Abstract
In the heart, Ca2+ released from the intracellular Ca2+ storage site, the sarcoplasmic reticulum (SR), is the principal determinant of cardiac contractility. SR Ca2+ release is controlled by dedicated molecular machinery, composed of the cardiac ryanodine receptor (RyR2) and a number of accessory proteins, including FKBP12.6, calsequestrin (CASQ2), triadin (TRD) and junctin (JN). Acquired and genetic defects in the components of the release channel complex result in a spectrum of abnormal Ca2+ release phenotypes ranging from arrhythmogenic spontaneous Ca2+ releases and Ca2+ alternans to the uniformly diminished systolic Ca2+ release characteristic of heart failure. In this article, we will present an overview of the structure and molecular components of the SR and Ca2+ release machinery and its modulation by different intracellular factors, such as Ca2+ levels inside the SR as well as phosphorylation and redox modification of RyR2s.We will also discuss the relationships between abnormal SR Ca2+ release and various cardiac disease phenotypes, including, arrhythmias and heart failure, and consider SR Ca2+ release as a potential therapeutic target.
Keywords: Ryanodine receptor, Calcium release, Calsequestrin, Junctin, Triadin, Cardiomyopathy, Arrhythmias
1. Introduction
Despite significant advances in treatments, cardiac diseases remain the leading cause of mortality and mor!bidity in Western nations. Development of new efficient treatment strategies requires understanding of basic mechanisms underlying cardiac function. Since the classical experiments of Dr. Sydney Ringer, Ca2+ has been recognized as a principal agent governing the mechanical activity of the heart (Ringer, 1883). On a beat-to-beat basis, Ca2+ derived from the intracellular Ca2+ storage site, the sarcoplasmic reticulum (SR), results in activation of contractile filaments and shortening of cardiac myocytes. Alterations in timing and magnitude of SR Ca2+ release result in different cardiac diseases such as arrhythmia and heart failure. SR Ca2+ release is controlled by specialized molecular machinery, composed of the cardiac ryanodine receptor (RyR2) and a number of accessory proteins, including FKBP12.6, calsequestrin (CASQ2), triadin (TRD) and junctin (JN). This multimolecular Ca2+ signaling complex is essential to normal Ca2+ cycling and is a site of potential pathologic failure. In this review, we will present an overview of the molecular organization of the Ca2+ release machinery and its modulation by different intracellular factors, such as Ca2+ levels inside the SR as well as phosphorylation and redox modification of RyR2s.We will also consider the relationships between abnormal SR Ca2+ release and various cardiac disease phenotypes, including, catecholaminergic polymorphic ventricular tachycardia, triggered and reentrant arrhythmias and heart failure. Finally we will consider SR Ca2+ release as a potential therapeutic target.
2. The sarcoplasmic reticulum
2.1. Structure and continuity
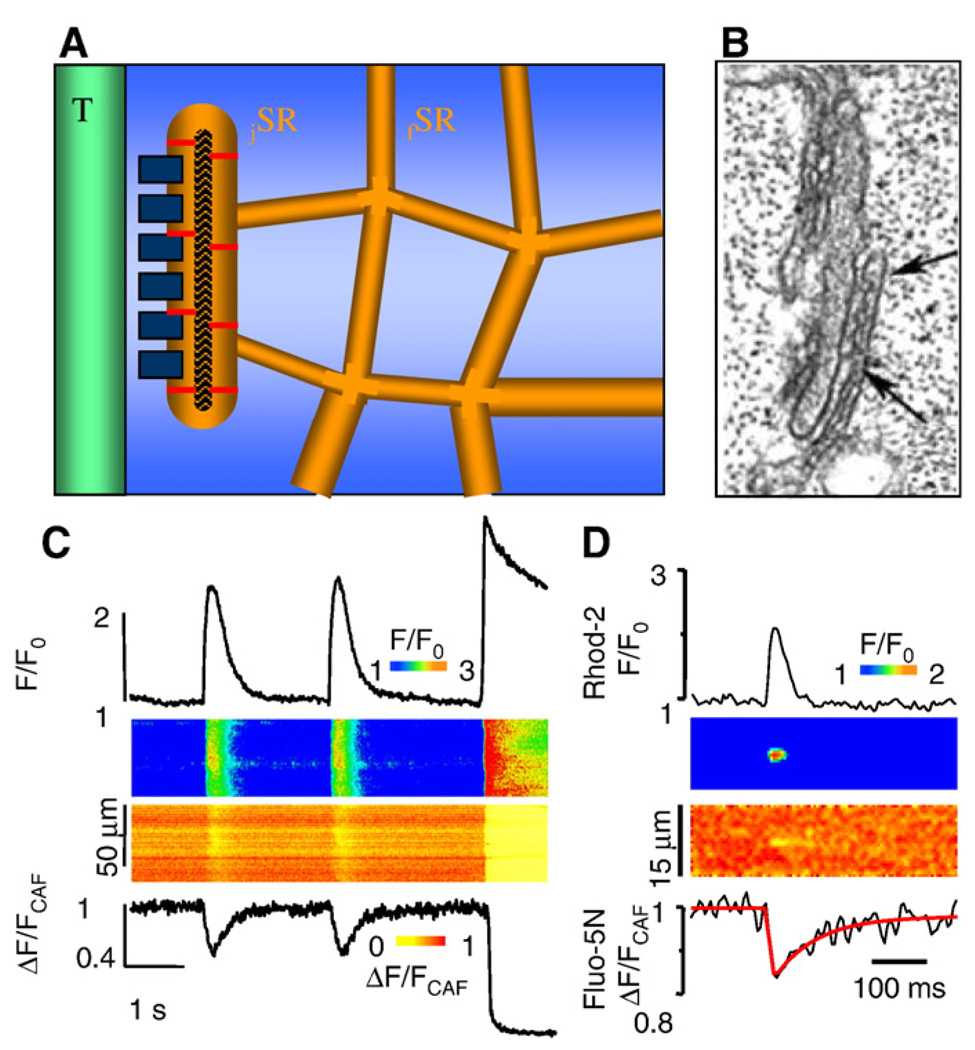
Striated muscle sarcoplasmic reticulum is a highly specialized form of endoplasmic reticulum (ER), which was described first in detail for both skeletal and cardiac muscle as an extensive network of interconnected cisternae and tubules throughout the sarcoplasm (Fawcett & McNutt, 1969; Sommer, 1995; Franzini-Armstrong & Protasi, 1997). Estimates of the total SR volume in ventricular myocytes of different mammalian species vary between 0.87 and 7.9% of cell volume, and it is commonly assumed to constitute 3.5% of cell volume (Bers, 2001). The SR consists of two distinct regions: junctional SR (jSR), which directly faces the transverse tubules, and free SR (fSR) which faces the myofibrils and does not form junctions with other membrane systems. See Fig. 1A and B. The total SR volume is divided approximately equally between jSR and fSR. In rabbit ventricular myocytes, jSR forms extended, flattened cisternae with an average diameter of 592 nm (Brochet et al., 2005). Each cistern carries 66 RyR2s clustered in one or two release units on the junctional membrane. The jSR contains an electron dense material which is formed by the Ca2+ binding protein calsequestrin (CASQ2) (Tijskens et al., 2003). The free SR is devoid of internal content, and its external surface exhibits densely distributed 8 nm particles representing the Ca2+ ATPase molecules. Although topologically continuous with the rest of the SR network, the connectivity of an average jSR cistern to fSR is limited to a few narrow passages; therefore, diffusional barriers for the free translocation of Ca2+ and other molecules exist between these compartments (Brochet et al., 2005). The restricted connections between jSR and fSR facilitates the generation of local luminal Ca2+ gradients, which are thought to play an important role in luminal Ca2+ signaling (Gyorke and Terentyev, 2008).
Fig. 1.
SR microanatomy and Ca2+ signaling. A. Diagram illustrating the relationship between the free SR (fSR) network, the junctional (jSR) cisternae, T tubules, feet and calsequestrin along with its anchoring filaments in a mammalian ventricular myocyte. B. Electron micrograph of junctional SR of a rabbit ventricular myocyte. Between the arrows RyR2s are seen as foot structures in the gap between jSR and T tubule membranes. C. Action potential-elicited cytosolic and intra-SR Ca2+ signals followed by caffeine-induced Ca2+ release and SR depletion. Upper: profiles and linescan images of cytosolic Ca2+ changes measured with Rhod-2; lower: profiles and linescan images of [Ca2+]SR changes monitored with Fluo 5N. D. Simultaneous measurements of local SR Ca2+ release (Rhod-2, upper) and depletion signals (Fluo 5N, lower) (i.e. sparks and blinks). Panel B from DXP Brochet, D Yong, De Maio A, WJ Lederer, C Franzini-Armstrong, H Cheng. Ca2+ blinks: rapid nanoscopic store calcium signaling. PNAS 2005; 102: 3099–3104. Copyright (2005) National Academy of Sciences, U.S.A.
2.2. SR as a Ca2+ storage and release organelle
The Ca2+ stored in and released from the SR is a critical determinant of cardiac contractility. Additionally intra-SR Ca2+ levels play a key role in SR-mediated Ca2+ signaling by controlling the functional activity of RyR2s. In mammalian cardiac myocytes, the total SR Ca2+ content has been estimated to be in the range of 50–150 µmol/ l in the cytosol (or 1.4–3 mmol/l of SR volume), considering that SR comprises 3.5% of cell volume (Bers, 2001). Due to the presence of substantial amounts of Ca2+ binding sites in the SR, only a fraction of this Ca2+ is free. According to various estimates, 50 to 90% of the total Ca2+ is bound to Ca2+ buffering proteins such as CASQ2 (Shannon & Bers,1997; Shannon et al., 2000), and the existence of large reserves of bound Ca2+ permits the SR to serve as an efficient intracellular Ca2+ storage site despite its minute space. Whereas bound Ca2+ contributes to the storage capacity of the SR, free Ca2+ plays an important role in store operation by setting thermodynamic limits on Ca2+ATPase Ca2+ transport activity, establishing the driving force for SR Ca2+ release, and modulating RyR2 activity. As measured directly by low affinity Ca2+ dyes loaded into the SR, the resting (diastolic) free [Ca2+]SR constitutes 1 to 1.5 mM and becomes only partially depleted (24% to 63%) during contraction (Shannon et al., 2003a). See Fig. 1C and D. Local depletion signals in individual SR cisternae during Ca2+ sparks (termed “blinks”) have been measured in myocytes from both normal and diseased hearts (Brochet et al., 2005; Kubalova et al., 2005). The occurrence of local SR Ca2+ depletion is consistent with restricted diffusion of Ca2+ from fSR to jSR (described above). Indeed, a rapid refilling of local jSR Ca2+ store from the bulk of the SR would be expected to prevent significant local depletion. Interestingly, as determined by luminal Ca2+ measurements with Fluo5N, Ca2+ diffusion through the SR network along the length of the myocyte is unexpectedly fast (60 µm2 s−1,(Guo et al., 2007). This reinforces the notion that diffusion barriers between jSR and the rest of the SR network must exist to explain jSR Ca2+ depletion during localized Ca2+ release events.
3. The Ca2+ release channel complex
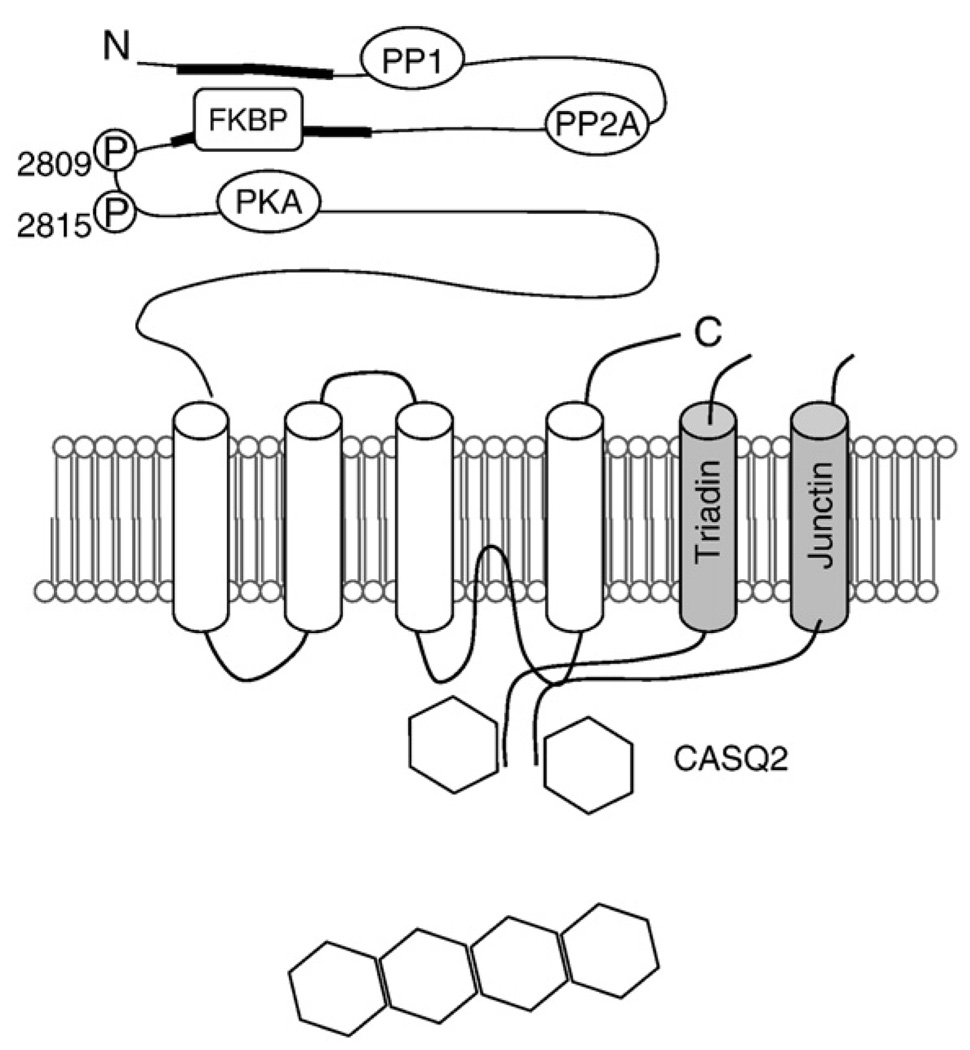
The cardiac Ca2+ release channel is a macromolecular complex composed of more than a dozen of proteins centered around the RyR2 (Franzini-Armstrong & Protasi, 1997; Zhang et al., 1997; Marx et al., 2000; Bers, 2004). See Fig. 2. The cardiac RyR2 is composed of four homologous subunits of 560KD that are encoded by a separate gene of three existing genes (Nakai et al., 1990; Fill & Copello, 2002). While a significant portion of the RyR2 extends into the SR lumen, the bulk of the RyR2 homotetramer is situated on the cytosolic side of the SR membrane (visible on EM micrographs and described as the “foot”); it carries multiple sites for cytosolic regulatory molecules (Ca2+, ATP, etc.) as well as docking sites for accessory regulatory proteins (FKBP12.6, calmodulin, sorcin, etc.). On the luminal side, the RyR2 is associated with a number of proteins, including TRD, JN and CASQ2. TRD and JN bind directly to RyR2 and each other and mediate interactions of CASQ with RyR2. The structure of RyR2 at an atomic resolution remains to be resolved. Structure–function studies, and analysis of mutational hot spots revealed several key regions, referred to as domains (Ikemoto & Yamamoto, 2002; George et al., 2007). Interactions between these domains (domain “unzipping”) are thought to underlie transitions between conformations corresponding to open and closed states of the channel, as well as transduction of regulatory events, and pathologic influences from both the cytosolic and luminal side.
Fig. 2.
Schematic presentation of the RyR2 channel complex. The domain structure of RyR2 with sites of interaction with auxiliary proteins and phosphorylation sites. Two major divergent regions located in the cytosolic portion are indicated. Calsequestrin, junctin, and triadin, proteins interacting with RyR and between themselves and RyR2 in the SR, are shown. PP, protein phosphatase; P, phosphorylation sites; CaM, calmodulin; CaMKII, calmodulin-dependent protein kinase II. Adapted from Gyorke and Terentyev, 2008.
3.1. FK binding protein 12.6
FK binding proteins (FKBPs) are immunophilins, and are so named based on binding to the immunosuppressant tacrolimus (originally named FK506) (Brillantes et al., 1994). In cardiac muscle, FKBP12 and FKBP12.6 are present (Jayaraman et al., 1992; Timerman et al., 1993; Timerman et al., 1996), and FKBP12.6 has been put forward as a key modulator of RyR2 function in both health and disease (Marx et al., 2000; Wehrens et al., 2005a; Yano et al., 2005). However, the role of FKBP12.6 in regulating RyR2 function remains highly controversial. FKBP12.6 is preferentially associated with RyR2 due to higher affinity (Timerman et al., 1996). The binding stoichiometry is one FKBP12.6 per RyR subunit, but the FKBP12.6 binding site in the RyR2 linear sequence has not been precisely identified. Dissociation of FKBP12.6 has been reported to result in increased RyR2 activity. It has been suggested that PKA phosphorylation at 2809 results in dissociation of FKBP12.6 from the RyR2 with a subsequent increase in the open probability of the RyR2 (Brillantes et al., 1994; Ahern et al., 1994; Kaftan et al., 1996). However, other studies have failed to detect any changes in RyR2 function upon dissociation of FKBP12.6 (Barg et al., 1997; Xiao et al., 2007). Furthermore, it has been reported that FKBP12.6 remains attached to RyR2 following phosphorylation (Stange et al., 2003; Xiao et al., 2004).
3.2. Calsequestrin
Calsequestrin (CASQ) is an intra-SR protein which has a low affinity and high-capacity for Ca2+ binding (MacLennan & Wong, 1971; Campbell et al., 1983; Mitchell et al., 1988; Beard et al., 2004; Gyorke & Terentyev, 2008). In the SR, calsequestrin exists as a mixture of monomers, dimers, and various multimers. When Ca2+concentrations exceed 1–10 mmol/l, CASQ monomers and dimers form linear oligomers, and these polymers (Park et al., 2004) can be detected as electron-dense filamentous matrices both in vitro and in vivo (Somlyo et al., 1981; Saito et al., 1984; Franzini-Armstrong et al., 1987). CASQ polymers are primarily located in the junctional SR (jSR) in both skeletal and cardiac muscle (isoforms CASQ1 and CASQ2, respectively) (Jorgensen & Campbell, 1984). Due to the proximity of CASQ matrices to RyR2, CASQ2 polymers are thought to provide the stores of releasable Ca2+ which activate myocyte contraction. In this regard, condensed CASQ represents the high Ca2+ binding capacity form of the protein and possesses at least twice the Ca2+ binding capacity of monomeric CASQ in both skeletal and cardiac muscle (Tanaka et al., 1986; Park et al., 2004). CASQ condensation reportedly involves head-to-tail oligomerization that occurs through front-to-front and back-to-back dimerization contacts involving the N-and C-terminal regions of the protein, respectively (Wang et al., 1998; Park et al., 2004). While the precise mechanism(s) of Ca2+ sequestration by CASQ is not known, it has been suggested that CASQ polymers provide electrostatically charged surfaces for absorption of Ca2+ (Wang et al., 1998).
In addition to serving as a Ca2+ storage site, cardiac calsequestrin (CASQ2) has also been shown to regulate RyR2 activity more directly via protein–protein interactions involving triadin (TRD) and junctin (Jn) (Gyorke et al., 2004; Gyorke & Terentyev, 2008; Terentyev et al., 2007). Both the N-terminal (Zhang et al., 1997; Kobayashi et al., 2000) and C-terminal regions of CASQ2 have been suggested as the triadin (and/or junctin) interaction sites (Shin et al., 2000). It has been proposed that CASQ2mediates the responsiveness of the RyR2 channel to luminal Ca2+ by serving as a luminal Ca2+ sensor (Gyorke et al., 2004; Terentyev et al., 2007; Gyorke & Terentyev, 2008). In particular, we demonstrated that both triadin 1 and junctin exert potentiatory influences on RyR2 activity (Gyorke et al., 2004). Thus, at low luminal Ca2+, CASQ2 is tightly bound to triadin 1 (junctin) and prevents it from activating the RyR2 channel. When luminal Ca2+ is increased, the binding of Ca2+ to CASQ2 disrupts the interactions of CASQ2with TRD thereby relieving the inhibitory influence of CASQ2 on the RyR2 channel. Thus, luminal Ca2+-dependent termination of Ca2+ release could be partly attributed to inhibition of the RyR2 by CASQ2 at lowered [Ca2+]SR. At the same time, when there is an increased [Ca2+]SR, the increase in the fraction of CASQ2-uninhibited RyR2 channels could contribute to the known enhancement of diastolic SR Ca2+ leak in cardiac myocytes. Modulation of channel function seems to be done by the monomer as inhibition occurs at low concentrations when CASQ2 exists as a monomer. In summary, CASQ2 appears to play at least two different roles in cardiac myocytes: as a Ca2+ storage reservoir in the SR, and as an active modulator of the Ca2+ release process, mediated by monomers.
3.3. Triadin and junctin
Triadin (TRD) and junctin (JN) are transmembrane proteins in the junctional SR that appear to bind directly to the RyR2, and to CASQ2 thereby anchoring CASQ2 to the RyR2 channel (Jones et al., 1995; Kobayashi & Jones, 1999; Kobayashi et al., 2000; Gyorke & Terentyev, 2008). Three isoforms of triadin (35, 40 and 92 kD) have been described of which triadin 1 (40 kD) is the predominant, representing more than 95% of the cardiac protein (Guo et al., 1996; Kobayashi & Jones, 1999). Junctin was identified as a 26-kDa CASQ2 binding protein in cardiac and skeletal muscle (Jones et al., 1995). Triadin 1 and junctin exhibit significant structural similarities. Both proteins are composed of a single membrane spanning domain, a short cytoplasmic N-terminal segment and a long, highly positively charged C-terminal domain situated in the lumen of the SR. The luminal domains of both proteins are characterized by the frequent occurrence of long stretches of alternating positively and negatively charged residues known as KEKE motifs (Jones et al., 1995; Kobayashi et al., 2000). These regions, in general denoted as protein–protein binding motifs, are thought to be involved in interactions between triadin 1 and junctin themselves and in interactions with RyR2 and CASQ2 (Zhang et al., 1997). In lipid bilayers both TRD and JN activate RyR2s [Gyorke 2004], and acute overexpression of TRD also results in increased RyR activity (Terentyev et al., 2005). The functions of TRD1 and JN have also been studied using genetic mouse models (Zhang et al., 2001; Kirchhefer et al., 2001; Kirchhefer et al., 2003; Kirchhefer et al., 2006; Yuan et al., 2007). However, the interpretation of the physiologic roles of TRD and JN from the transgenic models is complicated by various adaptive and pathological changes (e.g. hypertrophy and heart failure) accompanying chronic protein expression. Our current understanding, as described above (Section 3.2) is that the complex of CASQ, TRD and JN serves to regulate RyR2 activity in a [Ca2+]SR-dependent manner.
3.4. Other proteins
A number of other proteins interact with the RyR2 complex, including sorcin (Valdivia, 1998), homer (Pouliquin et al., 2006; Worley et al., 2007), and the histidine-rich Ca2+ binding protein (Kim et al., 2003). The specific role of these proteins in SR Ca2+ release regulation remains to be defined.
4. Modulation of sarcoplasmic reticulum Ca2+ release
4.1. Modulation of sarcoplasmic reticulum Ca2+ release by luminal Ca2+
Rather than merely serving as a passive pool of available Ca2+, intra-SR Ca2+ plays an active role in controlling the Ca2+ release process. With measurements of cell-wide Ca2+ release, this regulatory influence manifests itself as an increase in the fraction of total releasable Ca2+ from the SR at elevated Ca2+ loads and conversely as a decrease of fractional release at reduced SR Ca2+ loads (Bassani et al., 1995; Shannon et al., 2000). Increasing SR Ca2+ content also enhances the frequency of spontaneous Ca2+ release events, Ca2+ sparks (Cheng et al., 1996; Lukyanenko et al., 1996). The effects of luminal Ca2+ on SR Ca2+ release have been attributed to twomechanisms: 1) Activation of RyR2s on the cytosolic side of the channel at sites involved in CICR by Ca2+ passing through the channel; and 2) stimulation of RyR2s activity at distinct sites on the luminal side of the channel. The relative contribution of each mechanism to Ca2+ signaling in myocytes is difficult to define due to significant interdependence of the two mechanisms.
The stimulatory effects of luminal Ca2+ on RyR2 channels have been directly demonstrated in in vitro reconstitution studies(Lukyanenko et al., 1996; Xu & Meissner, 1998; Gyorke & Gyorke, 1998; Ching et al., 2000; Gyorke & Terentyev, 2008). Consistent with the existence of Ca2+ regulatory sites on both sides of the channel, two different mechanisms of action of luminal Ca2+ upon RyR2 have been described: 1) indirect, “feed-through” activation by luminal Ca2+ at the cytosolic activation site of the channel; and 2) direct, “true luminal” activation by Ca2+ on the luminal side of the channel. In single reconstituted RyR2s, these two mechanisms are readily distinguishable based on differences in the voltage-dependency of luminal Ca2+ effects and the dissimilar impact of luminal Ca2+ on channel gating behavior which are characteristic of these two mechanisms. For example, feed-through effects are characteristically larger at negative holding potentials than at positive holding potentials due to the reliance of the stimulatory action of luminal Ca2+ on luminal-to-cytosolic fluxes which are intrinsic to this mechanism (Xu & Meissner, 1998). Furthermore, feed-through activation increases RyR2 open probability by prolonging the lifetime of channel openings rather than by increasing their frequency. This is understandable considering that luminal Ca2+ can flow through the RyR2 channel and reach the cytosolic activation sites only when the channel is open; once the channel closes, Ca2+ accumulated near the cytosolic face of the RyR rapidly dissipates rendering the channel inactive. Compelling evidence for true luminal Ca2+ activation of RyR2s has been obtained under experimental conditions that prevent any luminal-to-cytosolic Ca2+ fluxes (measurements performed at highly positive holding potentials and millimolar cis [Ca2+]) and by using tryptic digestion of the luminal phase of the RyR2 channel to disable the putative luminal Ca2+ sensor (Gyorke & Gyorke,1998; Ching et al., 2000).
4.2. Modulation of the Ca2+ release channel by protein kinases and phosphatases
In addition to being governed by changes in cytosolic and SR luminal Ca2+, the RyR2 channels are targets for modulation by a number of intracellular ligands and signaling cascades, including phosphorylation pathways. The RyR2 is phosphorylated by PKA and CAMKII at Ser-2809 and by CAMKII at Ser-2815 (Marx et al., 2000; Wehrens et al., 2004). Recently a new PKA phosphorylation site has been reported at Ser-2830 (Xiao et al., 2005; Xiao et al., 2006), although this site has been disputed (Wehrens et al., 2006). In addition to these identified phosphorylation sites, RyR2 is phosphorylated by CAMKII on as many as five sites, based on 32P phosphate incorporation measurements (Rodriguez et al., 2003). The RyR2 is reportedly preassociated with PKA, and the protein phosphatases PP1 and PP2A through the anchoring protein mAKAP (Marx et al., 2000). In addition, RyR2 co-immunoprecipitates with CAMKII (Zhang et al., 2003) and PP2B (calcineurin, CN, (Bandyopadhyay et al., 2000), However, the molecular sites for these interactions remain to be identified. Thus, the molecular components involved in both phosphorylation and dephosphorylation of the RyR2 are anchored directly to the protein.
Despite clear evidence for RyR2 phosphorylation, the physiological consequences of this process remain highly controversial. PKA-dependent phosphorylation has been shown to either increase or decrease steady-state RyR2 open probability in different reports (Bers & Guo, 2005; Benkusky et al., 2004; Wehrens et al., 2004). Marks and collaborators have reported that PKA phosphorylation at Ser-2809 leads to dissociation of FKBP12.6 from the RyR2, in turn resulting in enhanced channel activity (Marx et al., 2000); however, other investigators find that FKBP12.6 still binds to RyR2 after PKA phosphorylation (Stange et al., 2003; Xiao et al., 2004). In intact myocytes, PKA stimulation has been shown to enhance SR Ca2+ release, an effect that could be attributed to phosphorylation of PLB and increased SR Ca2+ load as well as increased ICa. Interestingly, in PLB knock-out myocytes, RyR2 phosphorylation by PKA produced no effects on spontaneous local Ca2+ release signals [i.e. Ca2+ sparks, (Li et al., 2002)], indicating that the PKA-dependent increase in Ca2+ spark frequency is entirely attributable to PLB phosphorylation and the resulting increase in SR Ca2+load. A recent study showed that RyR2 is stimulated only during extreme hyperphosphorylation (i.e. all 4 subunits of the tetramer are phosphorylated at Ser-2809) (Carter et al., 2006). CAMKII, expressed as the delta isoform, has also been shown to produce disparate effects. Some authors have reported that exogenous CaMKII increases RyR2 activity (Witcher et al., 1991; Hain et al., 1994), whereas others have found that exogenous (Lokuta et al., 1995) and endogenous CaMKII reduces RyR2 opening or ryanodine-RyR binding (Takasago et al., 1991). Similarly, in intact voltage clamped myocytes some investigators have reported that both endogenous and exogenous CAMKII increase the amplitude of Ca2+ transients at a given SR Ca2+ content and ICa trigger (Bers & Guo, 2005), whereas others have reported that endogenous CAMKII and constitutively active CAMKII suppress Ca2+ transients and Ca2+ sparks (Wu et al., 2002; Yang et al., 2007). Thus, this remains an open area of investigation at the present time.
The action of protein kinases is opposed by dephosphorylating phosphatases. Three types of protein phosphatases, including PP1, PP2A and PP2B (calcineurin), have been shown to influence cardiac performance (Neumann, 2002). Overall, in the majority of studies, phosphatases reportedly downregulate SR Ca2+ release and contractile performance (Neumann et al., 1993; duBell et al., 1996, 2002; Carr et al., 2002; Santana et al., 2002). Application of PP1 and PP2A to permeabilized myocytes caused an acute activation of Ca2+ sparks followed by depletion of the SR Ca2+ content (Terentyev et al., 2003a), suggesting that depression of EC coupling by phosphatases may involve depletion of the SR Ca2+ stores due to increased activation of RyR2s. Consistent with this possibility, phosphatases have been shown to increase RyR2 activity in some (Lokuta et al., 1995; Terentyev et al., 2003a; Carter et al., 2006) but not all single channel studies (Hain et al., 1994). Overall, as summarized in this brief overview, the mechanisms and functional consequences of RyR2 phosphorylation remain to be conclusively elucidated.
4.3. Redox modulation
Ryanodine receptor channels are subject to modifications by oxidizing and reducing agents capable of interacting with thiolcontaining cysteine residues of the channel protein. The cardiac RyR contains approximately 90 cysteines per subunit, of which approximately 20 are in reduced state (Xu et al., 1998). These free cysteines are targets for several types of redox modifications, including disulfide crosslinking, S-nitrosylation and S-glutathionylation. In general, oxidizing conditions increase RyR2 activity and therefore stimulate SR Ca2+ release (Abramson & Salama, 1988; Eager et al., 1997; Zima & Blatter, 2006), while these effects are reversed by reducing reagents such as dithiothreitol (DTT). Although different classes of oxidizing agents tend to increase RyR2 activity, the underlying effects on channel gating are not uniform. For example, whereas oxidation increases the sensitivity of the channel to Ca2+ activation, S-glutathionylation decreases the sensitivity of the channel to inhibition by Mg2+ (Donoso et al., 2000). There is also evidence of time-dependent effects whereby prolonged exposure to high doses of oxidizing agents, after an initial stimulation of activity, can cause irreversible inactivation of RyRs (Eager et al., 1997; Marengo et al., 1998). Thus, the functional consequences of RyR2 thiol modification are dependent on the concentration of the oxidizing agent, exposure time and the nature of the chemical reaction involved in the modification. These different effects are likely to reflect participation of different sets of cysteine residues under different oxidizing conditions. The role of specific cysteine residues in RyR redox modifications has only started to be elucidated. It has been shown that redox modification involves only 12 of the total 100 cysteines on RyR1 (Aracena-Parks et al., 2006). Whereas some of these cysteines are non-specific targets for different redox agents, other sites are exclusively S-nitrosylated (Cys-1040, Cys-1303) or S-glutathionylated (Cys-1591 and Cys-3193). Endogenous oxidizing agents (reactive oxygen species or ROS) are continuously generated in the heart and are likely to exert tonic influences on intracellular targets/processes including SR Ca2+ release. ROS production has been shown to increase in various pathological settings such as ischemia-reperfusion and heart failure. Growing evidence suggests that modification of RyR2 by ROS contributes to the abnormal Ca2+ handling associatedwith these disease states (see section 6.7 below).
5. The Ca2+ release-uptake cycle
5.1. Activation of sarcoplasmic reticulum Ca2+ release
Myocardial excitation–contraction (EC) coupling begins with membrane depolarization activating voltage-dependent Ca2+ channels in the plasma membrane which allows a relatively small amount of Ca2+ to enter the cell. This Ca2+ serves as a trigger signal to activate Ca2+ release channels present in large clusters in the junctional sarcoplasmic reticulum (jSR). See Fig. 3A. The release of Ca2+ via groups of RyR2s has been visualized as Ca2+ sparks (Cheng et al., 1993). Sparks have been estimated to involve coordinated openings of 6 to 30 RyR2s (Bridge et al., 1999; Lukyanenko et al., 2000;Wang et al., 2001). Ca2+ sparks amplify the initial Ca2+ influx trigger signal and combine to produce an elevation of cell-wide myoplasmic [Ca2+], the Ca2+ transient. This increase in cytosolic Ca2+ concentration ([Ca2+]i) leads to activation of the contractile proteins and hence to generation of the heartbeat. Under certain conditions, Ca2+ release can be triggered by other mechanisms such as Ca2+ entry via reverse mode NCX (Sipido et al., 1997). Moreover, Ca2+ entry via these Ca2+ transport mechanisms can interact synergistically resulting in activation of large SR Ca2+ release at positive membrane potentials where ICa by itself is small. (Viatchenko-Karpinski et al., 2005; Sobie et al., 2008).
Fig. 3.
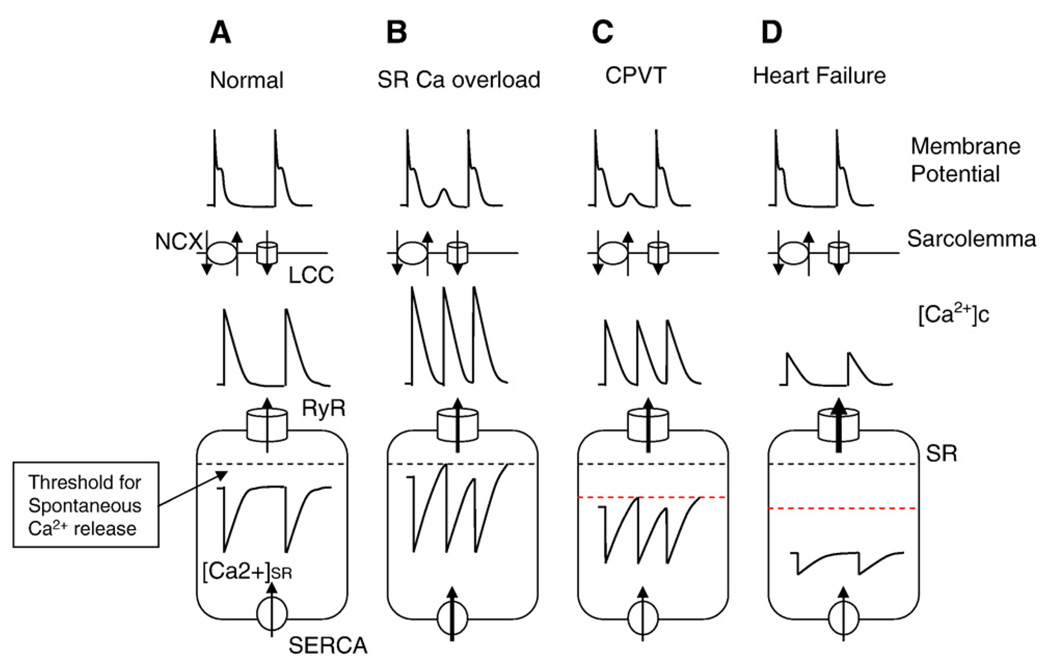
Ca2+ cycling in normalmyocytes andmyocytes under three different disease conditions: SR Ca overload, CPVT and heart failure. A. In normalmyocytes, Ca2+ influx through L-type Ca2+ channels (LCC) during the action potential activates RyR2s and initiates the release of Ca2+ stored in the SR on a beat-to-beat basis. Diastolic SR Ca2+ release is prevented due to RyR2 deactivation at reduced [Ca]SR; [Ca]SR is always below the threshold for activation of spontaneous Ca release (indicated by dashed line). B. Under conditions of Ca2+ overload, [Ca]SR reaches the threshold for activation of spontaneous Ca release, diastolic SR Ca2+ release occurs, resulting in arrhythmogenic DADs through stimulation of NCX. C. Abnormal RyR2 channel function caused by CPVT-linked mutations in RyR2 and CASQ2 result in a reduced threshold (indicated by red dashed line) for spontaneous Ca2+ release (i.e. perceived Ca2+ overload), diastolic Ca2+ release and DADs. D. In heart failure, acquired defects in RyR2 result in a more severe RyR2 deregulation ([Ca2+]SR threshold for spontaneous Ca2+ release is further reduced); excessive SR Ca2+ leak leads to depletion of the [Ca]SR and diminished systolic Ca2+ release and contraction.
5.2. Termination of sarcoplasmic reticulum Ca2+ release
Following its rapid activation, SR Ca2+ release robustly terminates leaving a substantial Ca2+ reserve of releasable Ca2+ in SR. The exact underlying causes of Ca2+ release termination remain to be defined (Stern & Cheng, 2004). Several mechanisms have been proposed, including inactivation of the RyR2 channel by elevated cytosolic Ca2+ (i.e. Ca2+-dependent inactivation); obligatory shift from high- to low modes of activity (i.e. adaptation); and RyR2 deactivation caused by decline in intra-SR [Ca2+] (i.e., luminal-Ca2+-dependent deactivation). In recent years, several lines of evidence have pointed to a prevailing role of luminal Ca2+ in controlling SR Ca2+ release (Terentyev et al., 2002; Sobie et al., 2002; Zima et al., 2008; Terentyev et al., 2008). For example, buffering [Ca2+]SR using low affinity exogenous Ca2+ buffers such as citrate or maleate prolonged the duration of Ca2+ release during both Ca2+ sparks and global Ca2+ transients (Terentyev et al., 2002). Subsequently, similar results were obtained by varying the expression level (increasing or decreasing) of the endogenous Ca2+ buffer CASQ2 in cardiac myocytes (Terentyev et al., 2003b). Recently, the role of this mechanism has received further support from the observation that Ca2+ sparks terminate at a constant free [Ca2+]SR regardless of the extent of [Ca2+]SR buffering by CASQ2 (Terentyev et al., 2008). Moreover, mutations in CASQ2 that enhance the responsiveness of RyR2s to luminal Ca2+ reduced the [Ca2+]SR threshold for local Ca2+ release termination. Thus, mounting experimental evidence suggests that luminal Ca2+-dependent changes in RyR2s contribute to SR Ca2+ release termination in cardiac myocytes.
5.3. Refractoriness of Ca2+ signaling
After activation, a shift in the gating mode of RyR2s closes the channels, which then remain closed in a time-dependent manner. There is substantial evidence indicating that changes in luminal Ca2+ govern not only termination of CICR, but also induce a refractory state that persists until recovery of [Ca2+]SR. (Terentyev et al., 2002, 2003b; Szentesi et al., 2004; Sobie et al., 2005). Thus, experimental interventions to slow SR-Ca2+ reuptake (either by SERCA-pump inhibitors or by increasing the intra-SR Ca2+ binding sites with either exogenous or endogenous buffers) slow recovery of Ca2+ signaling from refractoriness (Szentesi et al., 2004; Terentyev et al., 2002, 2003b). Moreover, accelerating [Ca2+]SR recovery following Ca2+ release either by stimulation of the SERCA pump or by reducing endogenous CASQ levels accelerated recovery of RyR2 refractoriness. Interestingly, restitution of refractoriness appears to lag behind [Ca2+]SR recovery (Wang et al., 2001; Sobie et al., 2005) suggesting that the relationship between recovery and luminal Ca2+ is non-linear and/or may involve some intermediate steps. Consistent with the latter possibility, modulation by luminal Ca2+ may involve a group of intermediate proteins including CASQ2, triadin and junctin (see Section 3.2 above).
5.4. Sarcoplasmic reticulum Ca2+ reuptake
During relaxation, rapid reuptake of Ca2+ into the SR is achieved by the SR Ca2+ ATPase (SERCA2a). The activity of SERCA is modulated by the phosphoprotein phospholamban (PLB) in a phosphorylation-dependent manner (Brittsan & Kranias, 2000). Dephosphorylated PLB inhibits SERCA activity whereas phosphorylation relieves the inhibition. Phosphorylation occurs at two sites, Ser 16 and Thr 17 by PKA and CAMK2 respectively. Within the SR a large fraction of Ca2+ is bound to CASQ, and this binding accelerates uptake by keeping free [Ca2+]SR at low levels. The ability of SERCA to attain a maximum thermodynamically allowable [Ca2+]SR is limited by the leak though RyRs which increases as a function of [Ca2+]SR as described below (Lukyanenko et al., 2001).
5.5. Luminal Ca2+-dependent leak
The SR exhibits a substantial diastolic Ca2+ leak in the form of spontaneous Ca2+ sparks. The frequency of sparks and the amount of leak is increased at elevated SR Ca2+ content and conversely is reduced at lowered SR Ca2+ content (Lukyanenko et al., 2001; Shannon et al., 2002). This leak limits the ability of the SR Ca2+ pump to attain the maximum thermodynamically allowable [Ca2+]SR and plays an important role in setting the SR Ca2+content. Mechanistically, the SR Ca2+ leak is a consequence of activation of RyRs by increased [Ca2+]SR. Increased SR Ca2+ leak plays an important role in various pathological states such as HF, in which it results in reduced SR Ca2+ content and weakened contractility (see below).
5.6. Balance of trans-sarcolemmal and sarcoplasmic reticulum Ca2+ fluxes
In order to maintain Ca2+ homeostasis, an equivalent amount of Ca2+ must enter the myocyte via ICa and then be removed from the myocyte on a beat-to-beat basis. Most of this Ca2+ is removed by the electrogenic Na+–Ca2+ exchanger that exchanges three Na+ ions for one Ca2+ ion. At the same time, the amount of Ca2+ released from the SR must be balanced by the amount of Ca2+ taken back up by SERCA. Deviation from the balance of trans-sarcolemmal and SR Ca2+ fluxes will lead to either gain or loss of Ca2+ in the cell and in the SR. This requirement for the homeostatic balance of cellular and SRCa2+ fluxes is an important factor in determining the SR Ca2+ content and release, and in shaping the response of SR Ca2+ release to pharmacological and/or pathophysiologic modulation.
By studying the effects of low concentrations of the RyR2 channel modulators (caffeine and tetracaine) on systolic Ca2+ release, Eisner and coworkers demonstrated that the SR Ca2+ release mechanism in cardiac myocytes exhibits an ability for self-regulation which allows myocytes to maintain a consistent amplitude of systolic Ca2+ transients, even when interventions alter RyR2 activity (Overend et al., 1998; Trafford et al., 2000). They attributed this self-regulation to a feedback loop consisting of SR Ca2+ release causing cellular Ca2+ extrusion; Ca2+ extrusion influencing SR Ca2+ load; and SR Ca2+ load affecting SR Ca2+ release (Eisner et al., 1998; Trafford et al., 2002). As susequently shown, the dependence of RyR gating on luminal Ca2+ is another important factor in determining Ca2+cycling, and is expected to both accelerate and increase the extent of restitution of Ca2+ transient amplitude after a change caused by a perturbed open probability of RyR2s (Lukyanenko et al., 2001; Shannon et al., 2002). It is to be noted, however, that the capacity of a myocyte to restore its initial Ca2+ transient amplitude following alterations in open probability of RyRs only operates over a limited range. Thus, if RyRs are hyperstimulated the SR becomes completely depleted and there is no SR Ca2+ release, and consequently only low amplitude steady-state Ca2+ transients. This situation occurs in certain pathological states such as HF and is examined in detail below.
6. Pathologies of cardiac sarcoplasmic reticulum Ca2+ signaling
6.1. Overview
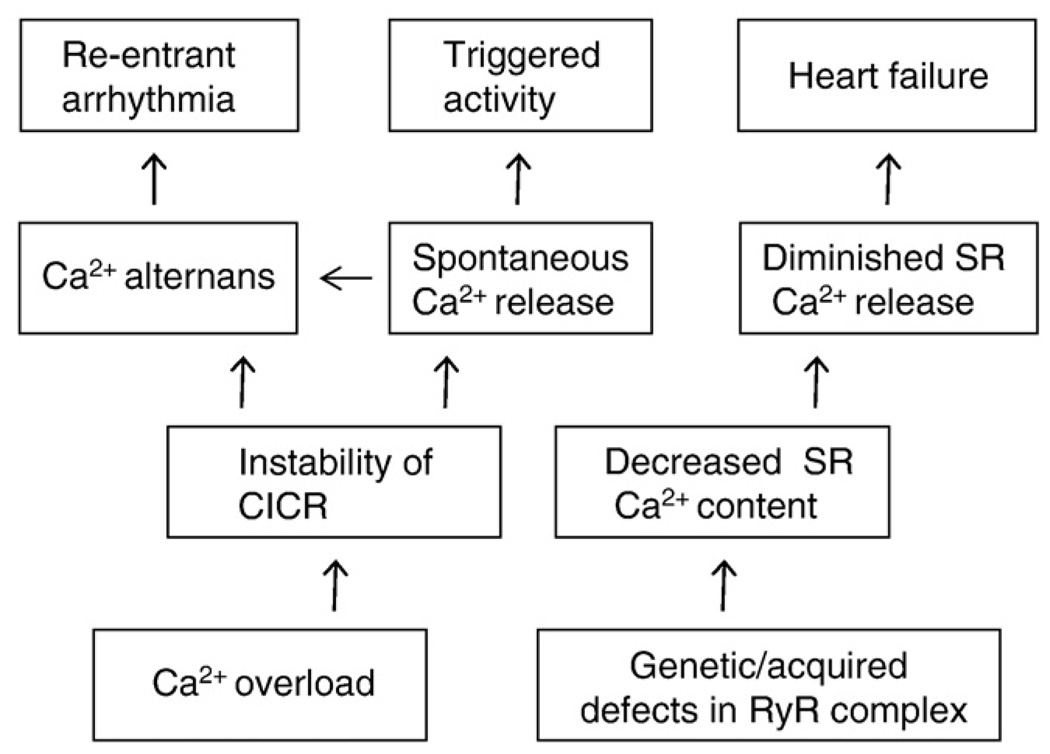
Abnormal RyR2-mediated SR Ca2+ release has been implicated in a spectrum of cardiac disease states ranging from triggered and reentrant arrhythmias to HF (Wehrens et al., 2004; George et al., 2007; Ter Keurs & Boyden, 2007; Laurita & Rosenbaum, 2008). A classification of cardiac diseases related to abnormal SR Ca2+ release and their hypothetical relationship to defective function is presented in Fig. 4. Ca2+-dependent arrhythmias of both triggered and reentry types are generally associated with instability of SR Ca2+ release whereas HF is associated with diminished systolic SR Ca2+ release. In general, loss of control of CICR can arise in two ways. Firstly, in a variety of conditions that lead to increased SR Ca2+ load (i.e. “Ca2+ overload”), sensitization of RyR2s by enhanced luminal Ca2+ contributes to generation of spontaneous Ca2+ releases or instabilities of SR Ca2+ release that manifest themselves as Ca2+ alternans (Fig. 3B; Fig. 4). Whereas spontaneous Ca2+ releases causes DADs and triggered arrhythmia, Ca2+ alternans can provide basis for heterogeneities in electrical repolarization and substrate for reentry arrhythmia. Secondly, acquired or genetic defects in RyR2s enhance the responsiveness of RyR2 to luminal Ca2+ such that the channel becomes hyperactive even at reduced SR Ca2+ content. This state of “perceived” Ca2+ overload (Gyorke & Terentyev, 2008) can also lead to Ca2+-dependent arrhythmias and has been implicated familial tachyarrhythmias associated with mutations in RyR2 or CASQ2 (Fig. 3C). Diminished SR Ca2+ release (e.g. as in HF) can result either from failure of EC coupling or exhaustion of SR Ca2+ stores due to an excessive leak via RyR2s (Fig. 3D). Thus, different diseases could result from different degrees of alteration in the same underlying mechanism, namely increased RyR activity such that a relatively small change could result in destabilization of CICR whereas a more severe deviation could lead to SR Ca2+ leak and SR store depletion. Clearly, other factors besides abnormal RyR function, such as changes in SERCA and NCX activity, also contribute to different disease phenotypes. The role of these factors has been extensively reviewed (e.g. Kranias and Bers, 2007; Periasamy et al., 2008) and is not discussed here.
Fig. 4.
Classification of Ca2+-dependent disease states based on mechanisms of alterations of Ca2+ handling.
6.2. Ca2+ overload and triggered arrhythmia
Excess intracellular Ca2+ (i.e. Ca2+ overload) is a characteristic feature of various pathological states such as metabolic inhibition, ischemia/reperfusion and digitalis toxicity. Ca2+ overload is associated with triggered afterdepolarizations, which can initiate sustained tachyarrhythmias (Ferrier et al., 1973; Kass et al., 1978; Pogwizd & Bers, 2004; Maltsev et al., 2006; Venetucci et al., 2008). See Fig. 3B. The causal relationships between Ca2+ overload and triggered activity are known to include the following sequence of events: (1) SR Ca2+ overload leads to generation of spontaneous Ca2+ release from the SR, (2) elevated Ca2+ induces depolarizing membrane currents that give rise to delayed afterdepolarizations (DADs), (3) DADs activate ectopic action potentials, and (4) ectopic action potentials can then initiate tachyarrhythmias. A brief discussion of these steps follows below.
6.3. Spontaneous Ca2+ release
Since the initial studies of Fabiato in skinned myocytes (Fabiato & Fabiato, 1972), spontaneous Ca2+ release or Ca2+ waves have been described by many investigators in various preparations. Ca2+ waves in general are associated with increased SR Ca2+content (Stern et al., 1988; Pogwizd & Bers, 2004; Venetucci et al., 2008). Moreover, it has been shown with both total SR Ca2+ content determinations (Diaz et al., 1997) and more directly using SR-entrapped Ca2+ indicators (Kubalova et al., 2004) that Ca2+ waves arise when [Ca2+]SR reaches a certain threshold value. See Fig. 3B. As visualized by confocal Ca2+ imaging, Ca2+ waves are initiated by local release events, Ca2+ sparks, that occur spontaneously in resting myocytes, and then propagate through saltatory activation of adjacent release sites (Cheng et al., 1996; Lukyanenko et al., 1999). The mechanisms for the transition from Ca2+ sparks to self-propagating Ca2+ waves have been examined. One possibility is that Ca2+ waves start and propagate when Ca2+ efflux via individual release sites becomes sufficient to raise Ca2+ at adjacent release sites above the threshold for CICR from a Ca2+-overloaded SR. However, this mechanism may not be sufficient by itself to account for Ca2+ wave generation. Experimental studies combined with mathematical modeling have suggested that propagation of Ca2+ waves relies on stimulatory effects of elevated luminal Ca2+ on RyR activity (Lukyanenko et al., 1999). As to the specific role of luminal Ca2+, two hypotheses have been considered: (1) increased luminal Ca2+ sensitizes the Ca2+ release channels to cytosolic Ca2+, thus enhancing the ability of cytosolic Ca2+ to activate adjacent release sites via CICR, and (2) Ca2+ transported from the wave-front into the adjacent SR elements raises luminal Ca2+ above a critical threshold level, resulting in activation of the release channels at a luminal site. Examination of the effects of SERCA inhibitors, such as thapsigargin, showed that Ca2+ waves elicited by local application of caffeine in myocytes preloaded with Ca2+ not only did not stop but even accelerated after rapid inhibition of SR Ca2+ uptake (Lukyanenko et al., 1999). At the same time, Ca2+waves could be elicited following sensitization of RyRs by agents such as caffeine — even in the absence of Ca2+ overload. These finding supported the role of CICR in wave propagation with luminal Ca2+ serving to sensitize RyRs. However, recently (Keller et al., 2007) reexamined the role of SERCA-mediated uptake in Ca2+ wave propagation using a photo-releasable SERCA inhibitor. Following inhibition of SR Ca2+ uptake these authors observed a reduction in Ca2+ wave velocity in contrast to previous findings. The reasons for these discrepant results remain undefined and additional experimentation will be required to resolve this issue. In summary, evidence exists to suggest that the effects of luminal Ca2+ on RyR2s play at least a sensitizing role in the generation of Ca2+ waves by promoting CICR. The intriguing possibility that [Ca2+]SR plays a more direct triggering role in Ca2+ wave generation will require further verification.
6.4. Afterdepolarizations and triggered arrhythmia
Triggered arrhythmias originate from afterdepolarizations and are so named as they are “triggered” by either abrupt or sustained abnormal variations in heart rate. Afterdepolarizations occur either during the action potential (early afterdepolarizations or EADs) or after termination of the action potential (delayed afterdepolarizations or DADs). Classically, EADs are considered to occur at slow heart rates, DADs at rapid heart rates.
Early afterdepolarizations are often associated with delayed cardiac repolarization (action potential prolongation or QT/QTc prolongation on the electrocardiogram), and have been implicated in the genesis of Torsades de Pointes arrhythmias (see below). It has been suggested that repolarization abnormalities either directly cause or provide a foundation for EADs [reviewed by (Roden, 1993)]. Postulated ionic mechanisms responsible for EADs include K+ channel block, enhanced late sodium current, reactivation of L-type calcium current during prolonged action potential plateau. However, most relevant to this review, abnormal release of calcium from intracellular SR stores during repolarization can lead to inward current via Na+/Ca2+ exchange and activation of EADs, even during rapid heart rates. This has been demonstrated in computer simulations (Huffaker et al., 2004) and in isolated myocytes when SR Ca2+ is increased (Spencer & Sham, 2003). In addition to NCX, window ICa has also been implicated in the development of EADs; however, SR Ca2+ release has been implicated as an important modulator of ICa inactivation and EADs, in a canine model of TdP (chronic AV block) (Antoons et al., 2007).
Delayed afterdepolarizations are typically attributed to “calcium overload” of cardiac myocytes, with the classic examples being inhibition of Na+–K+-ATPase with cardiac glycosides (e.g. digoxin) or beta-adrenergic stimulation (Rosen & Legato, 1985; Rosen, 1985). DADs are attributed to spontaneous Ca2+ release from the sarcoplasmic reticulum (SR), resulting in subsequent activation of a transient inward current via the Na+/Ca2+ exchanger. Elevations of cytosolic Ca2+ resulting from spontaneous SR Ca2+ release causes inward current to flow across the sarcolemma, producing transient depolarizations or DADs (Lederer & Tsien, 1976; Allen et al., 1984; Wier & Hess, 1984; Marban et al., 1986). These inward currents are predominantly due to the electrogenic NCX exchanging 1 Ca2+ for 3 Na+ ion (Schlotthauer & Bers, 2000). In most cases DADs are not of sufficient amplitude to reach the threshold to initiate an AP (see Fig. 5). This is because a Ca2+ wave at any time occupies only a fraction of the cell; therefore only a fraction of the total NCX molecules are exposed to elevated Ca2+. However, when several waves arise in the cell simultaneously (i.e. multifocal waves), the summation of the inward currents can depolarize the cell to threshold resulting in an AP (Capogrossi et al., 1987). In intact muscle, currents can spread from cell to cell through the gap junctions, summating over a region covering a group of myocytes (area comparable in size to the electrical space constant). When spontaneous Ca2+ release occurs asynchronously among cells, the likelihood that this summation could reach threshold to trigger an ectopic AP is low. However, spontaneous Ca2+ releases can be relatively synchronized following an AP. Such temporal concurrence of spontaneous release events could be due to similarly timed recovery of RyR2s from luminal-dependent refractoriness and simultaneous attainment of the [Ca2+]SR threshold for spontaneous Ca2+ release.
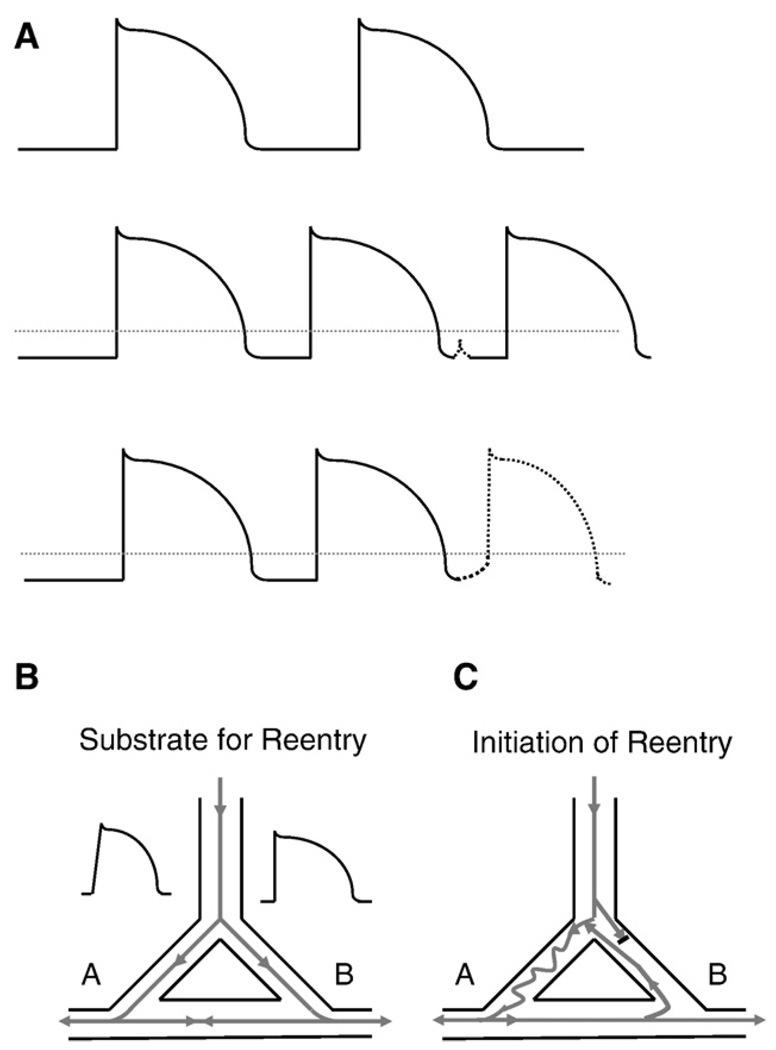
Fig. 5.
Substrate for re-entry and initiation of re-entry. A. Top panel: action potentials under control conditions. Middle panel: action potentials at a faster rate, with a delayed afterdepolarization (dashed line). Note that the delayed afterdepolarization does not reach the voltage threshold to trigger an action potential (threshold indicated by dotted line). Bottom panel: the delayed afterdepolarization is of sufficient amplitude to reach the threshold voltage and triggers an early action potential. B. The figure depicts an anatomic circuit which has the functional prerequisites to form a substrate for re-entry. Note that in pathway A, there is slowed conduction (represented by the slower upstroke of the action potential), while in pathway B, there is normal conduction but a prolongation of the action potential duration (and therefore prolongation of the refractory period) in the representative action potential. C. A properly timed premature beat excites the anatomic circuit and initiates a reentrant arrhythmia. Note that due to the slower conduction in pathway A, the impulse is very slowly conducted down pathway A. However, due to the longer refractory period which exists in pathway B, the premature beat is too early to excite pathway B, and conduction is blocked. As the premature impulse exits pathway A, the refractory period in pathway B has passed and the impulse excites pathway B in a retrograde manner. Due to the heterogeneities of conduction velocity and refractoriness, this anatomical circuit can maintain reentrant activation and thus, a tachyarrhythmia.
Afterdepolarizations of sufficient amplitude to reach the threshold voltage for a myocyte can initiate ectopic (triggered) beats. Arrhythmias can be induced by either isolated or sustained triggered beats. Notably, conduction of either EADs and DADs can give rise to ectopic (premature) beats which can initiate reentrant arrhythmias (see Fig. 5) resulting in sustained cardiac arrhythmias. Re-entry is thought to be the most common cause of sustained cardiac arrhythmias. Re-entrant arrhythmias require an underlying substrate; the substrate (predisposition) for re-entry is dispersion of both conduction velocities and refractory periods within a region of the heart. An illustration of the substrate and initiation of re-entry is shown in Fig. 5.
6.5. Catecholaminergic polymorphic ventricular tachycardia
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia characterized by exercise or stress-induced syncope and sudden death (Eldar et al., 2002; Laitinen et al., 2004; Napolitano & Priori, 2007). CPVT accounts for approximately 20% of all sudden deaths that occur in the absence of structural heart disease. Two genetic forms of CPVT have been described: a prevailing dominant form linked to mutations in RyR2s and a rare recessive form associated with mutations in CASQ2 (Postma et al., 2002; Lahat et al., 2004). Currently about 60 mutations in RyR2 and 7 mutations in CASQ2 have been linked to CPVT. Mutations in these proteins account only for about 50% of all cases of CPVT suggesting that genetic defects in other proteins can also lead to this syndrome. Notably, these different defects result in the same arrhythmic phenotype; and experimental work has reconciled the mechanisms of CPVT from these varying mutations.
Adenoviral expression studies of CPVT-associated CASQ2 mutants in cardiac myocytes have demonstrated that various mutations act by inducing irregular Ca2+ transients and arrhythmogenic DADs (Terentyev et al., 2003b; Gyorke & Terentyev, 2008). The mechanisms by which CASQ2 mutations alter regulation of SR Ca2+ release have been examined (Terentyev et al., 2006; Gyorke & Terentyev, 2008). Although various CASQ2 mutations affect different aspects of CASQ2 function, the results converge in a single common pathway to shorten RyR2 refractoriness and promote spontaneous Ca2+ release in the diastolic phase. See Fig. 3C. First, as described above, CASQ2 polymers are required for high-capacity Ca2+ binding, and certain mutations (Del.339–354) appear to disrupt CASQ2 polymerization (Terentyev et al., 2008). Reduced Ca2+ binding capacity of the SR results in accelerated recovery of [Ca]SR by the SERCA and thus premature functional recovery of RyR2 from luminal Ca2+-dependent deactivation. Secondly, interactions of CASQ2 with the RyR2 complex modulate the sensitivity of the RyR2 channel to luminal Ca2+. CASQ2 mutations [R33Q; (Terentyev et al., 2006)] alter the modulation of RyR2s by luminal Ca2+ without apparently altering the Ca2+ binding capacity of CASQ2. The resulting altered modulation of RyR2s by luminal Ca2+ (i.e. compromised ability of CASQ2 to deactivate the RyR2) with these mutations leads again to premature recovery of Ca2+ release from refractoriness.
The mechanisms by which CPVT-associated mutations in RyR2 alter RyR2 channel function have been investigated (George et al., 2007; Liu & Priori, 2008). Based on studies using a genetic mouse model, Marx and coworkers have suggested that RyR2s mutations lead to dissociation of the regulatory protein FKBP12.6 from RyR2 causing increased diastolic Ca2+ leak, DADs and arrhythmias (Wehrens et al., 2003). However, several aspects of this hypothesis have been disputed by other laboratories (George et al., 2007; Xiao et al., 2007; Liu & Priori, 2008). Alternatively, based on studies using a heterologous expression system, Chen and coworkers demonstrated that CPVT mutations increase the sensitivity of RyR2 to activation by luminal Ca2+ (Jiang et al., 2005). Similar to the effects of arrhythmogenic CASQ2 mutants, sensitization to luminal Ca2+ would reduce the [Ca2+]SR threshold for the generation of spontaneous Ca2+ release events and DADs. Thus, abnormal luminal modulation may be a common mechanism for genetically-distinct forms of CPVT.
Based on accumulated data it appears that CPVT can arise as a result of a genetic defect in any component of the Ca-storage-release machinery involved in controlling, sensing and responding to changes in luminal Ca2+. The defects affect the ability of RyRs to stay refractory, thus resulting in abnormal diastolic Ca2+ release and DADs. In the context of this mechanism, the role of adrenergic stimulation in CPVT can be explained by PKA-dependent stimulation of SERCA, which would accelerate SR Ca2+ store reuptake. This would further enhance the rate and extent of recovery of release sites from luminal Ca2+-dependent refractoriness exacerbating the arrhythmogenic effects of CASQ2/RyR2 mutations. As to the sites of origin within the heart, a recent study using epicardial mapping and a genetic mouse model (RyR2/RyR2.R4496C) of CPVT reported that focal arrhythmias arise predominantly in the His-Purkinje system (Cerrone et al., 2007).
6.6. Ca2+ alternans
Altered intracellular Ca2+ homeostasis is not only involved in triggered activity but also may be part of pathogenesis of reentrant arrhythmias. Instabilities in Ca2+ handling have been directly linked to heterogeneities in conduction and repolarization providing a substrate for reentrant excitation. These instabilities often manifest themselves as beat-to-beat variations in contractility and AP duration referred to as cardiac alternans and its cellular equivalent Ca2+ alternans (Weiss et al., 2006; Eisner et al., 2006; Laurita&Rosenbaum, 2008).When alternans is initiated it usually occurs in an identical phase, i.e. long–short–long APs, in all regions of the tissue (concordant alternans).When the heart rate is faster than the critical rate, a shift of phase occurs such that some regions continue to alternate in the long–short–long pattern while other regions alternate in the reverse pattern (discordant alternans). Spatially discordant alternans are highly arrhythmogenic and greatly amplify the heterogeneity of repolarization and electrical refractoriness producing conduction block and a substrate for reentrant excitation (Pastore et al., 1999; Qu et al., 2000). Cardiac alternans occurs in various pathologic settings including ischemia, electrolyte disbalances and HF (Eisner et al., 2006; Laurita & Rosenbaum, 2008). Moreover, T-wave alternans (electrocardiographic manifestation of cardiac alternans), is recognized as a reliable predictor of VF and sudden cardiac death in patients with HF (Rosenbaum et al., 1994).
Initially, it was thought that cardiac alternans has its origin in a non-linear relationship between the diastolic interval and electrical repolarization, resulting in periodic changes in AP duration. However, more recent studies have suggested that Ca2+ alternans might be a cause rather than a consequence of AP alternans (Chudin et al.,1999; Huser et al., 2000; Diaz et al., 2004;Weiss et al., 2006; Eisner et al., 2006; Laurita & Rosenbaum, 2008). The influence of the Ca2+ transient on AP is attributable to effects on Ca2+ dependent currents, such as the NCX current and ICa, during the AP plateau. The coupling between Ca2+ and membrane potential (Vm) can be either positive or negative. When a large Ca2+ transient increases the duration of the AP via stimulating the inward NCX current (concordant electrical and mechanical activity), the Ca2+–Vm coupling is referred to as positive. When the large Ca2+ transient decreases the duration of AP (discordant electrical and mechanical activity), the Ca–Vm coupling is said to be negative.
In general, Ca2+ alternans is thought to arise when the rate of myocyte Ca2+ cycling cannot keep up with heart rate. In various pathological settings, slowed Ca2+ cycling pushes the heart into alternans even at normal heart rates. Thus, when SERCA Ca2+ transport activity is decreased the SR Ca2+ store can be refilled only on an alternating basis. Increasing SR Ca2+ buffering capacity by overexpressing CASQ2 or introducing low affinity exogenous buffers into the SR should slow functional recharging of the Ca2+ store and has also been shown to cause alternans. The steepness of the relationship between SR Ca2+ content and SR Ca2+ release is another important factor in the development of alternans (Weiss et al., 2006; Eisner et al., 2006). As discussed above, the steepness arises as a result of the combination of stimulatory effects of luminal Ca2+ on RyR function, and the presence of the positive feedback loop inherent to CICR. With a steep Ca2+ release–[Ca2+]SR relationship, the SR will produce a large Ca2+ transient when the SR Ca2+ content is relatively high and a disproportionately small Ca2+ transient when the SR Ca2+ content is relatively low. An especially large Ca2+ transient will cause extrusion from the cell of an extra amount of Ca2+ resulting in an under-filled SR Ca2+ store and hence a smaller Ca2+ transient in the next cycle. This will decrease Ca2+ efflux and increase SR Ca2+ content to a larger extent, such that the Ca2+ transient will again be larger in the third cycle. In most disease conditions including acute ischemia and HF, alternans are thought to be caused by slowed Ca2+ cycling due to inhibition of SERCA (Weiss et al., 2006). However, we recently showed that in a post-infarction model of sudden cardiac death alternans is attributable to increased steepness of the Ca2+ release–[Ca2+]SR relationship (Belevych et al., 2008). In this model abnormal SR Ca2+ release regulation is caused by redox modification of RyRs and can be attenuated by reducing agents.
Given the non-linear dependence of Ca2+ release on [Ca]SR in the development of alternans, beat-to-beat variations in Ca2+ transients are expected to correlate with beat-to-beat variations in SR Ca2+ content. Indeed, such a correlation has been demonstrated experimentally by measuring both total SR Ca2+ content and free [Ca]SR in myocytes exhibiting Ca2+ alternans (Diaz et al., 2004). However, other investigators failed to observe consistent changes in [Ca]SR during alternans (Picht et al., 2006). These studies suggested that processes such as refractoriness control the number of available RyRs during each heart beat, and can produce alternans irrespective of changes in SR Ca2+ content. It is to be noted however, that RyR refractoriness itself appears to be controlled by [Ca]SR (Terentyev et al., 2002; Sobie et al., 2005; Gyorke &; Terentyev, 2008). Thus alterations in the number of available RyRs still would be expected to correlate with alterations in diastolic Ca2+ transients. One potential explanation as to why this is not observed in some studies is that changes are too small to be detected. Alternatively, recovery occurs with a time lag following [Ca]SR recovery, thus providing a window in which alternans can develop without significant alterations in diastolic [Ca]SR.
6.7. Heart failure
Systolic heart failure (HF) is an increasingly prevalent disease and occurs when the cardiac muscle is too weak to pump sufficient blood through the body. Patients with HF typically die either due to ventricular arrhythmias or progressive failure of cardiac mechanical function (pump failure). A recent analysis of the mode of death in patients with heart failure (arrhythmias vs. pump failure) suggests that during earlier (less severe) stages of heart failure, death is seven times more likely to occur from cardiac arrhythmias, while in end-stage HF, death is twice is likely to occur from pump failure (Mozaffarian et al., 2007). Although the pathophysiologic mechanisms of HF are diverse, highly complex and not fully defined, reduced Ca2+ transient amplitude and RyR2 dysfunction with elevated SR Ca2+ leak are increasingly recognized features of the disease process (Marx et al., 2000; Shannon et al., 2003b; Kubalova et al., 2005). Uncontrolled RyR2 gating and increased diastolic SR Ca2+ leak have been shown to cause a reduction in the SR Ca2+ content, thus limiting the ability of cardiac muscle to contract in myocytes from failing hearts (Belevych et al., 2007). Additionally, abnormal RyR2 activity could contribute to the pathogenesis of arrhythmia in patients with HF.
Similar to CPVT, elevated RyR2-mediated SR Ca2+ leak in HF is associated with altered responsiveness of RyRs to luminal Ca2+ (Kubalova et al., 2005). See Fig. 3D. In particular, luminal Ca2+ dependence is markedly left-shifted in RyR2s from failing hearts. As discussed for CPVT, this specific defect may explain why Ca2+ leak remains high even at reduced SR Ca2+ load despite the well established positive relationship between RyR2 activity and SR Ca2+ content. Thus, acquired and genetic alterations in RyR seem to affect RyR behavior in a similar manner. While it is apparent that HF-associated RyR2-mediated SR Ca2+ leak could contribute to 1) depleted SR Ca2+ stores and reduced contractility and/or 2) Ca2+-dependent arrhythmias, the underlying mechanisms of RyR2 dysfunction remains a subject of intense debate.
One proposed mechanism for abnormal RyR behavior involves hyperphosphorylation of RyR2 by PKA (Marx et al., 2000; Wehrens et al., 2004). RyR2 phosphorylation at Ser-2809 reportedly causes dissociation of FKBP12.6 followed by increased RYR activity (see above). Another possibility is that increased RyR activity is caused by CAMKII phosphorylation at Ser-2815 (Ai et al., 2005). However, both the functional effects of phosphorylation on RyR function and the role of abnormal RyR phosphorylation in HF require further clarification. An alternative mechanism for enhanced RyR2 activity is modification of the channel protein by reactive oxygen and/or nitrogen species generated during HF. ROS levels are markedly elevated in failing hearts (Belch et al.,1991; Sawyer et al., 2002; Giordano, 2005) and redox modification is known to stimulate RyR activity (see above). Preliminary results obtained in our laboratory suggest that sulfhydryl oxidation of RyRs contributes to enhanced SR Ca2+ leak in chronic heart failure (Gyorke et al., 2007). Importantly, oxidation impairs the luminal Ca2+ dependence of RyRs in amanner characteristic of HF. At the same time, reducing agents normalize SR Ca2+ leak, RyR activity and luminal Ca2+ sensitivity. Additional studies are required to establish the relative contribution of this mechanism to HF-induced abnormalities. In summary, evidence obtained using variousmodels of HF and in human HF suggests that RyR2s become excessively active i.e., “leaky” in HF. This increase in RyR2 activity has been linked to defective modulation of the channel by SR luminal Ca2+, a mechanism that normally operates to terminate SR Ca2+ release and keep the RyR2 channels closed during diastole (see above). Modest alterations in this control mechanism destabilize CICR causing arrhythmogenic dispersion of Ca2+ signaling, whereas more severe defects result in a massive diastolic leak and consequently the depleted SR Ca2+ stores characteristic of heart failure.
6.8. Atrial fibrillation
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. It has been estimated that the lifetime risk for the development of AF is 1 in 4 after reaching the age of 40 (Lloyd-Jones et al., 2004). Occasionally, AF occurs in the absence of detectable structural heart disease or any other recognizable risk factor. However, most commonly, AF occurs in the presence of known risk factors or diseases, including valvular heart disease, advanced age, hypertension, coronary artery disease, diabetes, hyperthyroidism, chronic obstructive pulmonary disease, and heart failure. AF is known to generally increase the risk of death, and specifically to increase the risk of death during heart failure.(Benjamin et al., 1998; Kammersgaard & Olsen, 2006) Recently, abnormal electrical activity originating in the sleeves of the pulmonary veins (PVs) has been implicated in the initiation of AF, particularly paroxysmal AF. However, non-PV atrial foci have also been clearly identified as a common cause of atrial arrhythmias in those with persistent AF(Haissaguerre et al., 2005a; Haissaguerre et al., 2005b). Thus, the initiation of AF is likely to be a multi-factorial process. Abnormalities in calcium handling have recently been implicated in the pathogenesis of AF. In atrial tissues from patients with chronic AF there are reported reductions in message for L-type Ca2+ channels and SERCA, most commonly without changes in message for phospholamban, CASQ, NCX or RyR2 (VanWagoner et al.,1999; Van Gelder et al.,1999; Brundel et al.,1999; Lai et al.,1999). However, RyR messagewas reportedly reduced in one study, and accompanied by reduced binding of 3H-ryanodine (Ohkusa et al., 1999). In vitro studies in normal tissues have found that activation of RyR2s with ryanodine (0.5–2 µM) results in increased spontaneous electrical activity in the PVs. As has been reported in the ventricle during HF, it has been suggested that hyperphosphorylation of RyR2s contributes to diastolic SR Ca2+ leak and may contribute to the initiation or maintenance of AF (Vest et al., 2005). In this study of atrial tissue from either dogs or humans with chronic AF, there was increased PKA-dependent phosphorylation of RyR2 at serine 2809, reduced FKBP12.6 bound to the RyR2 complex, and increased RyR2 channel open probability, compared to controls. In addition, treatment with JTV519 was reported to normalize AF-induced abnormalities in RyR2 function. More recently, reduced activity of SR-associated PP1 has been reported in atrial tissues from patients with chronic AF (Dobrev, 2006), which could contribute to increased phosphorylation of RyR2s. However in light of recent negative results on the role of FKBP in other disease states these findings should be viewed with caution.
7. Therapeutic strategies
7.1. Arrhythmia
Current antiarrhythmic treatments are often empiric and ineffective. One significant limitation to the effective prevention and treatment of arrhythmias is that the initiating and/or perpetuating event(s) are often unknown in a given patient. For arrhythmias of the atria or ventricles, pharmacologic antiarrhythmic therapies act by blocking fast inward sodium channels (slowing conduction) or blocking repolarizing potassium currents (prolonging refractoriness). Examples of clinically useful sodium channel blockers would include procainamide, lidocaine, mexiletine, flecainide or propafenone. Within these are drugs with pharmacology which varies from pure sodium channel block, (e.g. lidocaine), to procainamide which also prolongs refractoriness through effects on potassium channels, to propafenone which is also a non-selective beta-adrenergic blocker. Examples of clinically useful potassium current blockers include dofetilide, sotalol (also a non-selective beta-adrenergic blocker) and amiodarone (also a blocker of fast inward sodium current, slow inward calcium current, a non-selective beta-adrenergic blocker, and an antioxidant). Non-pharmacologic treatment with implantable cardioverter defibrillators has been shown to be more effective that optimal pharmacologic therapy in multiple clinical trials; although many patients receive a combination of pharmacologic and non-pharmacologic therapy for ventricular tachyarrhythmias (Carnes et al., 1998). Interestingly, the drug with the highest rate of efficacy is amiodarone which is the least pharmacologically selective.
However, for those diagnosed with specific forms of arrhythmic diseases, there may be targeted therapy. For example, catecholaminergic polymorphic ventricular tachycardia (CPVT) is typically treated initially with beta-adrenergic blockers (e.g. nadolol), with implantable cardioverter defibrillators also used for either primary or secondary prevention of cardiac arrest (Liu et al., 2007). Ideally, as arrhythmic mechanisms are better understood, additional targeted and specific therapies can be developed for the treatment and prevention of cardiac arrhythmias.
The antiarrhythmic effects of ryanodine have been investigated in multiple animal models. In canine models, ryanodine has been shown to reduce ouabain-induced (Ca2+ overload) arrhythmias (Lappi & Billman, 1993), but not ischemia-induced arrhythmias (Lappi & Billman, 1993). In addition, ryanodine has demonstrated efficacy in reducing early afterdepolarizations and Torsades de Pointes in a canine model of chronic AV nodal block (Verduyn et al., 1995). Similar to the findings in canines, ryanodine has been shown to reduce ouabain-induced arrhythmias in guinea pigs (Golovina et al., 1988; Zakharov et al., 1991). There is also evidence suggesting that ryanodine may reduce reperfusion-induced arrhythmias (Thandroyen et al., 1988; Hayashi et al., 1996). Thus, ryanodine, by reducing pathologic leak of Ca2+ from the SR, appears to have antiarrhythmic efficacy in multiple models of arrhythmogenesis, in particular when then there is increased SR Ca2+ load (e.g. ouabain toxicity). However, as might be expected, ryanodine is a profound negative inotrope (Thandroyen et al., 1988), and thus optimal therapy should aim to normalize, rather than simply inhibiting RyR2 function in order to maintain systolic function.
7.2. Heart failure
Heart failure is a constellation of symptoms (circulatory congestion, dyspnea, fatigue, and/or weakness) which results when cardiac output is not sufficient to meet the body's metabolic demands. One of the most common defects causing heart failure is impaired contractility of the ventricle (cardiomyopathy or ventricular systolic dysfunction). With respect to calcium cycling, hallmark characteristics of heart failure include impaired contractility, and low amplitude, broad calcium transients in isolated myocytes. Current therapies for heart failure are intended to reduce circulatory congestion, reduce cardiac hypertrophy and structural remodeling, reduce cardiac afterload and preload, and modify adrenergic signaling. Current therapeutic strategies for patients with current or previous symptoms of chronic systolic HF include a diuretic, beta-adrenergic blocker, an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, and in selected patients an aldosterone antagonist (Hunt et al., 2005). Other therapeutic strategies which may be appropriate for specific patient populations include an implantable cardioverter defibrillator, cardiac resynchronization therapy, digoxin and/or combined hydralazine and nitrate therapy. The ideal therapy for HF would be one that increases contractility while preserving relaxation (lusitropy) and avoiding proarrhythmia (the development of new arrhythmias or worsening of existing arrhythmias). Given the Ca2+-dependence of both arrhythmia initiation and contractility this remains a difficult and challenging goal.
7.3. Normalizing ryanodine receptor function as a treatment strategy for cardiac disease
Since RyR Ca2+ leak contributes to various disease phenotypes ranging from uncontrolled Ca2+ release and SR depletion, simply blocking RyRs is not a viable strategy for treating arrythmia or HF in general. Selectivity for RyR2s (relative to the RyR1 complexes in skeletal muscle) would be critical for any clinically viable therapy targeting RyR2 modulation. Additionally, implementing a successful therapy based on RyR2 targeting could be challenging due to the complex nature of store-mediated Ca2+ signaling. Indeed, inhibition of RyR2s may fix the leak, but has the potential to impair systolic function. Moreover, inhibition of RyR2s can also result in inhomogeneities of Ca2+ release that can be arrythmogenic (Diaz et al., 2002). Still RyR inhibitors, such as an RyR2-selective derivative of tetracaine, might prove to be useful for treating certain conditions when the SR Ca2+ leak is excessively large such as end-stage HF. Indeed, Eisner and coworkers (Venetucci et al., 2006) showed that tetracaine increased Ca2+ transients by increasing the SR Ca2+ load in myocytes exposed to isoproterenol. A caveat is that the therapeutic action of such an agent would be highly concentration-dependent such that at high concentrations it could inhibit systolic Ca2+ release.
RyR2 inhibitors could be potentially used in combination with other agents to enhance SERCA-mediated SR Ca2+ uptake thus restoring further SR Ca2+ content. Although potentially useful for treating end-stage HF where the SR Ca2+ content is greatly diminished, both strategies could be detrimental by increasing the risk of arrhythmia. For example, tetracaine can cause homogeneities in release, while adrenergic stimulation (which occurs in response to HF) in isolation can promote arrhythmias. Given these problems with using RyR2 antagonists, targeting the underlying causes of altered RyR2 function appears to be a more promising approach and developing such therapies might be the ultimate goal in treating RyR2-linked cardiac diseases. Currently several such strategies are emerging based on specific mechanisms proposed to underpin dysregulation of RyR2s in cardiac disease, including abnormal PKA and/or CAMKII phosphorylation and/or altered redox modification.
Much attention has been devoted to a Ca2+ channel blocker derivative with reported beneficial effects in HF and a range of reported intracellular targets such as PKC, mitochondrial K+ channels, and RyR2s. It has been suggested that JTV519 improves Ca2+ handling in both HF and CPVT by stabilizing FKBP12.6 binding to RyR2s thereby reducing the RyR-mediated SR Ca2+ leak (Yano et al., 2003; Wehrens et al., 2005b; Lehnart et al., 2006). However this mode of action of JTV519 has been disputed by other investigators, further adding to the uncertainties regarding the role of FKBP12.6 in HF-related alterations in RyRs. While most studies agree that JTV519 improves cardiac function in HF, its beneficial effects in CPVT are not supported by recent investigations (Liu et al., 2006; Hunt et al., 2007; George et al., 2007). This may indicate that the beneficial effects of JTV519 on cardiac function in HF do not involve RyRs, or that the RyR defects in CPVT and HF are caused by different mechanisms.
Given the recent evidence that RyR-mediated alterations in HF and in certain types of arrhythmia are mediated by CAMK2-phosphorylation of RyR (Ai et al., 2005; Grueter et al., 2007) targeting CAMK might be another strategy to normalize RyR function in cardiac disease. Finally, preventing/reversing pathologic RyR modification by reactive oxygen/nitrogen species has been recently proposed as a strategy to treat HF and arrhythmias caused by abnormal Ca2+ release. The ability of antioxidants to improve cardiac performance was demonstrated in a number of studies using animal models of HF (e.g. Mochizuki et al., 2007). However, a recent clinical trial examining xanthine oxidase inhibition was not successful (Hare et al., 2006). It is unclear to what extent this lack of success is due to the particular antioxidants used. A better understanding of the specific redox pathway abnormalities and the affected Ca2+ signaling mechanisms may enable the development of new rationally targeted therapeutic strategies.
8. Conclusion
In recent years there has been significant progress in understanding Ca2+ handling in the heart and how changes in Ca2+ homeostasis contribute to cardiac disease. Exploring the functional consequences of naturally occurring arrhythmogenic mutations in Ca2+ handling proteins such as RyR2 and CASQ2 has been especially instructive. These studies have led to a better understanding of the control of SR Ca2+ release at the molecular level in normal and disease settings. An important question for future studies is how derangement of one aspect of Ca2+ handling, i.e. Ca2+ release through RyR2s, can result in awide spectrum of disease phenotypes ranging from arrhythmogenic Ca2+ alternans and dispersed SR Ca2+ release to depleted SR Ca2+ stores in HF. A comprehensive understanding of these mechanisms may provide a conceptual framework for the development of individualized, targeted therapy of cardiac diseases.
Abbreviations
- AF
atrial fibrillation
- AP
action potential
- CAMKII
calmodulin-dependent protein kinase II
- CASQ
calsequestrin
- CN
calcineurin
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAD
delayed afterdepolarization
- DTT
dithiothreitol
- EAD
early afterdepolarization
- FKBP
FK binding protein
- fSR
free sarcoplasmic reticulum
- HF
heart failure
- JN
junctin
- jSR
junctional sarcoplasmic reticulum
- NCX
sodium–calcium exchanger
- PKA
protein kinase A
- PLB
phospholamban
- PV
pulmonary vein
- QTc
rate-corrected QT interval of the electrocardiogram
- RyR
ryanodine receptor
- SERCA
sarco-endoplasmic reticulum calcium ATPase
- TRD
triadin
- Vm
membrane voltage
References
- Abramson JJ, Salama G. Sulfhydryl oxidation and Ca2+ release from sarcoplasmic reticulum. Mol Cell Biochem. 1988;82:81–84. doi: 10.1007/BF00242520. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Junankar PR, Dulhunty AF. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994;352:369–374. doi: 10.1016/0014-5793(94)01001-3. [DOI] [PubMed] [Google Scholar]
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Allen DG, Eisner DA, Orchard CH. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoons G, Volders PG, Stankovicova T, Bito V, Stengl M, Vos MA, et al. Window Ca2+ current and its modulation by Ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block. J Physiol. 2007;579:147–160. doi: 10.1113/jphysiol.2006.124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Shin DW, Ahn JO, Kim DH. Calcineurin regulates ryanodine receptor/Ca(2+)-release channels in rat heart. Biochem J. 2000;352(Pt 1):61–70. [PMC free article] [PubMed] [Google Scholar]
- Barg S, Copello JA, Fleischer S. Different interactions of cardiac and skeletal muscle ryanodine receptors with FK-506 binding protein isoforms. Am J Physiol. 1997;272:C1726–C1733. doi: 10.1152/ajpcell.1997.272.5.C1726. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65:245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007 doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A, Terentyev D, Kubalova Z, Viatchenko-Karpinski S, Sridhar A, del Rio C, et al. Altered redox-modulation of ryanodine receptor associated with Catransient alternans in a canine model of sudden cardiac death. Biophys J. 2008;94 Suppl. S:491. [Google Scholar]
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- Benkusky NA, Farrell EF, Valdivia HH. Ryanodine receptor channelopathies. Biochem Biophys Res Commun. 2004;322:1280–1285. doi: 10.1016/j.bbrc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. 2 ed. Dordrect, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann NY Acad Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- Bridge JH, Ershler PR, Cannell MB. Properties of Ca2+ sparks evoked by action potentials in mouse ventricular myocytes. J Physiol. 1999;518(Pt 2):469–478. doi: 10.1111/j.1469-7793.1999.0469p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol. 2000;32:2131–2139. doi: 10.1006/jmcc.2000.1270. [DOI] [PubMed] [Google Scholar]
- Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci USA. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Deelman LE, Tieleman RG, et al. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc Res. 1999;42:443–454. doi: 10.1016/s0008-6363(99)00045-0. [DOI] [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH, Jorgensen AO, Mintzer MC. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem. 1983;258:1197–1204. [PubMed] [Google Scholar]
- Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res. 1987;61:498–503. doi: 10.1161/01.res.61.4.498. [DOI] [PubMed] [Google Scholar]
- Carnes CA, Mehdirad AA, Nelson SD. Drug and defibrillator interactions. Pharmacotherapy. 1998;18:516–525. [PubMed] [Google Scholar]
- Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, et al. Type 1 phosphatase, a negative regulator of cardiac functionc. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Colyer J, Sitsapesan R. Maximum phosphorylation of the cardiac ryanodine receptor at serine-2809 by protein kinase a produces unique modifications to channel gating and conductance not observed at lower levels of phosphorylation. Circ Res. 2006;98:1506–1513. doi: 10.1161/01.RES.0000227506.43292.df. [DOI] [PubMed] [Google Scholar]
- Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O'Connell R, Berenfeld O, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca(2+) dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz ME, Trafford AW, O'Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol. 1997;501(Pt 1):3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz ME, Eisner DA, O'Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–593. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- Diaz ME, O'Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- Dobrev D. Electrical remodeling in atrial fibrillation. Herz. 2006;31:108–112. doi: 10.1007/s00059-006-2787-9. [DOI] [PubMed] [Google Scholar]
- Donoso P, Aracena P, Hidalgo C. Sulfhydryl oxidation overrides Mg(2+) inhibition of calcium-induced calcium release in skeletal muscle triads. Biophys J. 2000;79:279–286. doi: 10.1016/S0006-3495(00)76290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBell WH, Lederer WJ, Rogers TB. Dynamic modulation of excitation–contraction coupling by protein phosphatases in rat ventricular myocytes. J. Physiol. 1996;493(Pt 3):793–800. doi: 10.1113/jphysiol.1996.sp021423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBell WH, Gigena MS, Guatimosim S, Long X, Lederer WJ, Rogers TB. Effects of PP1/PP2A inhibitor calyculin A on the E–C coupling cascade in murine ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H38–H48. doi: 10.1152/ajpheart.00536.2001. [DOI] [PubMed] [Google Scholar]
- Eager KR, Roden LD, Dulhunty AF. Actions of sulfhydryl reagents on single ryanodine receptor Ca(2+)-release channels from sheep myocardium. Am J Physiol. 1997;272:C1908–C1918. doi: 10.1152/ajpcell.1997.272.6.C1908. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW, Diaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Li Y, O'Neill SC. Alternans of intracellular calcium: mechanism and significance. Heart Rhythm. 2006;3:743–745. doi: 10.1016/j.hrthm.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Eldar M, Pras E, Lahat H. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Cold Spring Harbor Symp Quant Biol. 2002;67:333–337. doi: 10.1101/sqb.2002.67.333. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Excitation–contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972;31:293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, McNutt NS. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969;42:1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier GR, Saunders JH, Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973;32:600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Kenney LJ, Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell Biol. 1987;105:49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA, Zakharov SI, Bogdanov KY, Rosenshtraukh LV. Analysis of antiarrhythmic effect of ryanodine in guinea-pigs. J Mol Cell Cardiol. 1988;20:303–311. doi: 10.1016/s0022-2828(88)80064-6. [DOI] [PubMed] [Google Scholar]
- Grueter CE, Colbran RJ, Anderson ME. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J Mol Med. 2007;85:5–14. doi: 10.1007/s00109-006-0125-6. [DOI] [PubMed] [Google Scholar]
- Guo W, Jorgensen AO, Jones LR, Campbell KP. Biochemical characterization and molecular cloning of cardiac triadin. J Biol Chem. 1996;271:458–465. doi: 10.1074/jbc.271.1.458. [DOI] [PubMed] [Google Scholar]
- Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res. 2007;101:802–810. doi: 10.1161/CIRCRESAHA.107.152140. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Nishijima Y, Feldman DS, et al. Normalization of intracellular calcium signaling by chronic cardiac resynchronization involves redox modulation of ryanodine receptors. Circulation. 2007;116(II):86. [Google Scholar]
- Hain J, Nath S, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from skeletal muscle. Biophys J. 1994;67:1823–1833. doi: 10.1016/S0006-3495(94)80664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- Hare JM, Mangal B, Brown J, Fisher C, Freudenberger RS, Colucci WS, et al. Efficacy and safety study of oxypurinol added to standard therapy in patients with New York Heart Association Class III–IV Congestive Heart Failure (The OPT-CHF trial) J Card Fail. 2006;12(9):764. [Google Scholar]
- Hayashi H, Terada H, Katoh H, McDonald TF. Prevention of reoxygenation-induced arrhythmias in guinea pig papillary muscles. J Cardiovasc Pharmacol. 1996;27:816–823. doi: 10.1097/00005344-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Huffaker R, Lamp ST, Weiss JN, Kogan B. Intracellular calcium cycling, early afterdepolarizations, and reentry in simulated long QT syndrome. Heart Rhythm. 2004;1:441–448. doi: 10.1016/j.hrthm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed]
- Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation–contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N, Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front Biosci. 2002;7:d671–d683. doi: 10.2741/A803. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Brillantes AM, Timerman AP, Fleischer S, Erdjument-Bromage H, Tempst P, et al. FK506 binding protein associated with the calcium release channel (ryanodine receptor) J Biol Chem. 1992;267:9474–9477. [PubMed] [Google Scholar]
- Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, et al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- Jorgensen AO, Campbell KP. Evidence for the presence of calsequestrin in two structurally different regions of myocardial sarcoplasmic reticulum. J Cell Biol. 1984;98:1597–1602. doi: 10.1083/jcb.98.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftan E, Marks AR, Ehrlich BE. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circ Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- Kammersgaard LP, Olsen TS. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis. 2006;21:187–193. doi: 10.1159/000090531. [DOI] [PubMed] [Google Scholar]
- Kass RS, Lederer WJ, Tsien RW, Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Kao JP, Egger M, Niggli E. Calcium waves driven by “sensitization” wave-fronts. Cardiovasc Res. 2007;74:39–45. doi: 10.1016/j.cardiores.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Kim E, Shin DW, Hong CS, Jeong D, Kim DH, Park WJ. Increased Ca2+ storage capacity in the sarcoplasmic reticulum by overexpression of HRC (histidine-rich Ca2+ binding protein) Biochem Biophys Res Commun. 2003;300:192–196. doi: 10.1016/s0006-291x(02)02829-2. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Neumann J, Baba HA, Begrow F, Kobayashi YM, Reinke U, et al. Cardiac hypertrophy and impaired relaxation in transgenic mice overexpressing triadin 1. J Biol Chem. 2001;276:4142–4149. doi: 10.1074/jbc.M006443200. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Neumann J, Bers DM, Buchwalow IB, Fabritz L, Hanske G, et al. Impaired relaxation in transgenic mice overexpressing junctin. Cardiovasc Res. 2003;59:369–379. doi: 10.1016/s0008-6363(03)00432-2. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Hanske G, Jones LR, Justus I, Kaestner L, Lipp P, et al. Overexpression of junctin causes adaptive changes in cardiac myocyte Ca(2+) signaling. Cell Calcium. 2006;39:131–142. doi: 10.1016/j.ceca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi YM, Jones LR. Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium. J Biol Chem. 1999;274:28660–28668. doi: 10.1074/jbc.274.40.28660. [DOI] [PubMed] [Google Scholar]
- Kobayashi YM, Alseikhan BA, Jones LR. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J Biol Chem. 2000;275:17639–17646. doi: 10.1074/jbc.M002091200. [DOI] [PubMed] [Google Scholar]
- Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- Kubalova Z, Gyorke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, et al. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat H, Pras E, Eldar M. A missense mutation in CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Ann Med. 2004;36 Suppl 1:87–91. doi: 10.1080/17431380410032517. [DOI] [PubMed] [Google Scholar]
- Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS, et al. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33:1231–1237. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Laitinen PJ, Swan H, Piippo K, Viitasalo M, Toivonen L, Kontula K. Genes, exercise and sudden death: molecular basis of familial catecholaminergic polymorphic ventricular tachycardia. Ann Med. 2004;36 Suppl 1:81–86. doi: 10.1080/17431380410032599. [DOI] [PubMed] [Google Scholar]
- Lappi MD, Billman GE. Effect of ryanodine on ventricular fibrillation induced by myocardial ischaemia. Cardiovasc Res. 1993;27:2152–2159. doi: 10.1093/cvr/27.12.2152. [DOI] [PubMed] [Google Scholar]
- Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008;44:31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ, Tsien RW. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976;263:73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci USA. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- Liu N, Priori SG. Disruption of calcium homeostasis and arrhythmogenesis induced by mutations in the cardiac ryanodine receptor and calsequestrin. Cardiovasc Res. 2008;77:293–301. doi: 10.1093/cvr/cvm004. [DOI] [PubMed] [Google Scholar]
- Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- Liu N, Colombi B, Raytcheva-Buono EV, Bloise R, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Herz. 2007;32:212–217. doi: 10.1007/s00059-007-2975-2. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. J Physiol. 1995;487(Pt 3):609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke I, Gyorke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricularmyocytes. J Physiol. 1999;518(Pt 1):173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke I, Subramanian S, Smirnov A, Wiesner TF, Gyorke S. Inhibition of Ca(2+) sparks by ruthenium red in permeabilized rat ventricular myocytes. Biophys J. 2000;79:1273–1284. doi: 10.1016/S0006-3495(00)76381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Dynamic regulation of sarcoplasmic reticulum Ca(2+) content and release by luminal Ca(2+)-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mitchell RD, Simmerman HK, Jones LR. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988;263:1376–1381. [PubMed] [Google Scholar]
- Mochizuki M, Yano M, Oda T, Tateishi H, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Scavenging free radicals by lowdose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–398. doi: 10.1161/CIRCULATIONAHA.106.687103. [DOI] [PubMed] [Google Scholar]
- Nakai J, Imagawa T, Hakamat Y, Shigekawa M, Takeshima H, Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990;271:169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675–678. doi: 10.1016/j.hrthm.2006.12.048. [DOI] [PubMed] [Google Scholar]
- Neumann J. Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res Cardiol. 2002;97 Suppl 1:I91–I95. doi: 10.1007/s003950200036. [DOI] [PubMed] [Google Scholar]
- Neumann J, Boknik P, Herzig S, Schmitz W, Scholz H, Gupta RC, et al. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am J Physiol. 1993;265:H257–H266. doi: 10.1152/ajpheart.1993.265.1.H257. [DOI] [PubMed] [Google Scholar]
- Ohkusa T, Ueyama T, Yamada J, Yano M, Fujumura Y, Esato K, et al. Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J Am Coll Cardiol. 1999;34:255–263. doi: 10.1016/s0735-1097(99)00169-2. [DOI] [PubMed] [Google Scholar]
- Overend CL, O'Neill SC, Eisner DA. The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes. J Physiol. 1998;507(Pt 3):759–769. doi: 10.1111/j.1469-7793.1998.759bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, et al. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem. 2004;279:18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- Pouliquin P, Pace SM, Curtis SM, Harvey PJ, Gallant EM, Zorzato F, et al. Effects of an alpha-helical ryanodine receptor C-terminal tail peptide on ryanodine receptor activity: modulation by Homer. Int J Biochem Cell Biol. 2006;38:1700–1715. doi: 10.1016/j.biocel.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- Ringer S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol. 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM. Early after-depolarizations and torsade de pointes: implications for the control of cardiac arrhythmias by prolonging repolarization. Eur Heart J. 1993;14 Suppl H:56–61. doi: 10.1093/eurheartj/14.suppl_h.56. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- Rosen MR. Cellular electrophysiology of digitalis toxicity. J Am.Coll.Cardiol. 1985;5:22A–34A. doi: 10.1016/s0735-1097(85)80460-5. [DOI] [PubMed] [Google Scholar]
- Rosen MR, Legato MJ. Repolarization: physiological and structural determinants, and pathophysiological changes. Eur Heart J. 1985;6 Suppl D:3–14. doi: 10.1093/eurheartj/6.suppl_d.3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol. 2002;34:379–388. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Bers DM. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys J. 1997;73:1524–1531. doi: 10.1016/S0006-3495(97)78184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- Shin DW, Ma J, Kim DH. The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca(2+) and interacts with triadin. FEBS Lett. 2000;486:178–182. doi: 10.1016/s0014-5793(00)02246-8. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Maes M, Van de WF. Low efficiency of Ca2+ entry through the Na(+)–Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na(+)–Ca2+ exchange. Circ Res. 1997;81:1034–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- Sobie EA, Dilly KW, dos Santos CJ, Lederer WJ, Jafri MS. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie EA, Song LS, Lederer WJ. Local recovery of Ca2+ release in rat ventricular myocytes. J Physiol. 2005;565:441–447. doi: 10.1113/jphysiol.2005.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie EA, Cannell MB, Bridge JH. Allosteric activation of Na+–Ca2+exchange by L-type Ca2+ current augments the trigger flux for SR Ca2+ release in ventricular myocytes. Biophys J. 2008;94:L54–L56. doi: 10.1529/biophysj.107.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AV, Gonzalez-Serratos HG, Shuman H, McClellan G, Somlyo AP. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981;90:577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JR. Comparative anatomy: in praise of a powerful approach to elucidate mechanisms translating cardiac excitation into purposeful contraction. J Mol Cell Cardiol. 1995;27:19–35. doi: 10.1016/s0022-2828(08)80004-1. [DOI] [PubMed] [Google Scholar]
- Spencer CI, Sham JS. Effects of Na+/Ca2+ exchange induced by SR Ca2+ release on action potentials and afterdepolarizations in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2552–H2562. doi: 10.1152/ajpheart.00274.2003. [DOI] [PubMed] [Google Scholar]
- Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- Stern MD, Cheng H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium. 2004;35:591–601. doi: 10.1016/j.ceca.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Stern MD, Capogrossi MC, Lakatta EG. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988;9:247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ Res. 2004;95:807–813. doi: 10.1161/01.RES.0000146029.80463.7d. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ozawa T, Maurer A, Cortese JD, Fleischer S. Apparent cooperativity of Ca2+ binding associated with crystallization of Ca2+-binding protein from sarcoplasmic reticulum. Arch Biochem Biophys. 1986;251:369–378. doi: 10.1016/0003-9861(86)90084-6. [DOI] [PubMed] [Google Scholar]
- Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinskid S, Gyorke I, Terentyeva R, Gyorke S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J Physiol. 2003;552:109–118. doi: 10.1113/jphysiol.2003.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: mechanism for hereditary arrhythmia. Proc Natl Acad Sci USA. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, et al. Triadin overexpression stimulates excitation–contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ. Res. 2005;96:651–658. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, et al. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Vedamoorthyrao S, Oduru S, Gyorke I, Williams SC, et al. Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J Physiol. 2007;583:71–80. doi: 10.1113/jphysiol.2007.136879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, et al. Modulation of SR Ca release by luminal ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008 doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandroyen FT, McCarthy J, Burton KP, Opie LH. Ryanodine and caffeine prevent ventricular arrhythmias during acute myocardial ischemia and reperfusion in rat heart. Circ Res. 1988;62:306–314. doi: 10.1161/01.res.62.2.306. [DOI] [PubMed] [Google Scholar]
- Tijskens P, Jones LR, Franzini-Armstrong C. Junctin and calsequestrin overexpression in cardiac muscle: the role of junctin and the synthetic and delivery pathways for the two proteins. J Mol Cell Cardiol. 2003;35:961–974. doi: 10.1016/s0022-2828(03)00181-0. [DOI] [PubMed] [Google Scholar]
- Timerman AP, Ogunbumni E, Freund E, Wiederrecht G, Marks AR, Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993;268:22992–22999. [PubMed] [Google Scholar]
- Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, et al. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effecton systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522(Pt 2):259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Integrative analysis of calcium signalling in cardiac muscle. Front Biosci. 2002;7:d843–d852. doi: 10.2741/trafford. [DOI] [PubMed] [Google Scholar]
- Valdivia HH. Modulation of intracellular Ca2+ levels in the heart by sorcin and FKBP12, two accessory proteins of ryanodine receptors. Trends Pharmacol Sci1. 1998;9:479–482. doi: 10.1016/s0165-6147(98)01269-3. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Brundel BJ, Henning RH, Tuinenburg AE, Tieleman RG, Deelman L, et al. Alterations in gene expression of proteins involved in the calcium handling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:552–560. doi: 10.1111/j.1540-8167.1999.tb00712.x. [DOI] [PubMed] [Google Scholar]
- VanWagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- Venetucci LA, Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Reducing ryanodine receptor open probability as a means to abolish spontaneous Ca2+ release and increase Ca2+ transient amplitude in adult ventricular myocytes. Circ Res. 2006;98:1299–1305. doi: 10.1161/01.RES.0000222000.35500.65. [DOI] [PubMed] [Google Scholar]
- Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- Verduyn SC, Vos MA, Gorgels AP, van der ZJ, Leunissen JD, Wellens HJ. The effect of flunarizine and ryanodine on acquired torsades de pointes arrhythmias in the intact canine heart. J Cardiovasc Electrophysiol. 1995;6:189–200. doi: 10.1111/j.1540-8167.1995.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Terentyev D, Jenkins LA, Lutherer LO, Gyorke S. Synergistic interactions between Ca2+ entries through L-type Ca2+channels and Na+–Ca2+ exchanger in normal andfailing rat heart. J Physiol. 2005;567:493–504. doi: 10.1113/jphysiol.2005.091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Trumble WR, Liao H, Wesson CR, Dunker AKq, Kang CH. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol. 1998;5:476–483. doi: 10.1038/nsb0698-476. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, van der NR, Morales R, Sun J, et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- Wier WG, Hess P. Excitation–contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984;83:395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, et al. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium. 2007;42:363–371. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, et al. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Tian X, Jones PP, Bolstad J, Kong H, Wang R, et al. Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J Biol Chem. 2007;282:34828–34838. doi: 10.1074/jbc.M707423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys J. 1998;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, et al. Ca2+/calmodulin kinase II-dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ Res. 2007;100:399–407. doi: 10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest. 2005;115:556–564. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, et al. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- Zakharov SI, Bogdanov KY, Golovina VA. Effects of ryanodine on ouabain-induced spontaneous mechanical and electrical oscillations in guinea-pig heart. J Mol Cell Cardiol. 1991;23 Suppl 1:41–46. doi: 10.1016/0022-2828(91)90022-e. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- Zhang L, Franzini-Armstrong C, Ramesh V, Jones LR. Structural alterations in cardiac calcium release units resulting from overexpression of junctin. J Mol Cell Cardiol. 2001;33:233–247. doi: 10.1006/jmcc.2000.1295. [DOI] [PubMed] [Google Scholar]
- Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006 doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum ca release evokes long-lasting ca release events in ventricular myocytes: role of luminal ca in termination of ca release. Biophys J. 2008;94:1867–1879. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]