Abstract
Quorum-sensing (QS) regulates the production of key virulence factors in Pseudomonas aeruginosa and other important pathogenic bacteria. In this report, extracts of leaves and bark of Combretum albiflorum (Tul.) Jongkind (Combretaceae) were found to quench the production of QS-dependent factors in P. aeruginosa PAO1. Chromatographic fractionation of the crude active extract generated several active fractions containing flavonoids, as shown by their typical spectral features. Purification and structural characterization of one of the active compounds led to the identification of the flavan-3-ol catechin [(2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol]. The identity of catechin as one of the active molecules was confirmed by comparing the high-pressure liquid chromatography profiles and the mass spectrometry spectra obtained for a catechin standard and for the active C. albiflorum fraction. Moreover, standard catechin had a significant negative effect on pyocyanin and elastase productions and biofilm formation, as well as on the expression of the QS-regulated genes lasB and rhlA and of the key QS regulatory genes lasI, lasR, rhlI, and rhlR. The use of RhlR- and LasR-based biosensors indicated that catechin might interfere with the perception of the QS signal N-butanoyl-l-homoserine lactone by RhlR, thereby leading to a reduction of the production of QS factors. Hence, catechin, along with other flavonoids produced by higher plants, might constitute a first line of defense against pathogenic attacks by affecting QS mechanisms and thereby virulence factor production.
Pseudomonas aeruginosa is a gram-negative bacterium infecting insects, plants, animals, and humans (65). As an opportunistic pathogen, P. aeruginosa is a major cause of nosocomial diseases and mortality in immunocompromised patients and particularly in patients with cystic fibrosis, diffused panbronchitis, and pulmonary deficiencies (21, 54). Successful infection of diverse hosts is due to the profusion and diversity of virulence factors secreted by P. aeruginosa such as proteases, exopolysaccharides and redox-active compounds, as well as to its capacity to form biofilms (9, 60, 62).
Many pathogenic bacteria trigger the production of their virulence factors in a population density-dependent manner, a cell-to-cell communication mechanism known as quorum sensing (QS) (24). This mechanism enables bacteria to detect their population density through the production, release, and perception of small diffusible molecules called autoinducers and to coordinate gene expression accordingly (7, 9, 13, 24, 84). In P. aeruginosa, two QS systems (las and rhl) drive the production (by the synthetases LasI and RhlI) and the perception (by the transcription factors LasR and RhlR) of the acyl-homoserine lactones (AHL) N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), respectively (9, 62). Once LasR interacts with 3-oxo-C12-HSL, it induces the las system (by increasing lasI expression) and triggers the production of LasB elastase, LasA protease, Apr alkaline protease, and exotoxin A (9, 25, 26, 73, 79, 80). The las system is also required for the development of P. aeruginosa biofilms (16). RhlR interacts with C4-HSL, resulting in an enhancement of the production of rhamnolipids, pyocyanin, LasB elastase, hydrogen cyanide, and cytotoxic lectins (9, 12, 42, 57, 85). The rhl system is also regulated, at the transcriptional and posttranscriptional levels, by the las system in a hierarchical manner (62). In P. aeruginosa, a third QS system, based on quinolone signals, interacts with the AHL systems in an intricate way (61, 81). Since QS systems regulate fundamental virulence processes in many pathogenic bacteria, interfering with this cell-to-cell communication mechanism is a rational strategy to attenuate their virulence with the hope of developing a new drug arsenal to counterbalance the emergence of antibiotic-resistant pathogens (8, 29, 52). Anti-QS compounds have been identified from chemical synthesis programs (34, 55) and from natural sources (28, 30, 44, 75). Moreover, many higher plants have been shown to contain QS mimics and/or anti-QS activities (1, 2, 10, 27, 40, 77, 78).
Ethnobotanical searches have evidenced that members of the Combretaceae family (Viridiplantae) are commonly used in traditional medicine, representing close to 90 medical indications including bacterial infections, fever, leprosy, and pneumonia (23, 53). In this plant family, species of the genus Combretum, which is the largest genus (with about 370 species), are known to contain a wide variety of secondary metabolites (23) and have been screened for their antimicrobial, antifungal, or antiviral activities (23, 46-48, 53). To the best of our knowledge, Combretum species have not been screened for their capacity to inhibit QS mechanisms in bacteria. However, two other Combretaceae species (Conocarpus erectus and Bucida buceras) have been identified as having anti-QS activities (1, 2), but the active compounds have still to be identified. C. albiflorum (Tul.) Jongkind, which is endemic in Madagascar and formerly known as C. phaneropetalum (Baker) (36), was screened for the presence of compounds reducing the production of extracellular virulence factors that are regulated by QS mechanisms. The screening of samples from C. albiflorum for their capacity to inhibit the production of violacein in Chromobacterium violaceum CV026 and pyocyanin in P. aeruginosa PAO1 generated several active fractions containing flavonoid-like compounds, among which the flavan-3-ol catechin. Catechin was found to have a negative impact on the transcription of several QS related genes (i.e., lasI, lasR, rhlI, rhlR, lasB, and rhlA), confirming the inhibitory effect of catechin on QS mechanisms.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
C. violaceum CV026 was grown in liquid LB medium at 28°C (51). P. aeruginosa PAO1 was obtained from the Pseudomonas Genetic Stock Center (strain PAO0001 [http://www.pseudomonas.med.ecu.edu/]) and was grown in liquid LB cultures (5 ml) supplemented with 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS; pH 7.0) at 37°C as described elsewhere (55). Plasmids (listed in Table 1) were introduced in P. aeruginosa PAO1 as described previously (76), and positive clones were selected on solid LB medium containing carbenicillin (300 μg/ml) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Mutant strains ΔPA1430 (ID 17281), ΔPA1432 (ID 11174), ΔPA3476 (ID 32454) and ΔPA3477 (ID 3452) were obtained from the Transposon Mutant Collection (Department of Genome Sciences, University of Washington-http://www.gs.washington.edu/labs/manoil/libraryindex.htm) and grown in LB medium supplemented with tetracycline according to (35). Escherichia coli biosensor strains harboring LasR- and RhlR-based plasmids pAL105 and pAL101 and control plasmids pAL106 (LasR−) and pAL102(RhlR−) (see Table 1) were grown in LB medium supplemented with tetracycline and chloramphenicol as described elsewhere (43). When required, the medium was supplemented with 10 μM (final concentration) of 3-oxo-C12-HSL or C4-HSL from Sigma.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| Strains | ||
| C. violaceum CV026 | Violacein-negative, cviI mini-Tn5 mutant of C. violaceum | 51 |
| P. aeruginosa PAO1 | Wild type (strain PAO0001; http://www.pseudomonas.med.ecu.edu/) | |
| P. aeruginosa ΔPA1430 | P. aeruginosa transposon mutant ID 17281 | 35 |
| P. aeruginosa ΔPA1432 | P. aeruginosa transposon mutant ID 11174 | 35 |
| P. aeruginosa ΔPA3476 | P. aeruginosa transposon mutant ID 32454 | 35 |
| P. aeruginosa ΔPA3477 | P. aeruginosa transposon mutant ID 3452 | 35 |
| E. coli JLD271 | E. coli K-12 ΔlacX74 sdiA271::Cam | 43 |
| Plasmids | ||
| pQF50 | Broad-host-range promoterless lacZ transcriptional fusion vector; Cbr | 34 |
| pβ01 | pQF50-derivative containing PlasB-lacZ transcriptional fusion | 34 |
| pβ02 | pQF50-derivative containing PrhlA-lacZ transcriptional fusion | 34 |
| pβ03 | pQF50-derivative containing PlasI-lacZ transcriptional fusion | 34 |
| pLP170 | Broad-host-range lacZ transcriptional fusion vector that contains an RNase III splice sequence positioned between the multiple cloning site and lacZ; Cbr | 62 |
| pPCS223 | pLP170-derivative containing PlasI-lacZ transcriptional fusion | 80a |
| pPCS1001 | pLP170-derivative containing PlasR-lacZ transcriptional fusion | 62 |
| pLPR1 | pLP170-derivative containing PrhlI-lacZ transcriptional fusion | 80a |
| pPCS1002 | pLP170-derivative containing PrhlR-lacZ transcriptional fusion | 62 |
| pAL101 | pSB401-derivative containing rhlR+ rhlI::luxCDABE; Tetr | 43 |
| pAL102 | pSB401-derivative containing rhlI::luxCDABE; Tetr | 43 |
| pAL105 | pSB401-derivative containing lasR+ lasI::luxCDABE; Tetr | 43 |
| pAL106 | pSB401-derivative containing lasI::luxCDABE; Tetr | 43 |
| pTB4124 | pQF50-derivative containing PaceA-lacZ transcriptional fusion | 41 |
Cbr, carbenicillin resistance; Tetr, tetracycline resistance.
β-Galactosidase and luminescence measurements.
Transcription of the QS genes was assayed by using PAO1-derived strains harboring promoter-lacZ fusions (Table 1). PAO1 reporter strains were prepared as described for pyocyanin and elastase quantification (see below). PAO1 strains (50 μl) were grown in 1 ml of LB medium at 37°C with agitation (175 rpm) supplemented with 10 μl of catechin (4 mM final) or dimethyl sulfoxide (DMSO; 1% [vol/vol], final concentration) and incubated for 8 and 18 h. After incubation, cell growth was assessed by spectrophotometry (i.e., the optical density at 600 nm [OD600]) using a SpectraMax M2 device (Molecular Devices), and the absorbance of the medium (OD600) after centrifugation of the bacteria (16,000 × g) was used as a blank. The sample used for cell growth assessment was used to perform the β-galactosidase assay with o-nitrophenyl-β-d-galactopyranoside as previously described (92). Promoterless lacZ fusion strains were used as controls. E. coli biosensor strains were grown in LB medium for 24 h, and 50-μl portions were subcultured for 8 h at 37°C in 1 ml of LB medium (the starting OD600 ranged between 0.02 and 0.025 corresponding to ∼5 × 106 CFU) supplemented with 10 μl of DMSO (1% [vol/vol] final), 10 μl of catechin dissolved in DMSO (4 mM, final concentration), 10 μl of the appropriate homoserine lactone (10 μM, final concentration), or 10 μl of catechin (4 mM, final concentration) and the appropriate homoserine lactone (10 μM, final concentration). C4-HSL was added to pAL101 and pAL102, while 3-oxo-C12-HSL was added to pAL105 and pAL106. After incubation for 8 h at 37°C with agitation (175 rpm), 200 μl of culture was transferred to 96-well OptiPlate-96 F plates from Perkin-Elmer, and the luminescence of each sample was measured by using a TopCount NXT device from Perkin-Elmer. The LasR− (pAL106) and RhlR− (pAL102) biosensors were used for background subtraction, and the OD600 values were measured to account for the differences in cell density. All experiments were performed in six replicates (n = 6). The statistical significance of each test was evaluated by conducting Student t tests using GraphPad Prism software, and P values of ≤0.05 were considered significant.
C. albiflorum sample preparation, chromatography, and electrospray ionization mass spectrometry (ESI-MS) analysis.
Dried powdered samples (5 g) of leaf and stem bark of C. albiflorum (Tul.) Jongkind (Combretaceae) were macerated overnight at room temperature in MilliQ water (25 ml). After centrifugation and filtration on Whatman paper, followed by lyophilization, the resulting residue was dissolved in 1 ml of MilliQ water and loaded onto a Sephadex LH-20 (GE Healthcare Life Sciences) column (2.5 by 7.0 cm) that was eluted successively with 100 ml of water (fractions 1 to 10), water-ethanol (9:1) (fractions 11 to 14), water-ethanol (8:2) (fractions 15 to 18), water-ethanol (1:1) (fractions 19 to 22), water-ethanol-acetone (1:0.5:0.5) (fractions 23 to 24), and water-acetone (1:1) (fractions 25 to 27). Fractions were evaporated and stored at −20°C until required for further analysis.
One active fraction (fraction 21) was further fractionated by HPLC using a reverse-phase C18 column (Symmetry 300, 2.4 by 150 mm) that was eluted with a gradient of water-methanol containing 1% acetic acid (from 0 to 100% methanol in 30 min). Analytical high-pressure liquid chromatography (HPLC) was performed by using a Waters apparatus equipped with a 626 pump, a 626 controller, and a 996 photodiode array detector. Samples were analyzed on an HPLC apparatus (Waters 600 system) coupled to both UV (Waters 2487 detector) and mass (Micromass Waters VG Quattro II mass spectrometer) detectors. A BIO Wide Pore C-18 column (250 by 4.6 mm; 5 μm) was used, and the solvent elution consisted of a linear gradient of water and acetonitrile (from 5 to 100% acetonitrile in 30 min) at a flow rate of 1 ml/min. After UV detection at 215 and 254 nm, the column eluate was split (LC Packings splitter), and 0.1 ml/min was directed to the mass spectrometer fitted with an ESI interface. For mass detection, analyte ionization was achieved by using the positive electrospray mode. The ESI parameters were as follows: nebulizing gas (N2, 20 liters/h), drying gas (N2, 250 liters/h), source temperature (80°C), cone voltage (35 V), and capillary voltage (35 kV). The total ion current scanning was from m/z 115 to 1,000 with 1 s/scan.
Commercially available catechin [(2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol] and epicatechin standards used for HPLC and ESI-MS analysis or for QS inhibition tests in C. violaceum CV026 or P. aeruginosa PAO1 were purchased from Sigma and dissolved in DMSO. The addition of catechin, epicatechin, or DMSO did not result in an increase of the LB broth pH (i.e., the anti-QS effect observed did not result from an opening of the lactone ring of the QS molecules through alkaline hydrolysis) (1).
Quantitative analysis of violacein production in C. violaceum CV026.
Inhibition of violacein production in C. violaceum CV026 by plant extracts (10 μl), chromatographic fractions (10 μl), or the authentic catechin and epicatechin standards (both at 4 mM final concentrations) was tested by using a liquid assay according to previously reported protocols (11, 51). Violacein production was induced in C. violaceum CV026 by adding N-hexanoyl-l-homoserine lactone (HHL; Sigma) at a final concentration of 3 μM. For violacein quantification, an overnight culture of C. violaceum CV026 was diluted 100 times in 250 μl of LB medium that were subsequently incubated for 18 h at 28°C with agitation (150 rpm) and supplemented with the appropriate sample. After assessment of bacterial growth by measuring the OD600 by using a SpectraMax M2 device (Molecular Devices), violacein contents were quantified as described previously (11). Briefly, cells were centrifuged (16,000 × g) and washed twice with fresh LB medium. After centrifugation, cells were suspended in 200 μl of ethanol and sonicated. Cell debris were discarded by centrifugation (16,000 × g), and the absorbance (A575) of the solution was measured. The statistical significance of each test (n = 4) was evaluated by conducting Student t tests using the GraphPad Prism software, and a P value of ≤0.01 was considered significant.
Quantitative analysis of pyocyanin and elastase production in P. aeruginosa wild-type and mutant strains.
Inhibition of pyocyanin and elastase production was assessed according to previously described procedures (34, 55). P. aeruginosa PAO1 or mutant cells (single colony from LB agar plates) were grown for 18 h in 5 ml of LB-MOPS medium (37°C and agitation at 175 rpm). The cells were washed twice in fresh LB-MOPS medium to eliminate the homoserine lactones, and the pellets were suspended in LB-MOPS medium. Then, 50-μl portions of the cell suspension were added to 1 ml of LB-MOPS (in order to obtain a starting OD600 of the LB-MOPS medium ranging between 0.020 and 0.025 and corresponding to ∼107 CFU/ml) supplemented with 10 μl of plant extract or authentic catechin standard (4 mM [final concentration] or as stated in the text) dissolved in DMSO and, when appropriate, with 3-oxo-C12-HSL or C4-HSL. After 8 h of growth, samples were taken to assess the growth (OD600). After centrifugation (16,000 × g), the absorbance at 600 nm of the medium was used as blank, and 900 μl was used for pyocyanin determination (A380). Elastase production was assessed through the measurement of elastase activity using elastin-Congo red (A495). The statistical significance of each test (n = 4) was evaluated by conducting Student t tests using GraphPad Prism software, and a P value of ≤0.01 was considered significant.
Biofilm formation and quantification.
P. aeruginosa PAO1 was grown overnight in LB medium at 37°C with agitation. After growth, the culture was diluted with TB (tryptone broth) medium (OD600 of ∼0.02), and 50 μl of the diluted culture was added to 940 μl of TB medium supplemented with 10 μl of DMSO or catechin (4 mM, final concentration). PAO1 cells were incubated statically for 18 h at 37°C in 24-well polystyrene plates. After incubation, planktonic bacteria were discarded, and the biofilms were washed three times with phosphate-buffered saline buffer. Washed biofilms were fixed with 1 ml of methanol (99%). After 15 min, the methanol was discarded, and the plates were dried at room temperature. Crystal violet (0.1% in water) was then added to each well (1 ml/well), and the plates were incubated for 15 min at room temperature. Crystal violet was then discarded, and stained biofilms were washed three times with 1 ml of water. Acetic acid (33% in water) was added to the stained biofilms (2 ml) in order to solubilize the crystal violet, and the absorbance of the solution was read at 590 nm with a SpectraMax M2 device (Molecular Devices). The statistical significance of each test (n = 6) was evaluated by conducting Student t tests and using the GraphPad Prism software; a P value of ≤0.01 was considered significant.
Assessment of P. aeruginosa PAO1 viability.
P. aeruginosa PAO1 cells incubated with DMSO or catechin (4 mM) for 8 and 18 h were stained with SYTO-9 (3.34 mM) (Molecular Probes/Invitrogen) and propidium iodide (PI) (20 mM) (Molecular Probes/Invitrogen). P. aeruginosa PAO1 cultures were adjusted to an OD600 of 0.10 to 0.15, and a 100-μl mix of SYTO-9 and PI was added to 100 μl of diluted bacteria according to the Molecular Probes protocol. The cells were transferred to a 96-well OptiPlate-96 F (Perkin-Elmer), and the fluorescence intensities were determined (excitation, 485 nm; emission, 530 nm [SYTO-9] and 630 nm [PI]) by using a SpectraMax M2 device (Molecular Devices). Ratios between the green and red fluorescences (530 nm/630 nm) were compared to assess the cytotoxicity of catechin and epicatechin. DMSO-supplied cultures were used as controls. The statistical significance of each test (n = 6) was evaluated by conducting Student t tests using the GraphPad Prism software, and a P value of ≤0.01 was considered significant.
RESULTS
C. albiflorum extracts reduce the production of QS-regulated factors.
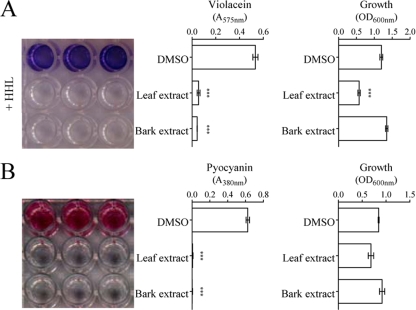
The leaves and bark of Combretum spp. are predominantly used in traditional medicine, while their fruits do not feature in traditional uses due to their toxicity to humans (23). Therefore, leaf and bark aqueous extracts of C. albiflorum (Tul.) Jongkind (Combretaceae) were screened for the occurrence of QS inhibitors. Assays were carried out using the C. violaceum CV026 reporter strain mutated in the AHL synthase gene cviI. This strain is able to produce violacein when the natural inducer HHL is supplied to the growth medium (51). Filter-sterilized extracts were added to noninduced (data not shown) or HHL-induced (Fig. 1A) C. violaceum CV026 cultures. When C. albiflorum extracts were added, violacein was not produced in either noninduced or HHL-induced C. violaceum CV026 cultures compared to the control cultures, indicating that C. albiflorum extracts do not contain HHL mimic compounds (data not shown) but contain QS inhibitory compounds (Fig. 1A). To evaluate the degree of inhibition of violacein production, violacein was extracted and quantified. As shown in Fig. 1A, both extracts inhibited violacein production by more than 80%. However, the growth of C. violaceum CV026 was inhibited by ca. 50% in the presence of leaf extract, whereas bark extract had no growth-inhibiting effect (Fig. 1A). These extracts were also tested on P. aeruginosa PAO1, where the extracellular virulence factor pyocyanin can be easily detected (62). As shown in Fig. 1B, both extracts abolished the QS-dependent production of pyocyanin. As observed with C. violaceum CV026, the bark extract had no effect on P. aeruginosa PAO1 growth, while the leaf extract did but to a lesser extent than with CV026 (Fig. 1B).
FIG. 1.
Screening of C. albiflorum leaf and bark extracts for anti-QS activity using C. violaceum CV026 (A) and P. aeruginosa PAO1 (B). (A) Growth and violacein production by C. violaceum CV026 when HHL is supplied at a final concentration of 3 μM and in the presence of C. albiflorum extracts. (B) Growth and pyocyanin production by P. aeruginosa PAO1 when C. albiflorum extracts are added to the growth medium. DMSO-supplied cultures were used as controls. Violacein and pyocyanin were extracted as described in Materials and Methods and quantified by spectrophotometry (575 nm for violacein and 380 nm for pyocyanin). Bacteria growth was assessed at 600 nm. All graphs show the OD values associated with each parameter measured, and each test was performed four times. ***, Data that are statistically different (P ≤ 0.01).
Chromatographic fractionation of the C. albiflorum bark extract and identification of flavonoid-containing active fractions.
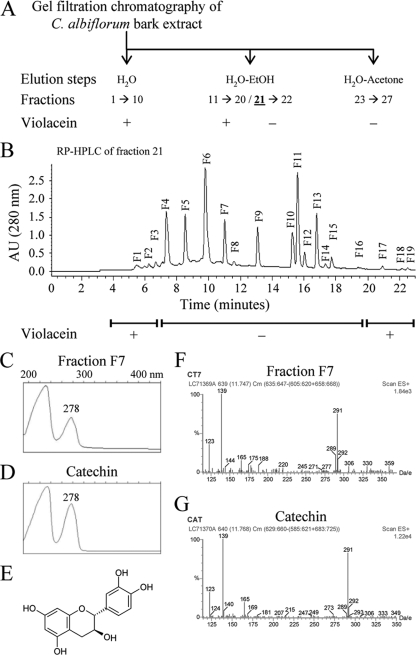
In order to identify the inhibitory compound(s) responsible for the QS inhibition activity, the C. albiflorum aqueous bark extract was subjected to gel filtration chromatography (Fig. 2A). Twenty-seven fractions were collected, and the last seven eluted fractions (fractions 21 to 27) were found to inhibit violacein production (Fig. 2A). The first active fraction (fraction 21) was further fractionated, using reversed-phase HPLC (RP-HPLC), into 19 subfractions, and subfractions F4 to F16 inhibited violacein production (Fig. 2B). Fraction 21 was chosen because (i) preliminary thin-layer chromatography showed that this fraction contained few different compounds compared to the following fractions, (ii) growth of the bacteria was not affected, and (iii) the amount of residue obtained allowed the further chemical characterization of the active compound(s) (data not shown). The UV spectra of these subfractions showed that most of the peaks presented typical flavonoid-type UV spectra profiles, i.e., two main absorption bands comprised between 240 to 270 nm and 320 to 380 nm, respectively (31; data not shown). These data suggest that the active fractions of the C. albiflorum bark extract contain flavonoid-like structures interfering with violacein production in C. violaceum CV026 without any bactericidal or bacteriostatic activity.
FIG. 2.
Chromatographic scheme of C. albiflorum bark extract fractionation steps used to isolate and identify the anti-QS compounds and identification of catechin as the main compound present in the active fraction F7. (A) Gel filtration fractionation of the crude C. albiflorum bark extract. The column was successively eluted with water, water-ethanol, and water-acetone, and 27 fractions were collected. These fractions were added to HHL-induced C. violaceum CV026, and inhibition of violacein production was observed starting from fraction 21. (B) RP-HPLC profile of fraction 21 (280 nm). Nineteen subfractions were collected and tested on HHL-induced C. violaceum CV026, and inhibition of violacein production was observed with fractions F4 to F16. AU, arbitrary unit. (C) UV spectrum of fraction F7. (D) UV spectrum of an authentic standard of catechin showing the characteristic absorption maximum of catechin at 278 nm. (E) Chemical structure of catechin with the two benzene cycles connected by the heterocycle typical of flavonoids. (F and G) ESI-MS of fraction F7 (F) and of the authentic standard of catechin (G) with the main peaks (Da/e) at 123, 139, and 291.
Catechin is one of the C. albiflorum flavonoid inhibiting the production of violacein in C. violaceum CV026 and pyocyanin in P. aeruginosa PAO1.
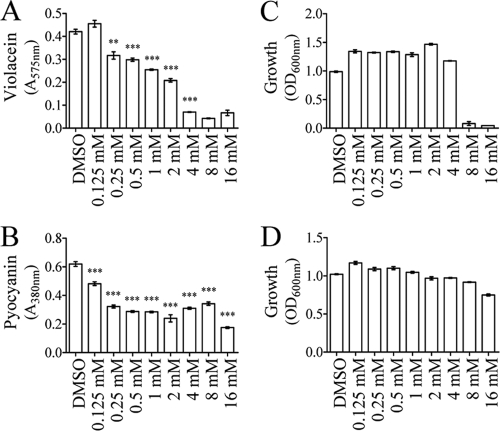
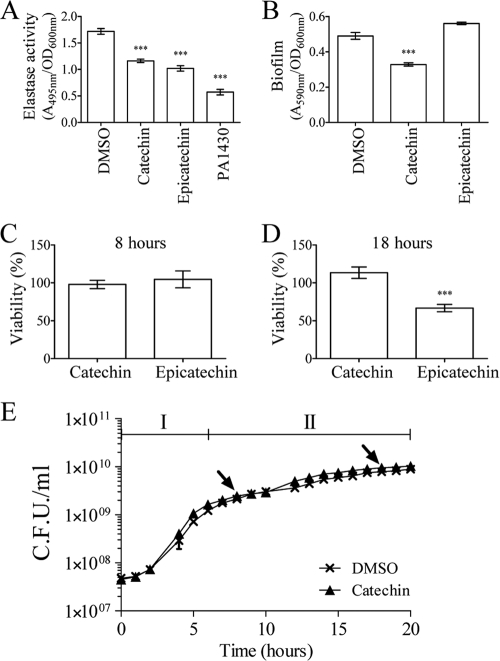
The peak purity of the HPLC subfractions F4 to F16 was assessed using the Waters Millennium chromatography manager. The presence of a single peak in subfractions F7 (with a retention time of 11 min) and F9 (with a retention time of 13 min) prompted us to further investigate the structural characterization of the QS inhibitory compound present in these subfractions. First, as shown in Fig. 2C and D, the UV spectrum of the major peaks in subfractions F7 is similar to that of catechin (of which the structure is given in Fig. 2E). Second, analysis of a standard of catechin in the same HPLC conditions showed that catechin has the same retention time as the compound present in subfraction F7 (data not shown). Third, coinjection of the catechin standard with subfraction F7 showed no difference in the retention times, which further supported the presence of catechin in this subfraction (data not shown). Fourth, the ESI-MS analysis indicated that the MS spectrum of subfraction F7 was similar to that of authentic catechin standard showing an identical quasimolecular ion at m/z (Da/electron [e]) 291 with ion at 139 and 123 (Fig. 2F and G, respectively) in agreement with data reported previously (22). Epicatechin, which is the epimer of catechin, has the same UV and mass spectra but is found in fraction F9, as demonstrated by HPLC separation of an epicatechin standard (which had a retention time of 13 min, as did fraction F9) and coinjection of epicatechin and fraction F9 in the same HPLC conditions (data not shown). Finally, the catechin standard (Fig. 2E) inhibited violacein production when added to HHL-induced liquid cultures of C. violaceum CV026, as did subfraction F7, further confirming that catechin is the QS inhibitory compound. Indeed, as shown in Fig. 3, serial dilutions of the catechin standard were tested on HHL-induced cultures of C. violaceum CV026 in order to determine the range of concentrations inhibiting violacein production with a limited effect on bacterial growth (Fig. 3A and C). When concentrations of catechin higher than 4 mM were used, C. violaceum CV026 growth was strongly reduced and, as a consequence, no violacein production occurred (Fig. 3A and C). From 0.25 to 4 mM, violacein production was significantly affected by catechin (Fig. 3A), an effect that was not associated with a decrease in growth of C. violaceum CV026 (Fig. 3C). Serial dilutions of catechin were also tested on P. aeruginosa PAO1. As shown in Fig. 3B, concentrations between 0.125 and 16 mM had a significant negative effect on pyocyanin production without affecting P. aeruginosa PAO1 growth (Fig. 3D). The epicatechin standard, tested at 4 mM, had an inhibitory effect on pyocyanin production in P. aeruginosa PAO1, as did subfraction F9, while this compound had a bactericidal effect on C. violaceum CV026 (data not shown). The effect of catechin and epicatechin (at 4 mM) was also tested on elastase production in P. aeruginosa PAO1 by performing an elastolysis assay as described in Material and Methods. As shown in Fig. 4A, after 8 h, the addition of catechin (as well as epicatechin) reduced the production of elastase by ca. 30%. For comparison, the production of pyocyanin by the ΔPA3476 (ΔrhlI) and ΔPA3477 (ΔrhlR) mutants was lower than in the wild-type strain PAO1 by 69% ± 6% and 73% ± 4%, respectively, whereas the production of elastase (as measured by the elastolysis assay) by the ΔPA1432 (ΔlasI) and ΔPA1430 (ΔlasR) mutants was lower than in the wild-type strain PAO1 by 53% ± 5% and 76% ± 6%, respectively.
FIG. 3.
Effect of serial dilutions of an authentic standard of catechin on violacein (A) and pyocyanin (B) production in C. violaceum CV026 and P. aeruginosa PAO1, respectively. (C and D) Growth of both bacteria assessed at 600 nm. Violacein and pyocyanin were extracted as described in Materials and Methods and quantified by reading the OD values of the solution at 575 nm (A) and 380 nm (B), respectively. DMSO-supplied cultures were used as controls. The statistical significance of each test (n = 4) was evaluated by conducting Student t tests, and a P value of ≤0.01 was considered significant. ***, Data that are statistically different (P ≤ 0.01).
FIG. 4.
Effect of authentic standards of catechin (4 mM) and epicatechin (4 mM) on elastase production (A), biofilm formation (B), P. aeruginosa PAO1 viability after 8 h (C) and 18 h (D) of culture, and P. aeruginosa PAO1 growth kinetics (E). DMSO-supplied cultures were used as controls. Elastase production by P. aeruginosa PAO1 (A) was quantified through an elastolysis assay as described in Materials and Methods. No difference in cell densities (OD600) was observed between the different treatments (data not shown). Elastase production by the ΔlasR mutant strain PA1430 was used as a comparison to assess the potency of catechin. (B) Biofilm formation by P. aeruginosa PAO1 after 18 h of incubation was assessed by using crystal violet. (C and D) The viability of P. aeruginosa PAO1 cells was assessed by using SYTO-9 and PI as described in Materials and Methods after 8 h (C) and 18 h (D) of culture. DMSO-supplied cultures were used as controls, and their viability was used to plot the viability of catechin- and epicatechin-treated cultures. The statistical significance of each test (n = 6) was evaluated by conducting Student t tests, and a P value of ≤0.01 was considered significant. ***, Data that are statistically different (P ≤ 0.01). (E) Growth kinetics of DMSO- and catechin-treated P. aeruginosa PAO1. The growth kinetics were divided into two phases: phase I was between 0 and 6 h (corresponding to the exponential growth phase), and phase II was between 6 and 20 h (corresponding to the stationary phase). Doubling times were calculated for both phases by using GraphPad Prism software (n = 6). Arrows indicate the time points at which samples were taken for pyocyanin, elastase, biofilm, or gene expression analysis.
Effect of catechin on biofilm formation in P. aeruginosa PAO1.
P. aeruginosa PAO1 can switch to a biofilm mode of growth. Biofilms are matrixes of polysaccharides in which bacteria are enmeshed and protected from the environment, increasing their resistance to antibiotics and the immune system, thereby increasing their capacity to remain in the infected host (15, 56). Since biofilm formation is partially controlled by QS mechanisms (16), the effect of catechin and epicatechin on P. aeruginosa PAO1 biofilm formation was assessed after 18 h of growth. As shown in Fig. 4B, catechin reduced biofilm formation by ca. 30%, while epicatechin had no effect. To determine whether the effect of both compounds on pyocyanin, elastase, and biofilm was due to a drop in cell viability, P. aeruginosa PAO1 viability was assessed after 8 and 18 h. As shown in Fig. 4C and D, none of the compounds had an effect after 8 h of incubation (Fig. 4C) but, after 18 h, epicatechin-treated P. aeruginosa PAO1 cells had a viability of 66%, whereas catechin-treated cells were not affected in their viability compared to DMSO-treated cells (Fig. 4D). Consistently, catechin had no significant effect on P. aeruginosa PAO1 growth parameters, as shown in Fig. 4E. Indeed, the doubling times for control and catechin-treated P. aeruginosa PAO1 cells were, respectively, 0.94 ± 0.13 and 0.92 ± 0.03 h during the first phase of the kinetics (phase I) and 8.02 ± 0.24 and 7.46 ± 0.30 h during the second phase of the kinetics (phase II).
Catechin affects lasI, lasR, rhlI, and rhlR and downstream genes expression.
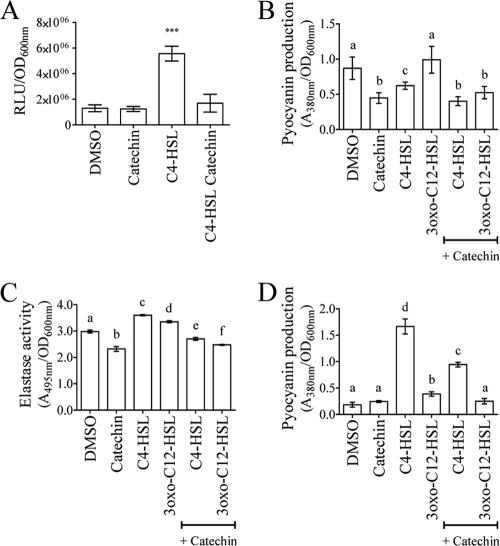
If the diminution of pyocyanin and elastase production and biofilm formation was due to an interference of catechin with QS mechanisms, this should be reflected in the expression of the main regulatory genes involved in QS mechanisms in P. aeruginosa PAO1. The effect of catechin on QS systems was therefore further characterized by evaluating the expression of the AHL synthetase genes lasI and rhlI and the QS regulator genes lasR and rhlR. Salicylic acid, which is known to affect QS signaling in P. aeruginosa and Agrobacterium tumefaciens (6, 64, 87, 89, 90), was used as a positive control. As shown in Table 2, after 8 h, the addition of catechin resulted in a significantly reduced expression of the regulatory genes of the las system by 40% (lasI) and 20% (lasR). The regulatory genes of the rhl system were also significantly affected. The expression of rhlI and rhlR was reduced by 44 and 38%, respectively. After 18 h of incubation, these reductions were of 26% for lasI and 52% for lasR, while the expression of rhlI and rhlR was reduced by 58 and 55%, respectively (Table 2). Compared to salicylic acid, catechin had an equivalent or a more pronounced effect on the expression of these genes after 8 and 18 h of incubation (Table 2). Elastolysis measurements showed that catechin likely reduced elastase production by ca. 30% (Fig. 4A). To strengthen this observation, the expression of the lasB gene, encoding LasB elastase (58, 79, 86), was quantified. As shown in Table 2, the addition of catechin reduced the expression of lasB by ca. 36% after 8 h and by 27% after 18 h, whereas salicylic acid was more effective after 8 h (ca. 40% of repression) than after 18 h. This reduction of lasB expression is in accordance with the concomitant reduction in expression of the las system. The effect of catechin on the expression of the rhlA gene, contributing to the production of rhamnolipids (58), was also investigated. As shown in Table 2, after 8 h of incubation, the addition of catechin or salicylic acid reduced rhlA expression by 23 and 21%, respectively. Salicylic acid had a greater impact on rhlA expression (20% reduction) than catechin after 18 h, which seemed not effective anymore (only 10% reduction [Table 2]). These results highlight that the expression of QS genes is affected by catechin (as well as salicylic acid) to various extents depending on the QS system, likely as a result of the complex, redundant, and hierarchical mechanisms underlying this cell-to-cell communication process (18). This reduction in QS gene expression was not linked to a drop in cell viability (data not shown), cell growth retardation (data not shown), or nonspecific reduction of the transcription machinery of the bacteria. Indeed, P. aeruginosa PAO1 grown in the presence of catechin (4 mM, final concentration) had growth characteristics similar to those of DMSO-treated cells. Finally, the activity of the aceA promoter (regulating the expression of the isocitrate lyase gene PA2634) from P. aeruginosa (41) was studied in strain PAO1 grown in the presence or absence of catechin. As shown in Table 2, the addition of catechin had no effect on the transcription of the aceA gene, indicating that catechin affects the expression of QS-related genes without affecting the transcription machinery of P. aeruginosa PAO1. To determine whether catechin could interfere with the perception of C4-HSL or 3-oxo-C12-HSL by their cognate receptors (i.e., RhlR or LasR, respectively), E. coli biosensor strains were used. As shown in Fig. 5A, the addition of catechin to the C4-HSL-treated pAL101 E. coli biosensor strain reduced the expression of the lux operon, indicating that catechin interferes with the capacity of RhlR to interact with the promoter of the rhlI gene (driving lux expression). The effect of catechin on the induction of the pAL105 biosensor strain was minor (data not shown), suggesting that catechin might mainly affect the rhlRI system, the effect on the las system being a side effect of the reduction of the rhl system. Besides, the effect of catechin could not be compensated by the addition of exogenous homoserine lactones. Indeed, as shown in Fig. 5B, the production of pyocyanin in the wild-type strain PAO1 could not be restored by the addition of homoserine lactones. In addition, as shown in Fig. 5C, the addition of HSL to catechin-treated wild-type strain PAO1 did not enhance the production of elastase as evaluated by the elastolysis assay. Consistent with the effect of catechin on the pAL101 biosensor (Fig. 5A), pyocyanin production was significantly reduced after 8 h of growth in the ΔPA3476 mutant supplemented with catechin (4 mM, final concentration) and C4-HSL compared to the C4-HSL-treated mutant cells, where pyocyanin production was optimal (Fig. 5D). A slight induction of pyocyanin production was observed when 3-oxo-C12-HSL was added to this mutant, but the addition of catechin reduced this production to basal levels (Fig. 5D).
TABLE 2.
Effect of catechin and salicylic acid on P. aeruginosa PAO1 QS genes expression after 8 and 18 h of incubation
| Time (h) | Culture condition | Gene expression (mean Miller units ± SD)a |
||||||
|---|---|---|---|---|---|---|---|---|
| lasIb | lasR | lasB | rhlI | rhlR | rhlA | aceA | ||
| 8 | DMSO | 521 ± 31 | 6,055 ± 220 | 3,286 ± 526 | 9,403 ± 634 | 7,520 ± 415 | 2,782 ± 145 | 610 ± 107 |
| Salicylic acid (4 mM) | 455 ± 71 | 4,659 ± 369* | 2,022 ± 282* | 7,804 ± 905† | 4,944 ± 673* | 2,208 ± 230* | ND | |
| Catechin (4 mM) | 314 ± 42* | 4,892 ± 321* | 2,116 ± 181† | 5,298 ± 654* | 4,629 ± 729* | 2,150 ± 118* | 525 ± 116 | |
| 18 | DMSO | 1,505 ± 222 | 4,199 ± 945 | 453 ± 34 | 2,718 ± 575 | 5,096 ± 1,323 | 2,754 ± 73 | 2,029 ± 143 |
| Salicylic acid (4 mM) | 1,338 ± 152 | 1,719 ± 472* | 459 ± 13 | 1,114 ± 134* | 2,582 ± 741* | 2,233 ± 187* | ND | |
| Catechin (4 mM) | 1,107 ± 371* | 2,002 ± 371* | 332 ± 23* | 1,132 ± 214* | 2,276 ± 663* | 2,525 ± 163* | 2,221 ± 46 | |
Gene expression was measured as the β-galactosidase activity of the lacZ gene fusions expressed in Miller units. *, Significance at P < 0.01; †, significance at P < 0.05. ND, not determined.
Similar results were obtained with plasmids pβ03 and pPCS223. The results shown were obtained with plasmid pPCS223.
FIG. 5.
Effect of catechin on exogenous addition of homoserine lactones. (A) Effect of catechin on the perception of C4-HSL by RhlR. The pAL101 and pAL102 E. coli biosensor strains were incubated for 8 h with DMSO (1% [vol/vol]), catechin (4 mM), C4-HSL only (10 μM), or C4-HSL (10 μM) plus catechin (4 mM). Samples were taken to record the luminescence of the culture in relative light units (RLU) and to measure the OD600. The pAL102 strain was used as background. The statistical significance of each test (n = 6) was evaluated by conducting Student t tests, and a P value of ≤0.01 was considered significant. (B and C) Production of pyocyanin (B) and elastase (C) by the wild-type strain PAO1 after the addition of C4-HSL or 3-oxo-C12-HSL (10 μM) in the presence or absence of catechin after 8 h of incubation. Pyocyanin (A380) and elastase (A495) were quantified as described in Materials and Methods, and values were corrected for the difference in cell density (OD600). (D) Pyocyanin production in the ΔrhlI mutant (PA3476) after the addition of C4-HSL or 3-oxo-C12-HSL (10 μM) in the presence or absence of catechin after 8 h of incubation. ***, Data that are statistically different (P ≤ 0.01). The different letters above the histograms indicate that the data are statistically different from each other (P ≤ 0.01).
DISCUSSION
Plants used by traditional healers for the treatment of various diseases constitute an important source of chemical diversity for the potential identification and development of new drugs useful as antimicrobials. Here, it has been shown that extracts from the leaves and bark of the Malagasy plant C. albiflorum reduce the production of two QS-controlled factors in two different types of bacteria, namely, violacein in C. violaceum CV026 and pyocyanin in P. aeruginosa PAO1 (Fig. 1). Nevertheless, the leaf extract affected significantly the growth of both bacterial species and was not further investigated. Combretum species are indeed known to contain many secondary metabolites such as tannins, flavonoids, terpenoids, and stilbenoids that have been associated to their antimicrobial potential (5, 23, 46-49, 53). Further analytical investigations of the bark extract (that had no effect on bacteria growth) showed that flavonoids (possibly acting synergistically) are likely responsible for these activities and that catechin is one of the active molecules among other bark components with an effect on QS (Fig. 2). The decrease in production of violacein and pyocyanin could not be attributed to any bactericidal or bacteriostatic effect of catechin on P. aeruginosa PAO1 or C. violaceum CV026 (Fig. 3). This observation is consistent with other reports showing that catechin has no effect on different gram-positive and gram-negative bacteria, including P. aeruginosa (3, 39), or increases growth of Lactobacillus hilgardii (3).
Compounds from other natural sources having an anti-QS activity include also l-canavanine from Medicago sativa which interferes with QS in Sinorhizobium meliloti likely through hindering the folding of the QS regulator protein ExpR at concentrations comprised between 56 μM and 5.6 mM (40); patulin (8 μM) from Penicillium coprobium and penicillic acid (147 μM) from P. radicicola, which both decreased QS-controlled gene expression in P. aeruginosa PAO1 (with patulin accelerating LuxR turnover and increasing sensitivity of P. aeruginosa biofilms to tobramycin when used at 3.5 and 8 μM, respectively) (68); garlic extract (used at 2%) and isolated components and some of their derivatives (10, 59, 67); various antibiotics or drugs (including salicylic acid used at concentrations ranging from 1 to 30 mM), which decrease the expression of QS-related genes and corresponding virulence factors (6, 64, 74, 87, 89, 90); the well-known and characterized furanones synthesized by the Australian red alga Delisea pulchra (28, 44, 45); manoalide, manoalide monoacetate, and secomanoalide used at micromolar concentrations from the marine sponge Luffariella variabilis (75); and the polyphenol curcumin (used at 1.5 and 3 μg/ml), which attenuates P. aeruginosa PAO1 pathogenicity by reducing the production of QS regulated virulence factors and biofilm formation (70). Quenchers of QS mechanisms produced by eukaryote organisms is likely not limited to these compounds since QS inhibitory activities have also been found in numerous plant extracts including Chlamydomonas reinhardtii, Pisum sativum, M. sativa, M. truncatula, Allium sativum, Callistemon viminalis, bean sprout, chamomile, Daucus carota, Capsicum chinense, B. buceras, and C. erectus (1, 2, 10, 27, 38, 40, 50, 67, 77, 78). Interestingly, the two last plant species belong to the Combretaceae plant family and have been shown to reduce the expression of the las and rhl genes, as well as the production of various virulence factors in P. aeruginosa PAO1 (1). Preliminary thin-layer chromatography characterization of the crude extracts of these plants revealed that they contain multiple active compounds (1). In the present study, C. albiflorum fractionation by HPLC and UV spectrum analysis revealed that several compounds (flavonoids in particular) are probably responsible for the activity of the crude extract and that the synergistic action of these compounds might explain the strong inhibitory activity observed in C. violaceum CV026 and P. aeruginosa PAO1. Whether the compounds present in B. buceras and C. erectus are the same or belong to the flavonoids remains an open question.
QS mechanisms can be inhibited in various ways: through signal mimicry (such as with furanones or synthetic signal analogs) resulting in a decrease in QS gene expression, through enzymatic degradation of homoserine lactones, through antiactivator proteins or negative transcriptional regulator homologs (such as the LasR and RhlR homolog QscR in P. aeruginosa) or through small regulatory RNAs (29, 82). In P. aeruginosa, the QS hierarchy is a complex process (18, 71, 72) with the las system regulating the rhl system through LasR, and recent evidence suggest that the rhl system is only delayed in a las negative background and that RhlR controls lasI expression and quinolone synthesis (a third signal in P. aeruginosa) (18, 72). Elastase (LasB) is mainly under the control of the lasI-lasR system (25), while pyocyanin production (12, 83), as well as rhamnolipid production (rhlA) (58), is under the control of the rhlI-rhlR system, which contributes to a lesser extend to the control of lasB expression (12, 58). Biofilm formation involves different stages consisting in attachment, proliferation and differentiation, and QS is thought to be involved mainly in this last stage, the las system being the main QS regulator of biofilm (16). The addition of catechin affected all of these processes to different extents (Table 2), and the use of E. coli biosensors suggests that catechin acts by interfering with the perception of C4-HSL by RhlR (Fig. 5A), which likely explains the reduction in rhlR, rhlI, and rhlA and, to some extent, lasB expression and the reduction in pyocyanin production. Nevertheless, it might be that, as suggested for the extracts of B. buceras and C. erectus (1), catechin affects also an upstream QS regulator such as GacA, Vfr, VqsR, RsaL, or QscR (4, 14, 17, 19, 32, 37, 63, 69).
Catechin derives from the phenylpropanoid pathway and is classified among the flavan-3-ols of the flavonoid family of polyphenols (20, 88). (−)-Epigallocatechin gallate (resulting from the esterification of epigallocatechin and gallic acid) has been demonstrated (at 100 μM) to repress the expression of lasB from P. aeruginosa in P. putida cells hosting the pKR-C12 plasmid (carrying a translational lasB-GFP fusion and the lasR gene under the control of a lac-type promoter) and the expression of luxI from Photobacterium fischeri in E. coli cells hosting the pSB403 plasmid (carrying the luxR gene and the luxI promoter fused to the luxCDABE operon) (33). More recently, using an in silico approach, it has been evidenced that baicalein, which is a flavone reported in Scutellaria baicalensis (a plant used in traditional Chinese medicine), inhibits the formation of biofilm by P. aeruginosa PAO1 when used at 200 μM and promotes the proteolysis of the signal reporter TraR protein from A. tumefaciens expressed in E. coli at concentrations ranging from 4 to 40 mM (91). Moreover, baicalein, whose chemical structure differs from catechin by its hydroxylation status and the carbonyl in the heterocycle, has been shown to have a high in silico TraR docking score (91), a characteristic catechin might also have. Both studies used reporter bacteria with a E. coli or P. putida genomic background differing from those of the target genes (i.e., luxI/luxR from P. fischeri, lasB from P. aeruginosa, and TraR from A. tumefaciens), while it seems that QS inhibitors should be used and screened in the specific organism targeted (66). Here, we have shown that the expression of P. aeruginosa QS genes is downregulated in the P. aeruginosa strain PAO1 genomic background, possibly as a result of the disruption of the perception of C4-HSL by RhlR, when catechin is added to the growth medium, which further supports the possible activity of some flavonoids, natural products widely found in plants, as QS inhibitors.
Consistent with a possible role of flavonoids in messing with QS communication among bacteria, a proteomic analysis of M. trunculata roots treated with nanomolar to micromolar concentrations of N-acyl homoserine lactones from the symbiont Sinorhizobium meliloti and from P. aeruginosa revealed that, among the 150 proteins whose accumulation was modified, the expression of proteins related to flavonoid metabolism is affected (50). In addition, by using transgenic Trifolium repens plants with a relevant GUS reporter fusion, these authors also showed that the exposure to 3-oxo-C12-HSL (produced by P. aeruginosa) substantially increased the expression of three chalcone synthase promoters involved in the flavonoid pathway. Although the exact consequence of these increased expressions is not known, these results and our data suggest a link between the flavonoid pathway in plant and bacterial QS signaling, playing important roles in the beneficial (symbiosis) or pathogenic outcomes of plant-prokaryote interactions. Hence, flavonoids might also constitute a first line of defense against pathogenic attacks by affecting QS mechanisms and thereby virulence factor production.
Acknowledgments
O.M.V. is a Postdoctoral Researcher of the FRS-FNRS (Fonds de la Recherche Scientifique, Belgium). M.B. is a Senior Research Associate of the FRS-FNRS. M.K. is indebted to the Coopération Universitaire pour le Développement-Coopération Universitaire Institutionnelle. S.R. is indebted to the Agence Universitaire de la Francophonie for a predoctoral fellowship.
We thank Barbara Iglewski from the Rochester University School of Medicine and Dentistry (United States) for kindly providing plasmids pLP170, pPCS223, pPCS1001, pLPR1, and pPCS1002; Junichi Kato from the Department of Molecular Biotechnology, Hiroshima University, Hiroshima, Japan, for kindly providing plasmids pQF50, pβ01, pβ02 and pβ03; Brian Ahmer from Ohio State University for providing the E. coli biosensor strains pAL101, pAL102, pAL105, and pAL106; and Frédéric Paulart from the Institute for Medical Immunology from the Université Libre de Bruxelles for helping with the use of the TopCount NXT.
Footnotes
Published ahead of print on 23 October 2009.
REFERENCES
- 1.Adonizio, A., K. F. Kong, and K. Mathee. 2008. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob. Agents Chemother. 52:198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adonizio, A. L., K. Downum, B. C. Bennett, and K. Mathee. 2006. Anti-quorum sensing activity of medicinal plants in southern Florida. J. Ethnopharmacol. 105:427-435. [DOI] [PubMed] [Google Scholar]
- 3.Alberto, M. R., M. E. Farias, and M. C. Manca De Nadra. 2001. Effect of gallic acid and catechin on Lactobacillus hilgardii 5w growth and metabolism of organic compounds. J. Agric. Food Chem. 49:4359-4363. [DOI] [PubMed] [Google Scholar]
- 4.Albus, A. M., E. C. Pesci, L. J. Runyen-Janecky, S. E. West, and B. H. Iglewski. 1997. Vfr controls quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angeh, J. E., X. Huang, I. Sattler, G. E. Swan, H. Dahse, A. Hartl, and J. N. Eloff. 2007. Antimicrobial and anti-inflammatory activity of four known and one new triterpenoid from Combretum imberbe (Combretaceae). J. Ethnopharmacol. 110:56-60. [DOI] [PubMed] [Google Scholar]
- 6.Bandara, M. B., H. Zhu, P. R. Sankaridurg, and M. D. Willcox. 2006. Salicylic acid reduces the production of several potential virulence factors of Pseudomonas aeruginosa associated with microbial keratitis. Investig. Ophthalmol. Vis. Sci. 47:4453-4460. [DOI] [PubMed] [Google Scholar]
- 7.Barnard, A. M., S. D. Bowden, T. Burr, S. J. Coulthurst, R. E. Monson, and G. P. Salmond. 2007. Quorum sensing, virulence, and secondary metabolite production in plant soft-rotting bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1165-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjarnsholt, T., and M. Givskov. 2008. Quorum sensing inhibitory drugs as next generation antimicrobials: worth the effort? Curr. Infect. Dis. Rep. 10:22-28. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnsholt, T., and M. Givskov. 2007. The role of quorum sensing in the pathogenicity of the cunning aggressor Pseudomonas aeruginosa. Anal. Bioanal. Chem. 387:409-414. [DOI] [PubMed] [Google Scholar]
- 10.Bjarnsholt, T., P. O. Jensen, T. B. Rasmussen, L. Christophersen, H. Calum, M. Hentzer, H. P. Hougen, J. Rygaard, C. Moser, L. Eberl, N. Hoiby, and M. Givskov. 2005. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 151:3873-3880. [DOI] [PubMed] [Google Scholar]
- 11.Blosser, R. S., and K. M. Gray. 2000. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 40:47-55. [DOI] [PubMed] [Google Scholar]
- 12.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Case, R. J., M. Labbate, and S. Kjelleberg. 2008. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J. 2:345-349. [DOI] [PubMed] [Google Scholar]
- 14.Chugani, S. A., M. Whiteley, K. M. Lee, D. D'Argenio, C. Manoil, and E. P. Greenberg. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 16.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 17.de Kievit, T., P. C. Seed, J. Nezezon, L. Passador, and B. H. Iglewski. 1999. RsaL, a novel repressor of virulence gene expression in Pseudomonas aeruginosa. J. Bacteriol. 181:2175-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekimpe, V., and E. Deziel. 2009. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155:712-723. [DOI] [PubMed] [Google Scholar]
- 19.Déziel, E., S. Gopalan, A. P. Tampakaki, F. Lépine, K. E. Padfield, M. Saucier, G. Xiao, and L. G. Rahme. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI, or the production of N-acyl-l-homoserine lactones. Mol. Microbiol. 55:998-1014. [DOI] [PubMed] [Google Scholar]
- 20.Dixon, R. A., D. Y. Xie, and R. B. Sharma. 2005. Proanthocyanidins: a final frontier in flavonoid research? New Phytologist 165:9-28. [DOI] [PubMed] [Google Scholar]
- 21.Driscoll, J. A., S. L. Brody, and M. H. Kollef. 2007. The epidemiology, pathogenesis, and treatment of Pseudomonas aeruginosa infections. Drugs 67:351-368. [DOI] [PubMed] [Google Scholar]
- 22.Du, Z. Z., P. J. Zhao, H. P. He, N. Zhu, X. J. Hao, and Y. M. Shen. 2005. The chemical constituents from the callus culture of Trewia nudiflora. Helv. Chim. Acta 88:2424-2429. [Google Scholar]
- 23.Eloff, J. N., D. R. Katerere, and L. J. McGaw. 2008. The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharmacol. 119:686-699. [DOI] [PubMed] [Google Scholar]
- 24.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 25.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao, M., M. Teplitski, J. B. Robinson, and W. D. Bauer. 2003. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant-Microbe Interact. 16:827-834. [DOI] [PubMed] [Google Scholar]
- 28.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez, J. E., and N. D. Keshavan. 2006. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 70:859-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gram, L., R. de Nys, R. Maximilien, M. Givskov, P. Steinberg, and S. Kjelleberg. 1996. Inhibitory effects of secondary metabolites from the red alga Delisea pulchra on swarming motility of Proteus mirabilis. Appl. Environ. Microbiol. 62:4284-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayer, R. J., and R. P. J. de Kok. 1998. Flavonoids and verbascoside as chemotaxonomic characters in the genera Oxera and Faradaya (Labiatae). Biochem. Syst. Ecol. 26:729-741. [Google Scholar]
- 32.Heurlier, K., F. Williams, S. Heeb, C. Dormond, G. Pessi, D. Singer, M. Camara, P. Williams, and D. Haas. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber, B., L. Eberl, W. Feucht, and J. Polster. 2003. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Naturforsch. C 58:879-884. [DOI] [PubMed] [Google Scholar]
- 34.Ishida, T., T. Ikeda, N. Takiguchi, A. Kuroda, H. Ohtake, and J. Kato. 2007. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol. 73:3183-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenthner, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 100:14339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jongkind, C. C. H. 1995. Prodromus for a revision of Combretum (Combretaceae) for Madagascar. Bull. Museum Natl. His. Nat. B Adansonia 17:191-200. [Google Scholar]
- 37.Juhas, M., L. Wiehlmann, B. Huber, D. Jordan, J. Lauber, P. Salunkhe, A. S. Limpert, F. von Gotz, I. Steinmetz, L. Eberl, and B. Tummler. 2004. Global regulation of quorum sensing and virulence by VqsR in Pseudomonas aeruginosa. Microbiology 150:831-841. [DOI] [PubMed] [Google Scholar]
- 38.Karamanoli, K., and S. E. Lindow. 2006. Disruption of N-acyl homoserine lactone-mediated cell signaling and iron acquisition in epiphytic bacteria by leaf surface compounds. Appl. Environ. Microbiol. 72:7678-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayser, O., and H. Kolodziej. 1997. Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 63:508-510. [DOI] [PubMed] [Google Scholar]
- 40.Keshavan, N. D., P. K. Chowdhary, D. C. Haines, and J. E. Gonzalez. 2005. l-Canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 187:8427-8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kretzschmar, U., V. Khodaverdi, J. H. Jeoung, and H. Gorisch. 2008. Function and transcriptional regulation of the isocitrate lyase in Pseudomonas aeruginosa. Arch. Microbiol. 190:151-158. [DOI] [PubMed] [Google Scholar]
- 42.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay, A., and B. M. M. Ahmer. 2005. Effect of sdiA on biosensors of N-acylhomoserine lactones. J. Bacteriol. 187:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manefield, M., R. de Nys, N. Kumar, R. Read, M. Givskov, P. Steinberg, and S. Kjelleberg. 1999. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145:283-291. [DOI] [PubMed] [Google Scholar]
- 45.Manefield, M., T. B. Rasmussen, M. Henzter, J. B. Andersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2002. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119-1127. [DOI] [PubMed] [Google Scholar]
- 46.Martini, N., and J. N. Eloff. 1998. The preliminary isolation of several antibacterial compounds from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 62:255-263. [DOI] [PubMed] [Google Scholar]
- 47.Martini, N. D., D. R. Katerere, and J. N. Eloff. 2004. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 93:207-212. [DOI] [PubMed] [Google Scholar]
- 48.Masoko, P., L. K. Mdee, L. J. Mampuru, and J. N. Eloff. 2008. Biological activity of two related triterpenes isolated from Combretum nelsonii (Combretaceae) leaves. Nat. Prod. Res. 22:1074-1084. [DOI] [PubMed] [Google Scholar]
- 49.Masoko, P., J. Picard, and J. N. Eloff. 2005. Antifungal activities of six South African Terminalia species (Combretaceae). J. Ethnopharmacol. 99:301-308. [DOI] [PubMed] [Google Scholar]
- 50.Mathesius, U., S. Mulders, M. Gao, M. Teplitski, G. Caetano-Anolles, B. G. Rolfe, and W. D. Bauer. 2003. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA 100:1444-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 52.McDougald, D., S. A. Rice, and S. Kjelleberg. 2007. Bacterial quorum sensing and interference by naturally occurring biomimics. Anal. Bioanal. Chem. 387:445-453. [DOI] [PubMed] [Google Scholar]
- 53.McGaw, L. J., T. Rabe, S. G. Sparg, A. K. Jager, J. N. Eloff, and J. van Staden. 2001. An investigation on the biological activity of Combretum species. J. Ethnopharmacol. 75:45-50. [DOI] [PubMed] [Google Scholar]
- 54.Mesaros, N., P. Nordmann, P. Plésiat, M. Roussel-Delvallez, J. Van Eldere, Y. Glupczynski, Y. Van Laethem, F. Jacobs, P. Lebecque, A. Malfroot, P. M. Tulkens, and F. Van Bambeke. 2007. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infect. 13:560-578. [DOI] [PubMed] [Google Scholar]
- 55.Muh, U., M. Schuster, R. Heim, A. Singh, E. R. Olson, and E. P. Greenberg. 2006. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob. Agents Chemother. 50:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parsek, M. R., and T. Tolker-Nielsen. 2008. Pattern formation in Pseudomonas aeruginosa biofilms. Curr. Opin. Microbiol. 11:560-566. [DOI] [PubMed] [Google Scholar]
- 57.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Persson, T., T. H. Hansen, T. B. Rasmussen, M. E. Skindersoe, M. Givskov, and J. Nielsen. 2005. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 3:253-262. [DOI] [PubMed] [Google Scholar]
- 60.Pesci, E. C., and B. H. Iglewski. 1997. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 5:132-135. [DOI] [PubMed] [Google Scholar]
- 61.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. G. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prithiviraj, B., H. P. Bais, T. Weir, B. Suresh, E. H. Najarro, B. V. Dayakar, H. P. Schweizer, and J. M. Vivanco. 2005. Down regulation of virulence factors of Pseudomonas aeruginosa by salicylic acid attenuates its virulence on Arabidopsis thaliana and Caenorhabditis elegans. Infect. Immun. 73:5319-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahme, L. G., F. M. Ausubel, H. Cao, E. Drenkard, B. C. Goumnerov, G. W. Lau, S. Mahajan-Miklos, J. Plotnikova, M. W. Tan, J. Tsongalis, C. L. Walendziewicz, and R. G. Tompkins. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasch, M., T. B. Rasmussen, J. B. Andersen, T. Persson, J. Nielsen, M. Givskov, and L. Gram. 2007. Well-known quorum sensing inhibitors do not affect bacterial quorum sensing-regulated bean sprout spoilage. J. Appl. Microbiol. 102:826-837. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen, T. B., T. Bjarnsholt, M. E. Skindersoe, M. Hentzer, P. Kristoffersen, M. Kote, J. Nielsen, L. Eberl, and M. Givskov. 2005. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 187:1799-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasmussen, T. B., M. E. Skindersoe, T. Bjarnsholt, R. K. Phipps, K. B. Christensen, P. O. Jensen, J. B. Andersen, B. Koch, T. O. Larsen, M. Hentzer, L. Eberl, N. Hoiby, and M. Givskov. 2005. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 151:1325-1340. [DOI] [PubMed] [Google Scholar]
- 69.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 70.Rudrappa, T., and H. P. Bais. 2008. Curcumin, a known phenolic from curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 56:1955-1962. [DOI] [PubMed] [Google Scholar]
- 71.Schuster, M., and E. P. Greenberg. 2007. Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genomics 8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuster, M., and E. P. Greenberg. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296:73-81. [DOI] [PubMed] [Google Scholar]
- 73.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skindersoe, M. E., M. Alhede, R. Phipps, L. Yang, P. O. Jensen, T. B. Rasmussen, T. Bjarnsholt, T. Tolker-Nielsen, N. Hoiby, and M. Givskov. 2008. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3648-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skindersoe, M. E., P. Ettinger-Epstein, T. B. Rasmussen, T. Bjarnsholt, R. de Nys, and M. Givskov. 2008. Quorum sensing antagonism from marine organisms. Mar. Biotechnol. 10:56-63. [DOI] [PubMed] [Google Scholar]
- 76.Smith, A. W., and B. H. Iglewski. 1989. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 17:10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teplitski, M., H. Chen, S. Rajamani, M. Gao, M. Merighi, R. T. Sayre, J. B. Robinson, B. G. Rolfe, and W. D. Bauer. 2004. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 134:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teplitski, M., J. B. Robinson, and W. D. Bauer. 2000. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant-Microbe Interact. 13:637-648. [DOI] [PubMed] [Google Scholar]
- 79.Toder, D. S., S. J. Ferrell, J. L. Nezezon, L. Rust, and B. H. Iglewski. 1994. lasA and lasB genes of Pseudomonas aeruginosa: analysis of transcription and gene product activity. Infect. Immun. 62:1320-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toder, D. S., M. J. Gambello, and B. H. Iglewski. 1991. Pseudomonas aeruginosa LasA: a second elastase under the transcriptional control of lasR. Mol. Microbiol. 5:2003-2010. [DOI] [PubMed] [Google Scholar]
- 80a.Van Delden, C., E. C. Pesci, J. P. Pearson, and B. H. Iglewski. 1998. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect. Immun. 66:4499-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wade, D. S., M. W. Calfee, E. R. Rocha, E. A. Ling, E. Engstrom, J. P. Coleman, and E. C. Pesci. 2005. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J. Bacteriol. 187:4372-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whitehead, N. A., M. Welch, and G. P. C. Salmond. 2001. Silencing the majority. Nat. Biotechnol. 19:735-736. [DOI] [PubMed] [Google Scholar]
- 83.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams, P., K. Winzer, W. C. Chan, and M. Camara. 2007. Look who's talking: communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1119-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winzer, K., C. Falconer, N. C. Garber, S. P. Diggle, M. Camara, and P. Williams. 2000. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 182:6401-6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolz, C., E. Hellstern, M. Haug, D. R. Galloway, M. L. Vasil, and G. Doring. 1991. Pseudomonas aeruginosa LasB mutant constructed by insertional mutagenesis reveals elastolytic activity due to alkaline proteinase and the LasA fragment. Mol. Microbiol. 5:2125-2131. [DOI] [PubMed] [Google Scholar]
- 87.Yang, L., M. T. Rybtke, T. H. Jakobsen, M. Hentzer, T. Bjarnsholt, M. Givskov, and T. Tolker-Nielsen. 2009. Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrob. Agents Chemother. 53:2432-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu, O., and J. M. Jez. 2008. Nature's assembly line: biosynthesis of simple phenylpropanoids and polyketides. Plant J. 54:750-762. [DOI] [PubMed] [Google Scholar]
- 89.Yuan, Z. C., M. P. Edlind, P. Liu, P. Saenkham, L. M. Banta, A. A. Wise, E. Ronzone, A. N. Binns, K. Kerr, and E. W. Nester. 2007. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc. Natl. Acad. Sci. USA 104:11790-11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan, Z. C., E. Haudecoeur, D. Faure, K. F. Kerr, and E. W. Nester. 2008. Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signal salicylic acid, indole-3-acetic acid and gamma-amino butyric acid reveals signalling cross-talk and Agrobacterium-plant co-evolution. Cell Microbiol. 10:2339-2354. [DOI] [PubMed] [Google Scholar]
- 91.Zeng, Z., L. Qian, L. Cao, H. Tan, Y. Huang, X. Xue, Y. Shen, and S. Zhou. 2008. Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 79:119-126. [DOI] [PubMed] [Google Scholar]
- 92.Zhang, X., and H. Bremer. 1995. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J. Biol. Chem. 270:11181-11189. [DOI] [PubMed] [Google Scholar]