Abstract
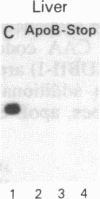
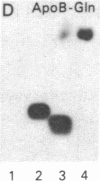
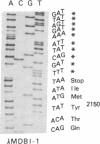
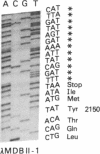
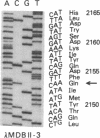
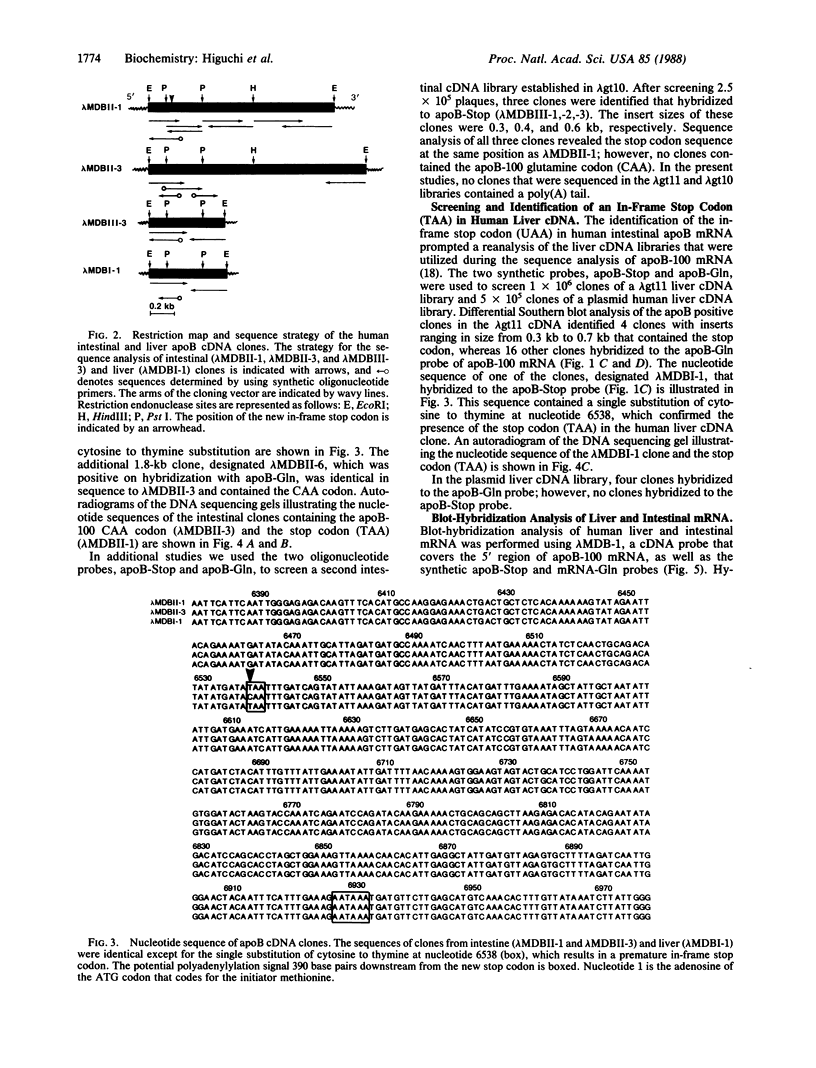
Human apolipoprotein B (apoB) is present in plasma as two separate isoproteins, designated apoB-100 (512 kDa) and apoB-48 (250 kDa). ApoB is encoded by a single gene on chromosome 2, and a single nuclear mRNA is edited and processed into two separate apoB mRNAs. A 14.1-kilobase apoB mRNA codes for apoB-100, and the second mRNA, which codes for apoB-48, contains a premature stop codon generated by a single base substitution of cytosine to uracil at nucleotide 6538, which converts the translated CAA codon coding for the amino acid glutamine at residue 2153 in apoB-100 to a premature in-frame stop codon (UAA). Two 30-base synthetic oligonucleotides (nucleotides 6523-6552 of apoB mRNA), designated apoB-Stop and apoB-Gln, were synthesized containing the complementary sequence to the stop codon (UAA) and glutamine codon (CAA), respectively. Analysis of intestinal apoB mRNA by hybridization with apoB-Stop and apoB-Gln probes and sequence analysis of apoB clones in two independent human small intestinal cDNA libraries established that intestinal apoB mRNA contained both the apoB mRNA that codes for apoB-100 and the apoB mRNA containing the premature in-frame stop codon, which codes for apoB-48. Investigation of hepatic apoB mRNA and two hepatic cDNA libraries by hybridization with the apoB-Stop and apoB-Gln synthetic probes as well as by cDNA sequencing revealed that liver apoB mRNA also contains both the apoB-100 mRNA and the apoB-48 mRNA containing the stop codon. The combined results from these studies establish that both human intestine and liver contain the two distinct apoB mRNAs, an mRNA that codes for apoB-100 and an apoB mRNA that contains the premature stop codon, which codes for apoB-48. The premature in-frame stop codon is not tissue specific and is present in both human liver and intestine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Dullaart R. P., Speelberg B., Schuurman H. J., Milne R. W., Havekes L. M., Marcel Y. L., Geuze H. J., Hulshof M. M., Erkelens D. W. Epitopes of apolipoprotein B-100 and B-48 in both liver and intestine. Expression and evidence for local synthesis in recessive abetalipoproteinemia. J Clin Invest. 1986 Nov;78(5):1397–1404. doi: 10.1172/JCI112727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge S. B., Hoeg J. M., Schneider P. D., Brewer H. B., Jr Apolipoprotein B synthesis in humans: liver synthesizes only apolipoprotein B-100. Metabolism. 1985 Aug;34(8):726–730. doi: 10.1016/0026-0495(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Elovson J., Huang Y. O., Baker N., Kannan R. Apolipoprotein B is structurally and metabolically heterogeneous in the rat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):157–161. doi: 10.1073/pnas.78.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Rogers M., Glickman J. N. Apolipoprotein B synthesis by human liver and intestine in vitro. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5296–5300. doi: 10.1073/pnas.83.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Monge J. C., Lee N., Law S. W., Brewer H. B., Jr, Sakaguchi A. Y., Naylor S. L. The human apoB-100 gene: apoB-100 is encoded by a single copy gene in the human genome. Biochem Biophys Res Commun. 1987 May 14;144(3):1332–1339. doi: 10.1016/0006-291x(87)91456-2. [DOI] [PubMed] [Google Scholar]
- Hospattankar A. V., Higuchi K., Law S. W., Meglin N., Brewer H. B., Jr Identification of a novel in-frame translational stop codon in human intestine apoB mRNA. Biochem Biophys Res Commun. 1987 Oct 14;148(1):279–285. doi: 10.1016/0006-291x(87)91107-7. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Morrisett J. D., Gotto A. M., Jr Lipoprotein structure and metabolism. Physiol Rev. 1976 Apr;56(2):259–316. doi: 10.1152/physrev.1976.56.2.259. [DOI] [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah K. V., Walker L. F., Borensztajn J., Schonfeld G., Getz G. S. Apolipoprotein B variant derived from rat intestine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3806–3810. doi: 10.1073/pnas.77.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner K. J., Law S. W., Brewer H. B., Jr The human apolipoprotein A-II gene: complete nucleic acid sequence and genomic organization. Nucleic Acids Res. 1985 Jun 25;13(12):4597–4608. doi: 10.1093/nar/13.12.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. W., Grant S. M., Higuchi K., Hospattankar A., Lackner K., Lee N., Brewer H. B., Jr Human liver apolipoprotein B-100 cDNA: complete nucleic acid and derived amino acid sequence. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8142–8146. doi: 10.1073/pnas.83.21.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. W., Gray G., Brewer H. B., Jr cDNA cloning of human apoA-I: amino acid sequence of preproapoA-I. Biochem Biophys Res Commun. 1983 Apr 15;112(1):257–264. doi: 10.1016/0006-291x(83)91824-7. [DOI] [PubMed] [Google Scholar]
- Law S. W., Lackner K. J., Hospattankar A. V., Anchors J. M., Sakaguchi A. Y., Naylor S. L., Brewer H. B., Jr Human apolipoprotein B-100: cloning, analysis of liver mRNA, and assignment of the gene to chromosome 2. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8340–8344. doi: 10.1073/pnas.82.24.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. W., Lee N., Monge J. C., Brewer H. B., Jr, Sakaguchi A. Y., Naylor S. L. Human ApoB-100 gene resides in the p23----pter region of chromosome 2. Biochem Biophys Res Commun. 1985 Sep 16;131(2):1003–1012. doi: 10.1016/0006-291x(85)91339-7. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Koren E., Singh S., Mok T. Presence of B-100 in rat mesenteric chyle. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1149–1156. doi: 10.1016/s0006-291x(84)80253-3. [DOI] [PubMed] [Google Scholar]
- Osborne J. C., Jr, Brewer H. B., Jr The plasma lipoproteins. Adv Protein Chem. 1977;31:253–337. doi: 10.1016/s0065-3233(08)60220-x. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks C. E., Hnatiuk O., Marsh J. B. Hepatic and intestinal contribution of two forms of apolipoprotein B to plasma lipoprotein fractions in the rat. Can J Biochem. 1981 Aug;59(8):693–699. doi: 10.1139/o81-096. [DOI] [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Variant forms of plasma apolipoprotein B. Hepatic and intestinal biosynthesis and heterogeneous metabolism in the rat. J Biol Chem. 1981 Apr 25;256(8):3615–3618. [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Scott T. M., Dubois B. W., Curtiss L. K., Witztum J. L. Parallel expression of the MB19 genetic polymorphism in apoprotein B-100 and apoprotein B-48. Evidence that both apoproteins are products of the same gene. J Biol Chem. 1986 Mar 5;261(7):2995–2998. [PubMed] [Google Scholar]