and their associated satellite sites with access to patients with both alpha and beta thalassemia syndromes. The TCRN performed a cross-sectional study to address the above questions. Particular emphasis was placed on the study of calcium and vitamin D metabolism, because of the recently described high prevalence of low bone mass across all thalassemia syndromes (Pollak et al, 2000; Vogiatzi et al, 2006; Vogiatzi et al, 2009). Finally, we sought to determine associations between endocrinopathies, anemia, transfusion history and chelation-related parameters, and thalassemia co-morbidities to better understand their evolution and etiology.
Methods
Study Protocol
TCRN patients of all thalassemia syndromes, age 6 and older, were eligible for this study. Exclusion criteria included pregnancy and any known pre-existing medical condition that is known to require chronic systemic administration of steroids. The protocol was approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all TCRN institutions. Informed consent, or assent in the case of a minor, was obtained.
Medical history was obtained by interview and review of medical records, and a complete physical examination was performed. A fasting morning blood sample was obtained for measurement of free thyroxine (T4) and TSH, insulin–like growth factor 1 (IGF1), insulin–like growth factor binding protein 3 (IGFBP3), serum gonadotropins (i.e. LH and FSH), testosterone (only in males), 25-hydroxy vitamin D (25 vit D), 1,25-dihydroxy vitamin D (1,25 vit D), intact parathyroid hormone (PTH), serum calcium, phosphorus, ferritin and transferrin receptor concentrations. A 24-hour urine collection was performed for measurement of calcium excretion. Dietary calcium intake was estimated from a 46-item self-completed food frequency questionnaire. Bone age was determined from x-rays of the left hand and wrist of participants younger than 20 years and read locally according to the method of Greulich and Pyle (1959).
Calculated Variables
Patients with beta thalassemia were classified as having TM if they had received 8 or more transfusions during the 12 months prior to entering the study or as TI if they had been transfused less than 8 times the year before.
Body mass index (BMI) was calculated as kg/m2. Anthropometric Z-scores were calculated relative to age- and gender-specific norms for Caucasians produced by the CDC from NHANES III data. Mid-parental heights were calculated as the average parental height plus or minus 6.5 cm for boys and girls, respectively. Presence of an endocrinopathy (i.e. hypogonadism, hypothyroidism, diabetes mellitus, growth hormone deficiency and hypoparathyroidism) was defined as having an identified clinical history or prescribed treatment. Among females, hypogonadism was defined as lack of menses after the age of 16, lack of spontaneous menarche, having a history of using hormone replacement therapy (HRT) for failure to proceed through puberty, loss of menses before age 40, or current use of HRT. Hypogonadism in males was determined by the prescription of HRT (testosterone or HCG) or by having a serum testosterone concentration lower than established norms for age. Inadequate gonadal steroid replacement in hypogonadal males was confirmed by having a serum testosterone concentration lower than established norms for age despite prescribed HRT. In addition to clinical history, hypoparathyroidism was defined as an intact PTH level below the normal range (10 ng/l) in the presence of albumin-adjusted hypocalcemia (serum calcium below 2.1mmol/l). Vitamin D deficiency was defined as a 25 vit D concentration of less than 27nmol/l, and insufficiency as between 27 and 75 nmol/l. 24-Hour urine calcium excretion above 4 mg/kg/d was consistent with hypercalciuria. Fasting hyperglycemia (a blood glucose level above 70mmol/l), having a history of fasting hyperglycemia or diabetes, having a history of prescribed therapy with oral hypoglycemics or insulin, or current therapy with oral hypoglycemics or insulin were used to determine the presence of diabetes. The diagnosis of growth hormone deficiency in children with growth failure was made after endocrine referral to a participating site and appropriate testing (i.e. growth hormone provocative test). Daily calcium intake (dietary + supplements) was considered adequate if it was above 800mg for the age group of 6-8y, 1300mg for 9-18y, 1000mg for 19- 50y and 1200mg for 51y and older.
Laboratory Assays
Urine and serum samples from each participant were stored at −80°C and analyzed as a batch at a central facility. Serum IGF1 and IGFBP3 were measured by solid-phase, enzyme-linked immunoassays (ELISA), 25 vit D by competitive radioimmunoassay following extraction, 1,25 vit D by column chromatography and radioimmunoassay, intact PTH by immunochemiluminometric assay, and free T4, LH, FSH, and TSH by high sensitivity heterogeneous sandwich separation assays. Testosterone was measured by a solid-phase, competitive radioimmunoassay. Serum ferritin levels were determined by a radioimmunoassay (RIA, T-14, Ramco Laboratories, Houston, TX, USA). Serum transferrin receptor concentrations were measured by an enzyme immunoassay (EIA; T-94, Ramco Laboratories).
Statistical Analysis
Very rare subgroups of participants (homozygous α thalassemia, n=3, and recipients of bone marrow transplants, n=6) were excluded from most analyses that included a thalassemia diagnosis as a predictor to avoid bias in variance estimates and model instability due to empty cells.
General linear models were used to model the effects of age, gender, race, thalassemia syndrome, hypogonadism, growth hormone deficiency, serum IGF1 and IGFBP3 concentrations, ferritin concentration, transferrin receptors, and hepatitis C on the following outcomes: height, weight, BMI, difference in height from midparental height, and difference between chronological age and bone age. Logistic regression was used to model the effect of the same variables on short stature (failure to thrive). Additional general linear models examined the effect of gender, thalassemia syndrome, hypogonadism, ferritin concentration, and growth hormone therapy on serum IGF1 and IGFBP3 concentrations, controlling for age as a covariate using linear splines with two knots at ages 11 and 20 years.
Logistic regression was used to model the effects of age, gender, thalassemia syndrome, and serum ferritin concentration on the following outcomes in patients over 10 years of age: hypogonadism, hypothyroidism, diabetes, and the presence of multiple endocrinopathies. The effect of various gonadal steroid replacement therapies on serum testosterone concentration was modeled with a general linear model, controlling for age, in medicated hypogonadal males. Additionally, a general linear model was also used to assess the effects of gender, thalassemia syndrome, hypogonadism, and ferritin concentration on fasting blood glucose concentration, controlling for age and hours fasting as covariates using linear splines with two knots each.
General linear models were used to determine the effects of age, race, and thalassemia syndrome on the following outcomes: dietary calcium intake, calcium supplementation, total calcium intake, vitamin D sufficiency, and serum concentration of intact PTH. Additional predictors of vitamin D sufficiency were season, and urinary calcium excretion. Analysis of variance was used to model the effect of vitamin D sufficiency and diagnosis (TM vs. TI) on intact PTH. A Fisher exact test and logistic regression were used to model the effect of diagnosis (TM vs. TI) and vitamin D sufficiency on hypercalciuria, respectively.
Analyses were generally exploratory with the aim of describing observed patterns in the data. Corrections for multiple comparisons were not made, and alpha ≤ 0.05 was considered statistically significant. All analyses were conducted using SAS (version 9.1.3, SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
A total of 361 subjects, 176 males (48.8%) and 185 females (51.2%), mean age 23.2 years (range 6.1 to 75.4 years) were enrolled in the study (Table I). Of these, 236 were diagnosed with beta TM, 43 with beta TI, 43 with E- β, 19 with HbH, and 20 with HbH/CS or other non-deletional mutations. There were three patients with Hz α thalassemia, who were not included in this analysis because of the small sample size. Details on the demographics of the participants, and their transfusion and chelation regimens are presented in Table 1.
Table I. Characteristics of study participants1.
| Characteristic | All participants (n = 361) |
Beta TM (n = 236) |
Beta TI (n = 43) |
E/beta (n = 43) |
HbH Disease (n = 19) |
HbH/CS (n = 20) |
|
|---|---|---|---|---|---|---|---|
| DEMOGRAPHICS | |||||||
| Age (years) | 23.2±12.1 (6.1 to 75.4) |
24.4±11.6 (6.1 to 53.2) |
26.3±16.2 (6.1 to 75.4) |
19.5±9.3 (7.0 to 48.2) |
15.1±8.7 (6.9 to 39.6) |
17.1±10.0 (6.2 to 51.2) |
|
| 06 to 10 years | 69 (19.1%) | 34 (14.4%) | 10 (23.3%) | 11 (25.6%) | 8 (42.1%) | 6 (30.0%) | |
| 11 to 19 years | 94 (26.0%) | 55 (23.3%) | 9 (20.9%) | 12 (27.9%) | 8 (42.1%) | 10 (50.0%) | |
| 20 years or older | 198 (54.8%) | 147 (62.3%) | 24 (55.8%) | 20 (46.5%) | 3 (15.8%) | 4 (20.0%) | |
| Gender | Male | 176/361 (48.8%) | 116/236 (49.2%) | 20/43 (46.5%) | 20/43 (46.5%) | 7/19 (36.8%) | 13/20 (65.0%) |
| Race | Asian | 152 (42.1%) | 78 (33.1%) | 6 (14.0%) | 40 (93.0%) | 10 (52.6%) | 18 (90.0%) |
| Caucasian | 186 (51.5%) | 154 (65.3%) | 30 (69.8%) | 1 (2.3%) | 1 (5.3%) | 0 (0.0%) | |
| Other | 23 (6.4%) | 4 (1.7%) | 7 (16.3%) | 2 (4.7%) | 8 (42.1%) | 2 (10.0%) | |
| ANTHROPOMETRICS | |||||||
| Height z-score | -1.26±1.17 (-5.08 to 1.81) |
-1.38±1.17 (-5.08 to 1.56) |
-0.52±0.95 (-2.17 to 1.81) |
-1.54±1.18 (-4.12 to 0.57) |
-0.91 ±1.11 (-3.10 to 1.24) |
-1.29±0.99 (-3.21 to 0.30) |
|
| Weight z-score | -0.80±1.23 (-4.57 to 2.78) |
-0.73±1.26 (-4.57 to 2.78) |
-0.51 ±0.92 (-2.80 to 1.20) |
-1.38±1.22 (-4.03 to 0.96) |
-0.76±1.59 (-3.72 to 2.06) |
-1.15±0.85 (-2.94 to 0.32) |
|
| BMI z-score | -0.17±1.09 (-4.52 to 2.71) |
-0.04±1.09 (-4.51 to 2.71) |
-0.28±0.89 (-3.08 to 1.22) |
-0.52±0.91 (-2.89 to 1.23) |
-0.48±1.77 (-4.52 to 1.93) |
-0.51 ±0.94 (-3.21 to 0.88) |
|
|
Bone-age (years, only if < 20 years) |
11.5±4.7 (4.5 to 20.0) |
11.7±4.6 (5.0 to 20.0) |
10.2±4.7 (5.0 to 20.0) |
10.8±4.1 (5.8 to 18.0) |
12.3±4.3 (7.8 to 20.0) |
13.1±7.9 (4.5 to 20.0) |
|
|
Chronologic age - bone age (years, only if < 20 years) |
0.60±1.51 (-4.44 to 4.54) |
0.59±1.46 (-2.73 to 4.54) |
0.88±1.12 (-0.87 to 3.49) |
1.17±1.39 (-1.27 to 4.05) |
-0.40±1.58 (-4.00 to 1.69) |
-0.00±2.66 (-4.44 to 1.82) |
|
| Height z-score – mid-parental height z-score, only if >20y | -0.73±1.24 (-4.82 to 2.63) |
-0.98±1.22 (-4.82 to 2.63) |
0.04±0.85 (-1.26 to 2.01) |
-0.01 ±1.19 (-3.58 to 1.23) |
0.57±0.70 (0.08 to 1.07) |
0.31 ±0.67 (-0.46 to 0.70) |
|
| TRANSFUSIONS & CHELATION | |||||||
|
Hemoglobin (g/l) |
97±14 (60 to 147) |
101+11 (73 to 147) |
91±18 (60 to 136) |
87±17 (62 to 121) |
95±09 (79 to 113) |
92±17 (68 to 124) |
|
|
Transferrin receptor (mg/l) |
26.1±17.6 (2.5 to 115.9) |
20.0±11.4 (2.5 to 66.3) |
42.6±18.1 (14.6 to 82.2) |
39.1 ±24.1 (6.7 to 115.9) |
16.3±5.8 (8.0 to 28.8) |
44.7±17.8 (6.3 to 66.8) |
|
|
Transfusion rate (mL/kg/month) |
9.8±6.9 (0.0 to 31.1) |
13.2±4.4 (0.9 to 31.1) |
2.5±5.6 (0.0 to 25.0) |
6.5±7.1 (0.0 to 25.3) |
N/A | 2.5±6.4 (0.0 to 21.3) |
|
|
Age started 8± transfusions/yr (years) |
3.1±6.5 (0.0 to 45.0) |
2.6±6.4 (0.0 to 45.0) |
10.5±10.2 (0.0 to 27.0) |
4.7±4.4 (0.0 to 17.0) |
N/A | 2.6±3.7 (0.0 to 8.0) |
|
| Years on 8± transfusions/yr | 17.8±10.9 (0.0 to 44.0) |
19.5±10.7 (1.0 to 44.0) |
3.8±2.7 (0.0 to 7.0) |
10.5±6.4 (0.0 to 25.0) |
N/A | 3.0±0.0 (3.0 to 3.0) |
|
|
Ferritin (μg/l) |
1662±1741 (10 to 12280) |
1992±1668 (94 to 11995) |
736±865 (17 to 3865) |
1898±2454 (14 to 12280) |
92±78 (10 to 311) |
680±1038 (67 to 3940) |
|
| Iron chelation | Unchelated | 95 (26.4%) | 8 (3.4%) | 28 (65.1%) | 23 (54.8%) | 19 (100.0%) | 17 (85.0%) |
| DFO | 241 (66.9%) | 207 (87.7%) | 14 (32.6%) | 19 (45.2%) | 0 (0.0%) | 1 (5.0%) | |
| ICL | 21 (5.8%) | 18 (7.6%) | 1 (2.3%) | 0 (0.0%) | 0 (0.0%) | 2 (10.0%) | |
| DFP | 3 (0.8%) | 3 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Age started DFO therapy | 9.0±9.2 (0.0 to 51.0) |
7.6±7.6 (0.0 to 46.0) |
21.7±12.3 (6.0 to 51.0) |
13.2±11.1 (0.0 to 43.0) |
N/A | 14.3±20.7 (2.0 to 45.0) |
|
| Years on DFO | 13.4±8.3 (-1.0 to 30.0) |
14.5±8.1 (0.0 to 30.0) |
7.7±8.2 (-1.0 to 22.0) |
7.1±6.2 (0.0 to 24.0) |
N/A | 2.0±1.7 (1.0 to 4.0) |
|
|
Prescribed DFO dose (mg/kg/day) |
32.2±11.5 (3.7 to 62.5) |
32.2±11.5 (3.7 to 62.5) |
27.7±11.2 (5.4 to 44.4) |
37.5±8.8 (22.5 to 56.9) |
N/A | 13.2±. (13.2 to 13.2) |
|
| THALASSEMIA COMPLICATIONS | |||||||
| Hypogonadism | Normal | 207 (58.5%) | 101 (43.9%) | 35 (81.4%) | 33 (78.6%) | 18 (94.7%) | 20 (100.0%) |
| Delayed puberty | 19 (8.2%) | 11 (9.3%) | 4 (10.3%) | 3 (8.1%) | 1 (5.3%) | 0 (0.0%) | |
| Hypogonadal | 128 (36.2%) | 118 (51.3%) | 4 (9.3%) | 6 (14.3%) | 0 (0.0%) | 0 (0.0%) | |
| Growth hormone deficiency | 33/344 (9.6%) | 29/223 (13.0%) | 2/42 (4.8%) | 2/42 (4.8%) | 0/18 (0.0%) | 0/19 (0.0%) | |
| Hypothyroidism | 31/358 (8.7%) | 28/234 (12.0%) | 2/43 (4.7%) | 0/42 (0.0%) | 0/19 (0.0%) | 1/20 (5.0%) | |
| Hypoparathyroidism | 5/358 (1.4%) | 5/234 (2.1%) | 0/42 (0.0%) | 0/43 (0.0%) | 0/19 (0.0%) | 0/20 (0.0%) | |
| Diabetes Mellitus | 33/358 (9.2%) | 33/234 (14.1%) | 0/43 (0.0%) | 0/42 (0.0%) | 0/19 (0.0%) | 0/20 (0.0%) | |
| 25(OH)Vitamin D | < 27nmol/l | 42 (12.0%) | 27 (11.7%) | 5 (12.2%) | 6 (14.3%) | 0 (0.0%) | 4 (20.0%) |
| 27 to 75nmol/l | 245 (69.8%) | 149 (64.8%) | 31 (75.6%) | 35 (83.3%) | 15 (83.3%) | 15 (75.0%) | |
| ≥ 75nmol/l | 64 (18.2%) | 54 (23.5%) | 5 (12.2%) | 1 (2.4%) | 3 (16.7%) | 1 (5.0%) | |
| Medicated heart disease | 20/359 (5.6%) | 20/235 (8.5%) | 0/43 (0.0%) | 0/42 (0.0%) | 0/19 (0.0%) | 0/20 (0.0%) | |
| Cirrhosis | 13/352 (3.7%) | 12/231 (5.2%) | 1/42 (2.4%) | 0/40 (0.0%) | 0/19 (0.0%) | 0/20 (0.0%) | |
| Hepatitis C | 84/359 (23.4%) | 75/235 (31.9%) | 3/43 (7.0%) | 6/42 (14.3%) | 0/19 (0.0%) | 0/20 (0.0%) | |
Values for continuous variables are mean±SD (range). Values for categorical variables are N/Total (%). Totals for some measures are less than the total number of participants due to missing data. DFO = deferoxamine ICL= deferasirox (oral iron chelator), DFP= deferiprone (oral iron chelator) BMI= Body mass Index
Anthropometric parameters
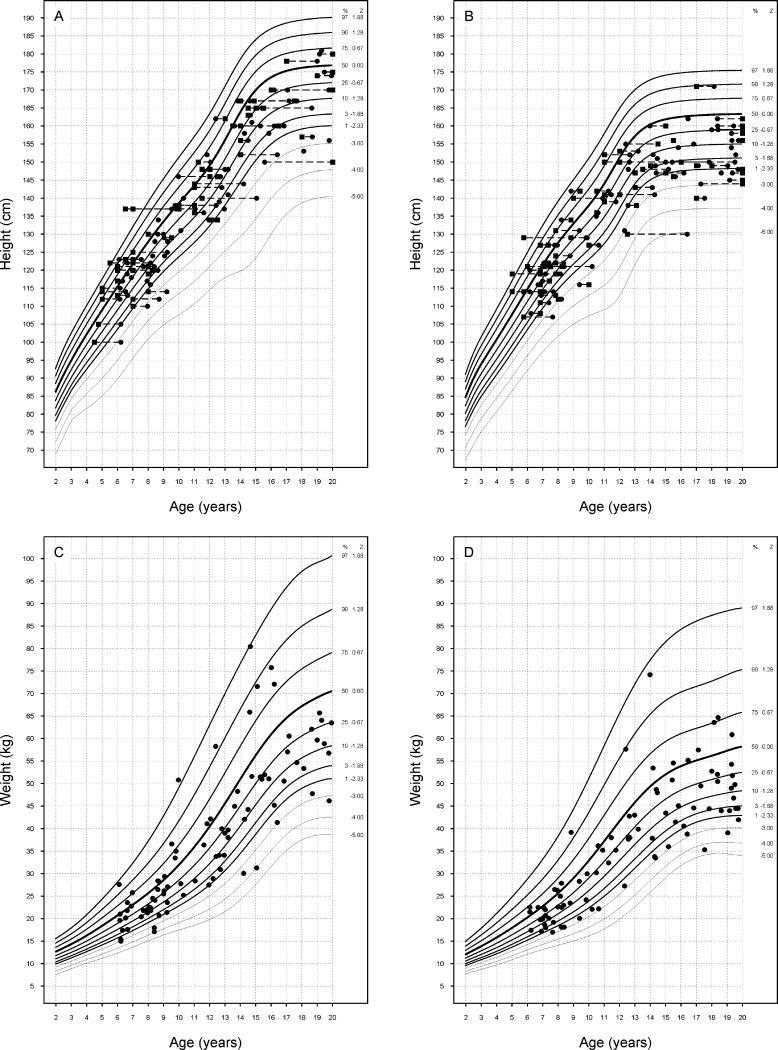
Growth abnormalities were common among children and adolescents (age <20 years): height z-score: -1.20 ± 1.16; mean ± SD; range -5.08 to 1.56 (Figure 1). Twenty-five percent were of short stature (height z-score < -2), compared to <3% of the general population. Their mean weight and BMI were in the normal range (weight z-score: -0.89 ± 1.25, range -4.47 to 2.78; BMI z-score: -0.26 ± 1.1; range -4.5 ± 2.71). The final height z-score (calculated in participants >20 years) was -1.34 ± 1.2; range -4.8 to 1.81. Short stature occurred in 28.5%. As in the case of children, mean adult weight and BMI were in the normal range (weight z-score = -0.72 ± 1.2, range − 4.57 to 2.48; BMI z-score = -0.07 ± 1.05, range -4.51 to 2.44). The difference of final height from midparental height was -0.72 ± 1.23 SDs or -4.3 ± 7.4 cm.
Figure 1.
Plots of height, bone age and weight of study participants. A. Stature of male participants vs. calendar age (●) and bone age (■). B: Stature of female participants vs. calendar age (●) and bone age (■). Individuals are connected by dashed lines. C: Weight of male participants vs. calendar age. D: Weight of female participants vs. calendar age.
The type of thalassemia syndrome had no effect on growth before the age of 20 years, final height or difference of final height from midparental height. Among children and adolescents (age < 20 years), hypogonadism was associated with decreased height (p = 0.04), weight (p < 0.01) and BMI (p = 0.02) in a model that included age, gender, race and type of thalassemia as co-variants. Growth hormone deficiency was also a significant negative predictor of height (p = 0.05) and weight (p = 0.03). Further multivariate analyses that, in addition, included IGF1 concentrations and serum ferritin and transferring receptor concentrations as co-variants showed a 1.3-fold increase in the odds of short stature with advancing age (p = 0.022) and a 4.5-fold increase in Asians compared to Caucasians (p = 0.045). Similar analyses in adults found that the only significant predictor of short stature was GH deficiency (OR = 3.8 [1.2.12.4]); p = 0.025).
Among participants younger than 20 years, bone age (BA) was close to chronological age (CA; Table 1). The difference between CA and BA was greater in patients with hypogonadism (p = 0.01) and among patients with beta thalassemias compared to HbH disease (p = 0.02). Gender, race, and serum IGF1 concentrations were not significantly associated with delayed BA.
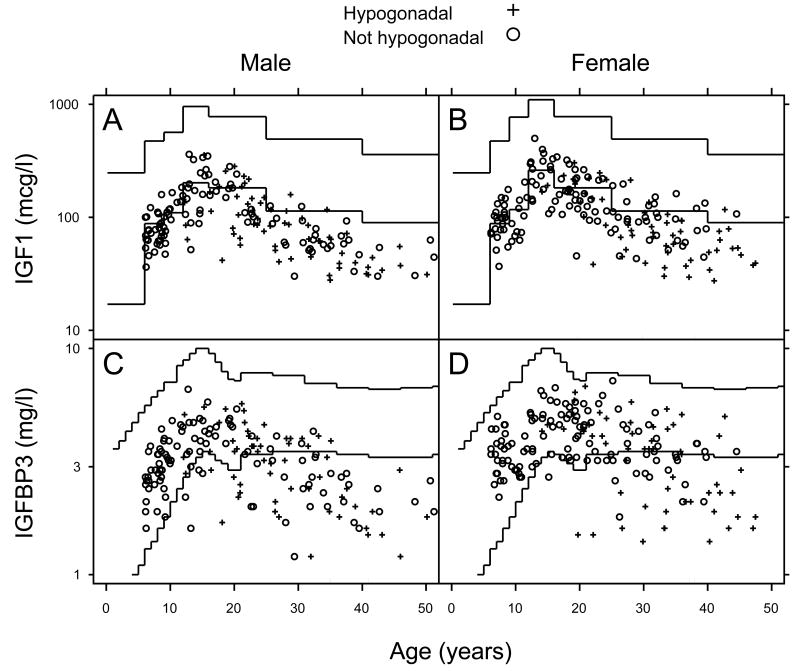
Serum IGF1 and IGFBP3 concentrations were plotted against normative values for sex and age (Figure 2). Seventy-one percent of all patients had IGF1 concentrations below normal (57.7%, 56.8% and 83.3% for the age groups 6 - 11, 12 - 19 and > 20 years, respectively). IGFBP3 concentrations were below normal in 34.4% of the subjects. Increased ferritin concentrations (p ≤ 0.001), male gender (p ≤ 0.02), the presence of hypogonadism (p ≤ 0.01), and TI (p ≤ 0.02) were all associated with lower serum IGF1 and IGFBP3 concentrations. Of interest, IGF1 and IGFBP3 concentrations were not found to be predictors of short stature.
Figure 2.
IGF1 and IGFBP3 vs. age stratified by gender and hypogonadal status. +: hypogonadal, o: not hypogonadal. IGF1 concentrations below normal range were found in 57.7% of children 6-11y, 56.8% of those 12-19y and 83.3% of subjects older than 20y. All children 6-11y had normal IGFBP3 levels. IGFBP3 concentrations below normal range were found in 12.5% of children 12-19y and 58.3% of subjects older than 20y.
Hypogonadism and other endocrinopathies
Hypogonadism was the most frequent endocrinopathy and affected both genders. It was present in 14.3% of females and 25.5% of males younger than 20 years. Its frequency was significantly higher after age 20 at 52.4% in woman and 60.0% in men.
Hypogonadism was more frequent in patients with beta TM compared to those with beta TI (p < 0.001) or those with E-beta (p = 0.006) (Table 1) among participants greater than 10 yrs old and after adjustment for age, gender, and serum ferritin levels. In addition to diagnosis, both age and serum ferritin concentration at the time of the study were associated with the development of hypogonadism. Specifically, the odds of hypogonadism increased by 13.9% for every 5 years of age (p < 0.001) and by 11.8% for every 1,000 ng/mL increase in ferritin concentration (p = 0.04).
The age of menarche was 15.7 ± 3.9 years for the overall group. For females who experienced normal puberty, menarche occurred at 13.3 ± 1.0 years (the youngest being11y). Girls with delayed puberty reached spontaneous menarche at 17.2 ± 1.2 years. Among those with a diagnosis of hypogonadism, menses were induced at 17.1 ± 4.0 years. Hypogonadal females were treated with either standard postmenopausal gonadal steroid replacement regimens (i.e. a combination of premarin and provera) or a birth control given not for contraception but as a form of gonadal replacement. Twenty-five percent of hypogonadal females were untreated at the time of the study. Among hypogonadal males, 36.2% were treated with either testosterone, given intramuscularly or transdermally by patch or gel, 7.25% were treated with HCG and 56.5% remained untreated. Of those treated, 27% had serum testosterone concentrations below the normal range indicating inadequate gonadal steroid replacement. The mean testosterone concentration among adequately replaced hypogonadal males was 672 ± 236 ng/dl (range 353 - 1102 ng/dl) vs. 113 ± 102 ng/dl (range 10 - 323 ng/dl) among poorly replaced patients. The type of steroid replacement made no difference on serum testosterone concentrations.
Growth hormone deficiency was the second most frequent endocrinopathy and was reported in 9.6% of the study participants, with a mean age at diagnosis of 14.3 years (range 5 – 28 years). Most (90.3%) of the growth hormone deficient patients (8.5% of all study participants) had been treated with growth hormone. There were no adults currently receiving growth hormone replacement.
Diabetes mellitus (DM) was seen only in TM, with a frequency of 14.1% and a mean age at diagnosis of 22 years, range 10 - 45 years. Of the diabetics, 29.5% were treated with an oral hypoglycemic and 47.1% with insulin. Analysis of fasting blood glucose concentrations in the non-diabetic, otherwise asymptomatic, participants identified 37 patients (13.9%) with impaired glucose tolerance (mean age 26 years, range 8.8 - 47.5 years) and 4 additional patients (1.5%) with diabetes. In the group of patients who were Hep C positive, 35.7% had either a diagnosis of diabetes or an abnormal fasting blood glucose (either in the diabetic or impaired glucose tolerance range), compared to 16.3% of patients without Hep C (p = 0.0001, chi-square test). The overall prevalence of hypothyroidism was 8.7% (mean age at diagnosis 23.8 years, range 10 – 44 years). Hypoparathyroidism occurred only in TM (2.1% prevalence; mean age at diagnosis, 19 years, range 12-30 years).
An increase of 5 years in age was associated with a 13.6% increase in the risk of hypothyroidism and 14% increase in the odds of DM using multivariate analysis. In the same analysis, the serum ferritin concentration at the time of the study was not a predictor of the above-mentioned endocrinopathies. However, the results differed when factors that affected the development of multiple combined endocrinopathies were considered. Patients with increasing serum ferritin concentrations (p = 0.05) and those with beta TM had higher rates of multiple endocrinopathies compared to beta TI, E-β and α hemoglobinopathies (p < 0.0001). Finally, increasing serum ferritin concentrations (p = 0.002), the presence of hypogonadism (p = 0.01) and having beta TM (p = 0.05) were all associated with higher fasting glucose levels in multivariate analysis.
Calcium Metabolism and Vitamin D
Measurements of serum 25 vit D concentrations revealed that 12.0% of the subjects were vitamin D deficient and 69.8% had insufficient levels. There were no differences in the prevalence of abnormal 25 vit D concentrations according to the thalassemia syndrome. 25 Vit D concentrations were lower among adolescents (47 ± 20 nmol/l in the 11- to 19-yr old group vs. 62 ± 21 nmol/l in the 6- to 10-year old group and 58.4 ± 31 nmol/l among the 20 years+ group; p = 0.0014) and among Asians (p = 0.02). As expected, 25 vit D was higher during the summer months (p = 0.0001). Patients with vitamin D deficiency had decreased urinary calcium excretion (p = 0.0004).
Total daily calcium intake (dietary and supplementation) was adequate across all thalassemia syndromes and age groups. Dietary calcium intake was lower among older subjects (p < 0.0001) and Asians (p = 0.018) and did not differ according to the type of thalassemia syndrome. Calcium supplementation was prescribed more frequently in older patients (p = 0.004) and in beta TM (p = 0.03), most likely reflecting efforts to treat patients with low bone mass. The total calcium intake in beta TM was 1,721 ± 924 mg/day.
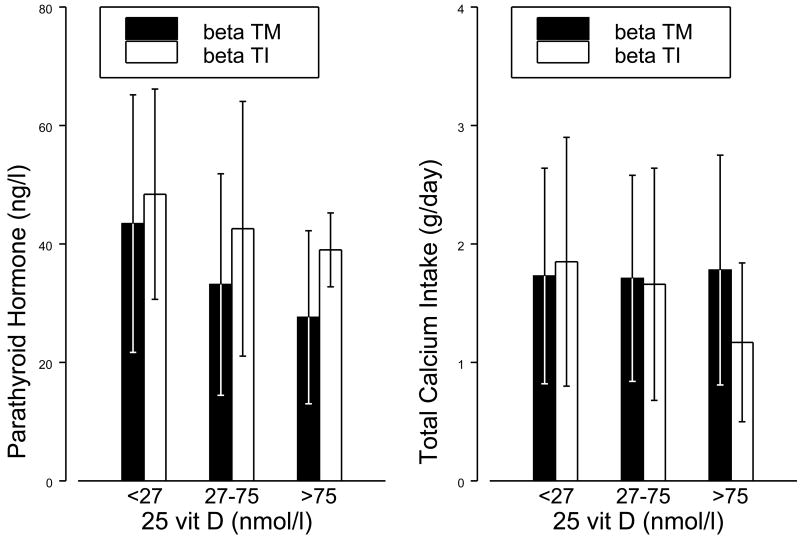
PTH levels decreased with increasing vit D concentrations (p<0.001), in a pattern similar to what has been described in the general population. Hypoparathyroidism was rare, and was seen in only 5 patients with beta TM. However, patients with beta TM had lower PTH concentrations compared to those with beta TI for the same 25 vit D levels (p = 0.02; Figure 3). This finding can not be attributed to differences in calcium intake, since total Calcium intake did not significantly vary according to the thalassemia diagnosis or vitamin D sufficiency (Figure 3). Hypercalciuria was present in 29% of patients with beta TM compared to none with beta TI (p = 0.002; Table II). Among patients with beta TM, the incidence of hypercalciuria was increased in vitamin D sufficient subjects compared to those with vitamin D deficiency (OR = 3.2, p = 0.007).
Figure 3.
Parathyroid hormone and total Calcium intake by vitamin D sufficiency and diagnosis. Parathyroid hormone is significantly lower in beta TM compared to beta TI (p=0.02), and decreases with vitamin D (p<0.001). Total Calcium intakes does not significantly vary by diagnosis (p=0.76) or vitamin D sufficiency (p=0.88).
Table II.
Calcium metabolism data and prevalence of hypercalciuria according to vit D sufficiency status and diagnosis1
| Mean ± SD or N (%) | ||||
|---|---|---|---|---|
| 25 vit D<27 (nmol/l) | 25 vit D =27-75 (nmol/l) | 25 vit D>75 (nmol/l) | ||
| All patients | N=43 | N=251 | N=66 | |
| Intact PTH (ng/l) | 48.67 ± 26.46 | 38.80 ± 20.70 | 28.61 ± 14.13 | |
| 1, 25 Vit D (pmol/l) | 97.86 ± 40.32 | 116.43 ± 42.15 | 134.86 ± 53.67 | |
| Serum Ca (mmol/l) | 2.25 ± 0.14 | 2.28 ± 0.14 | 2.31 ± 0.14 | |
| Total Ca intake (g/d) | 1.47 ± 0.95 | 1.63 ± 0.91 | 1.66 ± 0.93 | |
| % Hypercalciuria (>4mg/kg/24hrs) | 5/24 (21%) | 27/130 (21%) | 20/42 (48%) | |
| Beta TM | N=27 | N=150 | N=54 | |
| Intact PTH (ng/l) | 43.44 ± 21.75 | 33.16 ± 18.70 | 27.63 ± 14.60 | |
| 1, 25 Vit D (pmol/l) | 92.70 ± 40.40 | 112.21 ± 44.30 | 130.40 ± 56.10 | |
| Serum Ca (mmol/l) | 2.27 ± 0.14 | 2.29 ± 0.15 | 2.30 ± 0.13 | |
| Total Ca intake (g/d) | 1.73 ± 0.91 | 1.71 ± 0.87 | 1.78 ± 0.97 | |
| % Hypercalciuria (>4mg/kg/24hrs) | 5/21 (24%) | 27/115 (23%) | 20/40 (50%) | |
| Beta TI | N=5 | N=31 | N=5 | |
| Intact PTH (ng/l) | 48.40 ± 17.76 | 42.58 ± 21.50 | 39.00 ± 6.24 | |
| 1, 25 Vit D (pmol/l) | 75.61 ± 34.20 | 117.40 ± 41.70 | 139.18 ± 31.30 | |
| Serum Ca (mmol/l) | 2.23 ± 0.11 | 2.29 ± 0.13 | 2.30 ± 0.21 | |
| Total Ca intake (g/d) | 1.85 ± 1.05 | 1.66 ± 0.98 | 1.17 ± 0.67 | |
| % Hypercalciuria (>4mg/kg/24hrs) | 0/3 (0%) | 0/15 (0%) | 0/2 (0%) | |
Totals for some measures are less than the total number of participants due to missing data.
Discussion
We have defined differences in the rates of growth, endocrine and calcium related abnormalities in the various thalassemia syndromes in North America with current therapy. The results of this study indicate that patients with beta TM constitute a high risk group, as they manifest higher rates of hypogonadism, combined multiple endocrinopathies and worse fasting hyperglycemia than individuals with other thalassemia syndromes. All of these conditions were found to correlate with higher ferritin concentrations. In addition, patients with beta TM have subtle abnormalities in PTH secretion and high rates of hypercalciuria, which appear as newly-appreciated complications of this particular syndrome.
Hypogonadism remains the most common endocrinopathy (De Sanctis et al, 2004; Borgna-Pignatti et al, 2005; Shalitin et al, 2005) and is frequently under-treated. Among hypogonadal girls, the age of menarche was delayed to 17 years. Approximately half of the males were untreated, and of those who were receiving gonadal steroid replacement, 27% had low serum testosterone concentrations. We have previously reported a high incidence of low bone mass and fractures in this population and identified hypogonadism as a strong independent predictor of low bone mass (Vogiatzi et al, 2009). In light of the above, we advocate a more timely and aggressive hormone replacement therapy. The most beneficial gonadal steroid regimen for the treatment of low bone mass in thalassemia has yet to be established, however, and needs to be considered in future prospective studies.
Height was only mildly affected in the overall group. Weight and BMI were not decreased relative to height, suggesting that nutritional factors are unlikely to adversely affect stature in this cohort. Nevertheless, approximately 25% of children and adults present with growth failure, which occurs independent of the thalassemia syndrome, serum IGF1 and IGFBP3 levels and serum ferritin concentration. Similar recent studies report that short stature occurs in approximately 30-35% of beta TM patients (De Sanctis et al, 2004; Shalitin et al, 2005; Skordis et al, 2006). Rates of growth failure were similar among children and adults. This implies that the current transfusion and chelation therapies, which were introduced approximately 25 years ago, have not significantly improved growth in thalassemia.
Both normal and low vitamin D concentrations have been reported previously in thalassemia (Voskaridou et al, 2001; Napoli et al, 2006). In this study, we found that 12% of the participants had 25 vit D levels below 11ng/ml and 82% below 30ng/ml regardless of the thalassemia syndrome. The 25 vit D concentrations that are used as cut offs to define vitamin D deficiency and sufficiency have recently increased (Holick, 2007). Depending on the definition, the prevalence of vitamin D deficiency has been reported to range in the U.S. from 15% to 80% (Holick, 2007). A current review from the National Health and Nutrition Examination Survey (NHANES) III, a cross-sectional survey administered to a representative sample of U.S. civilians, documented that the prevalence of vitamin D deficiency was 6.6% in 2001-2004 using the standard of 25nmol/l (Ginde et al, 2009). According to same study, 70-80% of the population plotted below 75nmol/l (Ginde et al, 2009), which is close to the prevalence that we detected. Compared to children and adults with thalassemia, our adolescents had lower mean 25 vit D concentration at 47nmol/l. The recently reported mean concentration among U.S. adolescents is considerably higher at approximately 60-75nmol/l (Ginde et al, 2009; Saintonge et al, 2009). All together, our data indicate that patients with thalassemia in U.S. have rates of vitamin D abnormalities slightly higher than the general population with the exception of adolescents, which represents a high risk group for low vitamin D concentrations. Adolescence is a critical period for optimal bone accrual. Vitamin D abnormalities may contribute, therefore, to the low bone mass accrual that is seen among adolescents with thalassemia (Vogiatzi et al, 2009).
Overt hypoparathyroidism is rare in well-chelated thalassemia patients, while the presence of subtle abnormalities in PTH secretion is not well established (Chern & Lin, 2002; Even et al, 2007). In this study, we observed lower PTH concentrations in beta TM, after correcting for vitamin D concentrations and calcium intake, indicative of subclinical hypoparathyroidism. Of interest, patients with beta TM had high rates of hypercalciuria that worsened with increasing vitamin D concentrations. This is a new finding. Whether it is related to a renal tubular defect (Smolkin, 2008) and/or a contributing effect of subtle PTH dysfunction on calcium excretion cannot be determined at this point. The optimal vitamin D concentrations that are required for bone accrual and heath while minimizing hypercalciuria, have not been established in thalassemia. Finally, the presence and extent of renal tubular damage, the long-term consequences of hypercalciuria (i.e. increased risk for nephrolithiasis) as well as the development of appropriate treatment strategies need to be addressed in future studies.
Although the present study reports on a large number of patients with all thalassemia syndromes, the cross-sectional design limits our ability to determine the etiology of described endocrine and calcium abnormalities beyond the extent of determining certain associations. Determination of serum ferritin was the only methodology used to assess iron overload in this study. Since serum ferritin is a poor marker in heavily overloaded individuals, it is possible that the role of iron on the development of various endocrinopathies was under-estimated in this study. Regardless of these limitations, this study provides evidence that endocrinopathies in thalassemia are frequent and start early in life. We have identified patients with beta TM as a particularly vulnerable group for multiple endocrinopathies and adolescents as prone to vitamin D abnormalities regardless of their genotype. Our findings can be used to establish evidence based practice guidelines for regular endocrine evaluations starting in childhood as part of the comprehensive care of these patients. Since life expectancy in thalassemia has improved significantly with current transfusion and chelation, we need to better understand the long-term implications of these endocrine complications on the welfare of the aging patient.
Acknowledgments
Supported by a cooperative agreement with the National Heart, Lung, and Blood Institute, National Institutes of Health (U01-HL-65232 to Children's Hospital of Philadelphia, U01-HL-65233 to University Health Network Toronto General Hospital, U01-HL-65239 to Children's Hospital and Research Center at Oakland, U01-HL-65244 to Weill Medical College of Cornell University, U01-HL-65260 to Children's Hospital Boston, and U01-HL-65238 to New England Research Institutes). The study was also supported, in part, at Weill Medical College of Cornell University grant K08 HL088231 awarded to Maria G. Vogiatzi, at Children's Hospital of Philadelphia by NIH-NCRR grant UL1-RR024134, and at Children's Hospital Boston by NIH-NCRR grant M01-RR02172 and NIH grant 5K24HL004184-08 to Ellis Neufeld.
Appendix 1
This work was performed through the Thalassemia Clinical Research Network (TCRN). The authors would like to thank the patients who volunteered their time to participate in this study. The following TCRN sites and investigators also contributed to the study (listed in alphabetical order): Childrens Hospital, Boston: Ellis Neufeld, MD, PhD, Principal Investigator, Melody Cunningham, MD, Co-Principal Investigator; Childrens Hospital of Philadelphia: Alan R. Cohen, MD, Principal Investigator, Janet L. Kwiatkowski, MD, Co-Principal Investigator, Catherine S. Manno, MD, Coinvestigator, Marie Martin, RN, Nurse Coordinator, Debra Hillman, Regulatory Affairs Coordinator, Gail M. Jackson, CDT, Nutrition Assessment Program Coordinator, Maria J. Henwood-Storto, DO, Endocrinologist; Shannon H. Fourtner, MD, Endocrinologist; Childrens Hospital & Research Center Oakland: Elliott Vichinsky, MD, Principal Investigator, Dru Foote, NP, Study Coordinator, Eun-Ha Pang, Study Coordinator, Zahra Pakbaz, MD, CCD, Certified Clinical Densitometrist, Selma Holden, Study Coordinator; Toronto General Hospital: Nancy Olivieri, MD, Principal Investigator; U.T. Southwestern Medical Center: Charles T. Quinn, MD, Assistant Professor of Pediatrics, Elizabeth Shull, BSN, RN, Clinical Research Associate; Weill Medical College of Cornell University: Patricia J. Giardina, MD, Principal Investigator, Robert W. Grady, PhD, Co-Investigator, Jeffrey E. Mait and Dorothy Kleinert, NP, MPH, MA, Study Coordinators, Irina Chaikhoutdinov and Gladys Cintron, Data Coordinators, Sylvia Hom, DXA Technician, Hospital for Special Surgery; National Heart, Lung, and Blood Institute: Charles Peterson, MD, Project Officer; Data Coordinating Center, New England Research Institutes: Sonja McKinlay, PhD, Principal Investigator, Felicia Trachtenberg, PhD, Statistician, Haddy Jallow, MS, MPH, Project Coordinator.
References
- Al-Rimawi HS, Jallad MF, Amarin ZO, Al Sakaan R. Pubertal evaluation of adolescent boys with β-thalassemia major and delayed puberty. Fertility and Sterility. 2006;86(4):886–890. doi: 10.1016/j.fertnstert.2006.02.118. [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, Ghilardi R, Origa R, Piga A, Romeo MA, Zhao H, Cnaan A. Survival and complications of thalasemia. Ann NY Acad Sci. 2005;1054:40–47. doi: 10.1196/annals.1345.006. [DOI] [PubMed] [Google Scholar]
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patiens with beta thalassemia major. NEJM. 1994;331(9):567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- Calleja E, Shen JY, Lesser M, Grady RW, New MI, Giardina PJ. Survival and Morbidity in Transfusion Dependent Thalassemia Patients on Subcutaneous Desferrioxamine Chelation: Nearly Two Decades of Experience. NY Acad Sci. 1998;850:469–470. doi: 10.1111/j.1749-6632.1998.tb10524.x. [DOI] [PubMed] [Google Scholar]
- Caruso-Nicoletti M, De Sanctis V, Raiola G, Skordis N, Mancuso M, Coco M, Wonke B. No difference in pubertal growth and final height between treated hypogonadal and non-hypogonadal thalassemic patients. Horm Res. 2004;62:17–22. doi: 10.1159/000077703. [DOI] [PubMed] [Google Scholar]
- Chern JP, Lin KH. Hypoparathyroidism in transfusion-dependent patients with β-Thalassemia. J Ped Haematol. 2002;24(4):291–293. doi: 10.1097/00043426-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Maclin EA, Neufeld EJ, Cohen AR, Thalassemia Clinical Research Network Complcations of β-thalassemia major in North America. Blood. 2004;104(1):34–39. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- De Sanctis V, Katz M, Vullo C, Bagni B, Ughi M, Wonke B. Effect of different treatment regimes on linear growth and final height in beta-thalassaemia major. Clin Endocrinol (Oxf) 1994 Jun;40(6):791–8. doi: 10.1111/j.1365-2265.1994.tb02514.x. [DOI] [PubMed] [Google Scholar]
- De Sanctis V, Eleftheriou A, Malaventura C. Thalassemia International Federation Study Group on Growth and Endocrine Complications in Thalassemia. Prevalence of endocrine complications and short stature in patients with thalassemia major: a multicenter study by the Thalassemia International Federation (TIF) Pediatr Endocrinol Rev. 2004;2 2:249–55. [PubMed] [Google Scholar]
- Even L, Bader T, Hochberg Z. Nocturnal calcium, phosphorus and parathyroid hormone in the diagnosis of concealed and subclinical hypopathyroidsim. European Journal of Endocrinology. 2007;156:113–116. doi: 10.1530/eje.1.02316. [DOI] [PubMed] [Google Scholar]
- Gamberini MR, Fortini M, De Sanctis V, Gilli G, Testa MR. Diabetes mellitus and impaired glucose tolerance in thalassemia major: incidence, prevalence, risk factors and survival in patients followed in the Ferrara Center. Ped Endocrinol Rev. 2004;2S2:285–291. [PubMed] [Google Scholar]
- Ginde AA, Liu MC, Camargo CA. Demographic differences and trends in Vitamin D insufficiency in the US population, 1998 - 2004. Archives of Internal Medicine. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greuligh, Pyle . Radiographic atlas of skeletal development of the hand and wrist. 2nd. Stanford University Press; Stanford, CA: 1959. [Google Scholar]
- Holick MF. Vitamin D deficiency. The New England Journal of Medicine. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Jensen CE, Tuck SM, Old J, Morris RW, Yardumian A, De Sanctis V, Hoffbrand AV, Wonke B. Incidence of endocrine complications and clinical disease severity related to genotype analysis and iron overload in patients with B-thalassemia. Eur J Haematol. 1997;59:76–81. doi: 10.1111/j.1600-0609.1997.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Napoli N, Carmina E, Bucchieri S, Sferrazza C, Rini GB, Di Fede G. Low serum levels of 25-hydroxy vitamin D in adults affected by thalasemia major or intermedia. Bone. 2006;38:888–892. doi: 10.1016/j.bone.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Pollak RD, Rachmilewitz E, Blumenfeld A, Idelson M, Goldfarb AW. Bone mineral metabolism in adults with B-thalassemia major and intermedia. British Journal of Haematology. 2000;111:902–907. [PubMed] [Google Scholar]
- Roth C, Pekrun A, Bartz M, Jarry H, Eber S, Lakomek M, Schroter Short stature and failure of pubertal development in thalassemia major: evidence of hypothalamic neurosecretory dysfunction of growth hormone secretion and defective pituitary gonadotropin secretion. Eur J Pediatr. 1997;156:777–783. doi: 10.1007/s004310050711. [DOI] [PubMed] [Google Scholar]
- Saintonge S, Bang H, Gerber LM. Implications on a new definition of vitamin D deficiency in a multiracial US adolescent population: The National Health and Nutrition Examination Survey III. Pediatrics. 2009;123:797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- Shalitin S, Carmi D, Weintrob N, Phillip M, Miskin H, Kornreich L, Zilber R, Yaniv I, Tamary H. Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. Eur J Haematol. 2005;74:93–100. doi: 10.1111/j.1600-0609.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- Skordis N, Michaelidou M, Savva SC, Ioannou Y, Rousounides A, Kleanthous M, Skordos G, Christou S. The impact of genotype on endocrine complications in thalassemia major. Eur J Haematol. 2006;77:150–156. doi: 10.1111/j.1600-0609.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- Smolkin V, Halevy R, Levin C, Mines M, Sakran W, Ilia K, Koren A. Renal function in children with β-thalassemia major and thalassemia intermedia. Pediatr Nephrol Oct. 2008;23(10):1847–51. doi: 10.1007/s00467-008-0897-8. [DOI] [PubMed] [Google Scholar]
- Vogiatzi MA, Macklin EA, Fung EB, Vichinsky E, Oliveri N, Kwiatkowski JL, Cohen A, Neufeld E, Giardina PJ. Prevalence of fractures among the Thalassemia syndromes in North America. Bone. 2006;38:571–575. doi: 10.1016/j.bone.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi MA, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Oliveri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm I, Lane J, Schneider R, Fleisher M, Grady RW, Peterson C, Giardina PJ, Thalassemia Clinical Research Network Bone disease in thalassemia: a frequent and still unresolved problem. JBMR. 2009;24:543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskaridou E, Kyrtsonis MC, Tempos E, Skordili M, Theodoropoulos I, Bergele A, Diamanti E, Kalovidoulis A, Loutradi A, Loukopoulos D. Bone resorption is increased in young adults with thalassemia major. British Journal of Haematology. 2001;112:36–14. doi: 10.1046/j.1365-2141.2001.02549.x. [DOI] [PubMed] [Google Scholar]