Abstract
The basidiomycetous genus Wallemia is an active inhabitant of hypersaline environments, and it has recently been described as comprising three halophilic and xerophilic species: Wallemia ichthyophaga, Wallemia muriae, and Wallemia sebi. Considering the important protective role the fungal cell wall has under fluctuating physicochemical environments, this study was focused on cell morphology changes, with particular emphasis on the structure of the cell wall, when these fungi were grown in media with low and high salinities. We compared the influence of salinity on the morphological characteristics of Wallemia spp. by light, transmission, and focused-ion-beam/scanning electron microscopy. W. ichthyophaga was the only species of this genus that was metabolically active at saturated NaCl concentrations. W. ichthyophaga grew in multicellular clumps and adapted to the high salinity with a significant increase in cell wall thickness. The other two species, W. muriae and W. sebi, also demonstrated adaptive responses to the high NaCl concentration, showing in particular an increased size of mycelial pellets at the high salinities, with an increase in cell wall thickness that was less pronounced. The comparison of all three of the Wallemia spp. supports previous findings relating to the extremely halophilic character of the phylogenetically distant W. ichthyophaga and demonstrates that, through morphological adaptations, the eukaryotic Wallemia spp. are representative of eukaryotic organisms that have successfully adapted to life in extremely saline environments.
Hypersaline habitats had long been considered to be populated almost exclusively by prokaryotic organisms and the research on hypersaline environments had consequently been monopolized by bacteriologists. In 2000, the first reports appeared showing that fungi are active inhabitants of solar salterns (20). Until then, fungi able to survive in environments with a low amount of biologically available water (low water activity [aw]) were only known as contaminants of foods preserved with high concentrations of salt or sugar. Since their first discovery in salterns, many new species have been discovered in natural hypersaline environments around the world, including some species that were previously known only as food-borne contaminants. Due to these discoveries, fungi are now recognized as an integral part of indigenous halophilic microbial communities since they can grow and adjust across the whole salinity range, from freshwater to almost saturated NaCl solutions (49). Most fungi differ from the majority of halophilic prokaryotes (16): they tend to be extremely halotolerant rather than halophilic and do not require salt to remain viable, with the exception of Wallemia spp.
The order Wallemiales (Wallemiomycetes, Basidiomycota) was only recently introduced to define the single genus Wallemia, a phylogenetic maverick in the Basidiomycota (49). Until 2005, this genus contained only the species W. sebi. However, taxonomic analyses of isolates from sweet, salty, and dried foods (41) and from hypersaline evaporation ponds in the Mediterranean Sea, the Caribbean, and the Dead Sea (45, 49) have resolved this genus into three species: W. ichthyophaga, W. muriae, and W. sebi. The first two of these three Wallemia spp. require additional solutes in the growth media, and W. ichthyophaga is the most halophilic eukaryote described to date, since it cannot grow without the addition of 9% NaCl (wt/vol), and it still shows growth at aw of 0.77, equivalent to 30% NaCl (wt/vol) (49).
The survival, and especially the growth, of microorganisms in highly saline environments requires numerous adaptations (6, 18, 21, 34). The dominant representatives and the most thoroughly investigated halophilic fungi in hypersaline waters of the salterns are the black yeasts, and particularly the model organism Hortaea werneckii (20). An important level of adaptation of the black yeasts to high salinity is seen in their extremophilic ecotype, which is characterized by a special meristematic morphology and changes in cell wall structure and pigmentation (27). Other fungal osmoadaptations include the accumulation of osmolytes (27, 28, 40), the extrusion of sodium (5), modification of the plasma membrane (44) and the cell wall, and even changes in fungal colony morphology (27).
The fungal cell wall is the first line of defense against environmental stress; therefore, adaptation at the cell wall level is expected to have one of the most important roles for successful growth at a low aw (24, 32). The cell wall is essential for maintaining the osmotic homeostasis of cells, since it protects them against mechanical damage as well as high concentrations of salts (7). The central fibrillar glycan network of the cell wall is embedded in highly flexible amorphous cement, which allows considerable stretching with changing osmotic pressure (14, 29). Its balance between a rigid and a dynamic structure influences the shape of cells (14) and enables growth and hyphal branching (11).
Since the species within the genus Wallemia have been recognized only recently (49), little is known about their mechanisms of adaptation to high salinity. To investigate the effects of low and high NaCl concentrations on cell morphology, with particular emphasis on cell wall ultrastructure, we compared W. ichthyophaga, the most halophilic fungal species known thus far, with the related xerophilic W. muriae and W. sebi. Micrographs were prepared by using light, transmission, and scanning electron microscopy. The results reveal how this eukaryotic genus uses adaptations at the cell wall level for thriving in extremely saline environments.
MATERIALS AND METHODS
Organisms and culture conditions.
The fungi under study were maintained in the Culture Collection of Extremophilic Fungi (EXF) of the Department of Biology, Biotechnical Faculty, University of Ljubljana (Ljubljana, Slovenia) and in the Centraalbureau voor Schimmelcultures (CBS; Utrecht, The Netherlands). Wallemia ichthyophaga EXF-994 (CBS 113033) and Wallemia muriae EXF-951 (CBS 116628) were isolated from hypersaline waters of the Sečovlje Adriatic solar saltern in Slovenia. Wallemia sebi EXF-958 (CBS 818.96) was isolated from sunflower seeds in Sweden (49).
Growth conditions and determination of growth characteristics.
Cultures of W. ichthyophaga were maintained on solid malt extract medium (2% malt extract [19]) with 10% NaCl, and those of W. muriae and W. sebi were maintained on malt extract, yeast extract, and 50% glucose agar (MY50G; 2% malt extract, 0.5% yeast extract [39]); all were stored at 4°C.
These fungi were cultured in liquid and on solid yeast nitrogen base (YNB) medium [1.7 g of YNB, 5 g of (NH4)2SO4 per liter, 0.8 g of complete supplement mixture per liter, 20 g of glucose per liter, 20 g of agar per liter for solid medium (pH 7.0)]. For determination of the growth across the NaCl concentration range, the aw of the media was adjusted with NaCl [0 to 30% (wt/vol)]. The growth media were autoclaved at 121°C for 15 min. Cultures in liquid media were incubated in the dark at 28°C with constant shaking at 180 rpm. Cultures on solid YNB medium were incubated in the dark at 24°C.
For each of the Wallemia spp., growth was monitored at one low and one high salinity (W. ichthyophaga at 15 and 25% [wt/vol] NaCl; W. muriae and W. sebi at 5 and 20% [wt/vol] NaCl). Preculturing on the YNB medium of the same salinity as in the experiment was used to adapt the fungi to the media and growth conditions used. The cultures for the experiments were inoculated from precultures in the exponential phase of growth (inoculum was 1% of the final culture volume). The growth of the fungal strains in the liquid YNB medium was determined from samples every 2 days. The dry biomass weight was used, since the formation of densely interwoven mycelial masses referred to as cell pellets did not allow reliable measures of the optical density. The suspension of the pellets was filtered through nitrocellulose filter (pore size, 1.2 μm) and dried at 100°C to a constant weight. The growth curves were constructed from two independent experiments, each carried out in duplicate, and used to determine the final fungal biomass yield. Growth rates and generation times were calculated from the doubling times obtained from the growth curves during the exponential growth phase.
Sample preparation for light microscopy.
Cells from cultures grown to the mid-exponential growth phase were analyzed with an Olympus BX51 light microscope equipped with an Olympus DP12 digital camera. The cell sizes and sizes of hyphal compartment and multicellular clumps were measured in DP-Soft 3.2 (Olympus).
Sample preparation for transmission electron microscopy (TEM).
Cells from cultures grown to mid-exponential growth phase were filtered through nitrocellulose filter (pore size, 1.2 μm) and fixed in 2.5% glutaraldehyde and 4% paraformaldehyde buffered in 0.1 M sodium phosphate buffer (pH 7.2) for 2 h at room temperature. NaCl was added into the fixative to the same osmolarity as in the growth media. After fixing, the cells were rinsed three times with 0.1 M sodium phosphate buffer with descending concentrations of NaCl. This optimization of the fixation procedure was necessary since the standard fixation without NaCl caused osmotic shock and a collapse of the cell structure. Due to their small size, the samples were embedded in 3% agarose, and cut into small blocks of about 1 by 1 by 5 mm. Agarose embedding prevented cell loss during the processing and also allowed better penetration of chemicals into the fungal cells. Postfixing was performed in 1% OsO4 in distilled water with a drop of 0.1 M sodium phosphate buffer, for 24 h at 4°C. After three washes in distilled water, the samples were dehydrated through a graded series of ethanol solutions (vol/vol): 30% (10 min), 50% (twice for 10 min each time), 70% (twice for 10 min each time), 80% (twice for 10 min each time), 90% (twice for 10 min each time), absolute ethanol (three times for 10 min each time). Agar 100 resin (Agar Scientific) was used for embedding. Ultrathin sections (70 to 90 nm) were cut with a diamond knife and contrasted with uranyl acetate and lead citrate. The sections were analyzed with a Philips CM 100 transmission electron microscope (80 kV, with a Gatan Bioscan Camera 792 digital camera) using Digital Micrograph 3.3.1.
Sample preparation for standard and focused-ion-beam/scanning electron microscopy (FIB/SEM).
A focused-ion-beam scanning electron microscope is a system with both electron and ion beam columns, allowing the same sample to be investigated using either of the beams. A focused beam of ions, usually gallium, is used to image the sample in the chamber and to section the surface to expose the interior.
Cells from cultures grown to mid-exponential growth phase were filtered through a polycarbonate filter placed into a polypropylene filter holder, using a plastic syringe (Agar Scientific). All preparation steps, including the fixing and dehydration, were similar for TEM and for both standard and focused-ion-beam SEM. For standard and focused-ion-beam scanning electron microscopy, the samples were not embedded but were instead dried in 1,1,1,3,3,3-hexamethyldisilazan for 45 min at room temperature. After drying, the cells were mounted on aluminum stubs and coated with gold by magnetron sputtering (Bal-Tec SCD 050 sputter coater). The samples were analyzed with an FEI Strata DB 235 M scanning electron microscope or a Sirion 400 NC focused-ion-beam/scanning electron microscope (17).
Statistical analyses.
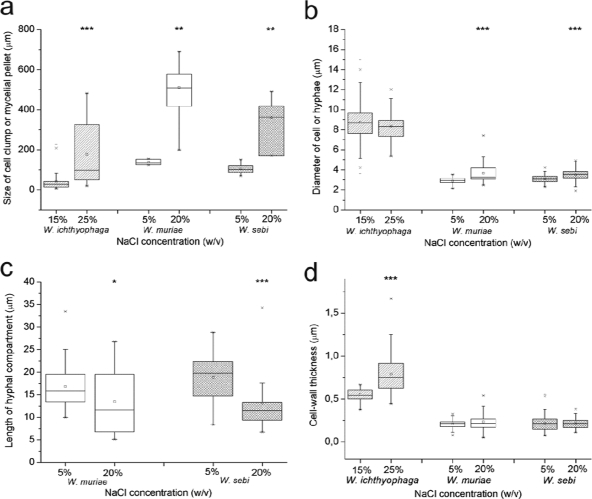
The number of samples analyzed is shown in legend to Fig. 3. The differences among the medians of the cell and cluster sizes, as well as the hyphal compartment lengths of all three of the Wallemia spp. at the low and the high salinities, were compared by using the Mann-Whitney U test. The differences between the medians of the cell wall thicknesses were compared by Kruskal-Wallis test, which determined whether the median of the cell wall thicknesses of W. ichthyophaga differed significantly from those of W. muriae and W. sebi. All calculations were done by using STAT-GRAPHICS Plus 4.0 statistics software for Windows. Statistical differences between two salinities were categorized into three groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
FIG. 3.
Morphological characteristics of Wallemia spp. at the low and high salinities. Box plots of morphological characteristics show median (line in box), 75th percentile (upper edge of box), 25th percentile (lower edge of box), and minimum and maximum data values (whiskers) and outliers (×). (a) Significant increases in size of the cell clumps (W. ichthyophaga, n = 162 [15%] and n = 43 [25%]) and mycelial pellets (W. muriae, n = 5 [5%] and n = 12 [20%]; and W. sebi, n = 9 [5%] and n = 4 [20%]) at the high salinities. (b) Nonsignificant changes in cell size of W. ichthyophaga (n = 113 [15%] and n = 36 [25%]) and significant increases in hyphal diameter of W. muriae (n = 35 [5%] and n = 51 [20%]) and W. sebi (n = 61 [5%] and n = 42 [20%]) at the high salinities. (c) Significant decrease for W. muriae (n = 38 [5%] and n = 19 [20%]) and W. sebi (n = 29 [5%] and n = 21 [20%]) in hyphal compartment length at the high salinity. (d) Significant differences in cell wall thickness of W. ichthyophaga (n = 40 [15%] and n = 71 [25%]) compared to W. muriae (n = 87 [5%] and n = 84 [20%]) and W. sebi (n = 120 [5%] and n = 118 [20%]) at the high salinities. Statistically significant differences are marked (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
RESULTS
Growth of Wallemia spp. at various salinities.
W. ichthyophaga, W. muriae, and W. sebi were grown in liquid YNB media supplemented with a broad range of NaCl concentrations. Growth tests showed that W. ichthyophaga grew from 9 to 30% (wt/vol) NaCl, whereas the W. muriae NaCl growth range extended from 4 to 25% (wt/vol) NaCl. W. sebi grew at up to 27% NaCl and was the only one of the tested species that also grew on media without NaCl (Table 1) .
TABLE 1.
Growth parameters of Wallemia spp. at low and high salinitiesa
| Organism | Growth range (% NaCl) | Specific growth rate (g g−1 h−1) |
Final biomass yield (mg/100 ml of medium) |
Duration of lag phase (days) |
|||
|---|---|---|---|---|---|---|---|
| LS | HS | LS | HS | LS | HS | ||
| W. ichthyophaga | 9-30 | 0.123 | 0.083 | 514.3 | 449.2 | 5 | 7 |
| W. muriae | 4-25 | 0.078 | 0.045 | 66.5 | 35.8 | 2 | 4 |
| W. sebi | 0-27 | 0.164 | 0.057 | 83.8 | 64.1 | 1 | 3 |
LS, low salinity: 5% NaCl for W. muriae and W. sebi and 15% NaCl for W. ichthyophaga. HS, high salinity: 20% NaCl for W. muriae and W. sebi and 25% NaCl for W. ichthyophaga.
According to their growth across the NaCl concentration range, we selected two NaCl concentrations for each of the species to compare the influence of salinity on the morphological characteristics of Wallemia spp. Therefore, all further investigations were carried out at a low NaCl concentration (15% NaCl for W. ichthyophaga and 5% NaCl for W. muriae and W. sebi) and a high NaCl concentration (25% NaCl for W. ichthyophaga and 20% NaCl for W. muriae and W. sebi). The growth parameters, including the specific growth rate, the final fungal biomass yield, and the duration of lag phase were obtained from the growth curves of each of these species at each of the above-mentioned salinities, and are presented in Table 1.
In the media with the high NaCl concentrations, the duration of the lag phase prolonged for 2 days in all three Wallemia spp. Furthermore, the growth rates and the final biomass yields of all of the three species were also reduced. At the high salinities, W. ichthyophaga reached 87% of its final biomass yield produced at the low salinity, whereas W. muriae and W. sebi reached only 54 and 77%, respectively, of the final biomass they produced at the low salinity.
Morphology and cell wall ultrastructural changes in W. muriae and W. sebi.
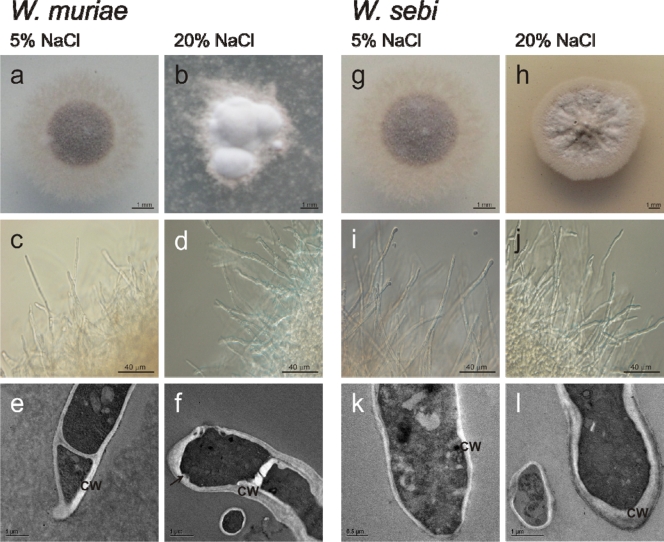
Since similar morphological characteristics were seen for the more closely related species of W. muriae and W. sebi, these two species are presented together. On solid YNB medium at 5 and 20% NaCl, the colonies of W. muriae (Fig. 1a and b; Table 2) and W. sebi (Fig. 1g and h) differed in size, color, and the extent of the area that was in direct contact with the medium.
FIG. 1.
NaCl effects on morphology of W. muriae and W. sebi. W. muriae (a, b, c, d, e, and f) and W. sebi (g, h, i, j, k, and l), showing colonies at 5% (a and g) and 20% (b and h) NaCl, hyphal morphology at 5% (c and i) and 20% (d and j) NaCl, and TEM micrographs at 5% (e and k) and 20% (f and l) NaCl. CW, cell wall; arrow, cell wall indentation.
TABLE 2.
Morphological characteristics of Wallemia spp.
| Organism | % NaCl in growth medium | Cell type or morphology | Colony morphology on solid medium | Presence or absence ofa: |
|
|---|---|---|---|---|---|
| Cell wall indentations | EPS | ||||
| W. ichthyophaga | 15 | Multicellular clumps | Light brown to yellowish/olive green; punctiform; heaped; soft; spreading deeply into the agar; yellow reverse (see Fig. 4a) | + | +++ |
| 25 | Multicellular clumps | White, yellowish to light green; heaped; soft; did not spread deeply into the agar; reduced area in contact with agar; light yellow reverse (see Fig. 4b) | ++ | ++ | |
| W. muriae | 5 | Hyphae | Brown; round shape with light margin; powdery (high no. of spores), dry; spreading deeply into the agar; brown reverse (see Fig. 1a) | - | + |
| 20 | Hyphae | Light brown to white; when white also dusty (high no. of spores); variable shape with light beige shaggy margin; heaped, powdery, dry; spreading into the agar; reduced area in contact with agar, pronounced aerial hyphae; brown reverse (see Fig. 1b) | + | + | |
| W. sebi | 5 | Hyphae | Light brown, with the center being lighter, with light tan hyphae; round shape with light shaggy marginal surface; powdery (high no. of spores), dry; spreading deeply into the agar; brown reverse (see Fig. 1g) | - | + |
| 20 | Hyphae | Rust brown with light margin, off white in the center; powdery, dry; spreading into the agar, not deeply; reduced area in contact with agar, brown reverse (see Fig. 1h) | + | + | |
-, Not present; +, present; ++, pronounced; +++, abundant.
In submerged culture, W. muriae and W. sebi grew in filamentous form at both low and high salinities (Fig. 1c, d, i, and j). Filamentous hyphae branched and intertwined into compact mycelial pellets, densely interwoven mycelial masses. At the low NaCl concentration, these pellets were darker and round shaped, whereas at the high salinity, they were light brown and variable in shape (Fig. 2a and b). Hyphal tips at the outer part of mycelial pellets were shorter at the high salinity (Fig. 2a and b). Furthermore, the morphology of the mycelial pellets at the low and high salinities differed significantly in size (W. muriae, P < 0.01; W. sebi, P < 0.01; Fig. 3a). At the high salinities, the mycelial pellets of W. muriae and W. sebi were four- and threefold larger, respectively (Fig. 3a). The high NaCl concentration resulted in a significant increase in hyphal diameter for both W. muriae and W. sebi and a decrease in the hyphal compartment length, which was significant for both species (Fig. 3b and c). Also, the hyphal branching was more pronounced, compared to the low salinity. These cells did not aggregate into multicellular clumps at any of the salinities tested. Indeed, scanning electron micrographs of W. muriae and W. sebi mycelial pellets (Fig. 2c) showed relatively smooth and regular hyphal surfaces at both of the salinities tested.
FIG. 2.
Mycelial pellet morphologies of W. sebi. (a and b) Morphology of W. sebi mycelial pellet at 5% (a) and 20% (b) NaCl. (c) SEM micrograph of W. sebi mycelial pellet at 5% NaCl.
The analyses of the transmission electron micrographs of W. muriae and W. sebi hyphae at the low salinity (5% NaCl) showed less variability in the hyphal diameters (Fig. 3b) and pronounced pigmentation of the cell wall. The cell wall thickness was ca. 0.2 μm. At the high salinity, there was a minor, and not significant, increase in cell wall thickness in both of these species (Fig. 3d). In addition, with both W. muriae and W. sebi, cell wall indentations appeared, and the intracellular vacuolation changed. In contrast to larger vacuoles in W. muriae and W. sebi grown at the low salinity, at the high salinity smaller but numerous vacuoles were observed. Independent of the NaCl concentrations in the growth media, the outer cell wall layers of both species were covered with a small amount of unpronounced fibrous extracellular polymeric substances (EPS) (Table 2; see Fig. 6b and c).
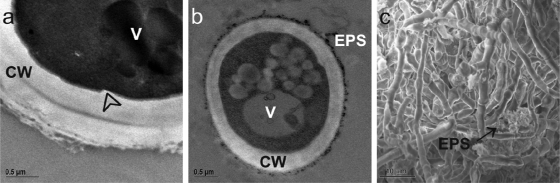
FIG. 6.
Morphology details of W. ichthyophaga, W. muriae, and W. sebi. (a) TEM micrograph of structured cell wall of W. ichthyophaga, when cultured in liquid YNB with 25% NaCl. (b) Unpronounced EPS of W. muriae at 5% NaCl. (c) SEM micrograph of W. sebi at 20% NaCl. Arrowhead, cell wall indentation; arrow, EPS (c); CW, cell wall; V, vacuoles.
Morphology and cell wall ultrastructural changes in W. ichthyophaga.
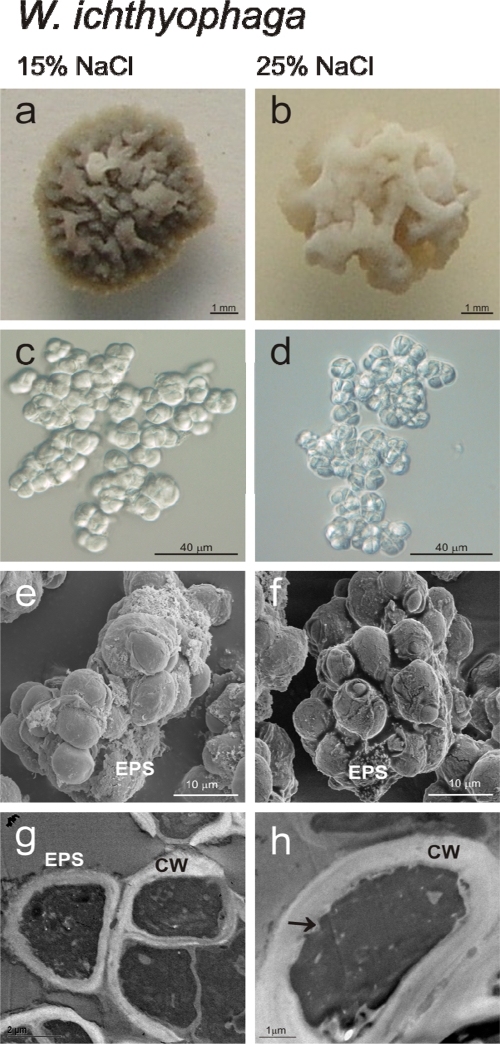
The W. ichthyophaga colonies on solid media at the low and high salinities also differed in size and color (Fig. 4a and b; Table 2). When grown in liquid medium, the most distinct morphological feature that distinguished W. ichthyophaga from W. muriae and W. sebi was the growth of W. ichthyophaga in multicellular clumps, composed of multiple spherical cells (Fig. 4c and d). These clumps were significantly larger at 25% NaCl (Fig. 3a). The high NaCl concentration resulted in a minor, but not significant, decrease in cell size (Fig. 3b). Scanning electron microscopy also revealed differences in the surface appearances of the multicellular clumps at the high salinity (25% NaCl). Here, the surfaces of the cells were more corrugated and less fully covered with EPS (Fig. 4e and f). Focused-ion-beam sectioning of these W. ichthyophaga clumps exposed the interior of the densely packed cells (Fig. 5). The image shown in Fig. 5 illustrates the presence of some EPS, which seals the spaces between the cells.
FIG. 4.
NaCl effects on morphology of W. ichthyophaga. (a and b) Colony morphology on solid YNB at 15% (a) and 25% (b) NaCl. (c and d) Multicellular clumps, when cultured in liquid YNB media at 15% (c) and 25% (d) NaCl. (e and f) SEM micrographs of multicellular clumps, when cultured in liquid YNB with 15% (e) and 25% (f) NaCl. (g and h) TEM micrographs of cells, when cultured in liquid YNB with 15% (g) and 25% (h) NaCl. CW, cell wall; arrow, cell wall indentation.
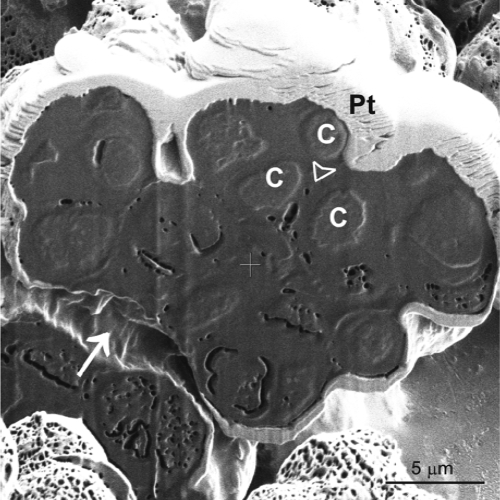
FIG. 5.
Morphology of multicellular clumps of W. ichthyophaga. Secondary electron micrograph of focused-ion-beam sectioned multicellullar clump of W. ichthyophaga, when cultured in liquid YNB with 25% NaCl. Pt, protective platinum layer against surface damage during focused-ion-beam sectioning; C, cells; arrow, space between two multicellular clumps; arrowhead, space between the cells in the clump.
TEM of the W. ichthyophaga cells sampled in the exponential growth phase at 15 and 25% NaCl showed significant differences in the cell wall thicknesses (Fig. 3d). At the low salinity, the cell walls were uneven (Fig. 4g and h), with thicknesses up to 0.6 μm, while at the high salinity the thicknesses increased to up to 1.6 μm (a 1.67-fold increase). A comparison of the median cell wall thicknesses of all three of the Wallemia spp. according to the Kruskal-Wallis test showed that at the high salinities, the cell wall thickness of W. ichthyophaga increased significantly (P < 0.001) compared to W. muriae and W. sebi (data shown in Fig. 3d). At both salinities, the thick cell walls of W. ichthyophaga were clearly structured in at least two distinct layers: a thinner, electron-dense outer layer, and a thicker, electron-translucent inner layer (Fig. 6a). At the low NaCl concentration with W. ichthyophaga, the outer cell wall layer was covered with a clearly visible fibrous layer that was wide and well defined and composed of EPS (Fig. 4g). At both the low and the high salinities, there were also cell wall indentations, which were more numerous at high salinity (Fig. 4 h, marked with an arrow).
DISCUSSION
Halophilic and halotolerant fungi show a surprisingly rich diversity and abundance in natural saline environments, such as salt crystals, food preserved with high concentrations of salt, and solar salterns (8-10, 20), where biologically available water is limited. The ability to grow at a low aw is apparent in 10 phylogenetically unrelated orders of fungi and is in most cases limited to a few species or to a single genus within an order. Moreover, the most representative of the xerophilic fungi belong to the division Ascomycota (16). However, the entire genus Wallemia, and therefore the entire phylogenetically old and separate order Wallemiales within the division Basidiomycota, is either xerophilic or xerotolerant. Two of the three Wallemia spp. show distinctly improved growth with NaCl as the solute (49). Their growth across increasing NaCl concentration ranges shown here confirm these previous findings of Zalar et al. (49). Indeed, W. ichthyophaga and W. muriae grew only in the presence of NaCl, whereas W. sebi grew on media without added NaCl. To our knowledge, W. ichthyophaga and W. muriae are the only fungal species that show a requirement for NaCl as a solute in the growth medium. Moreover, W. ichthyophaga was the only one of the three Wallemia spp. that also grew in saturated NaCl (i.e., 30% [wt/vol] NaCl).
The influence of high salinity on growth is seen in the growth characteristics of the Wallemia spp. (see Table 1). All three of these species had both lower specific growth rates and final biomass yields at the high salinities. Growth at high salinities requires the adaptation of cellular metabolism. The reductions in the final biomass yields indicate the high energy demands of life at high NaCl concentrations, even in such well-adapted species (36). However, the growth rates and the final biomass yields of W. ichthyophaga were higher at both the low and the high salinities than those of W. muriae and W. sebi. This suggests better adaptation of W. ichthyophaga to high NaCl concentrations in the growth medium.
Our data demonstrate that NaCl concentrations have an impact on the cell morphology of the Wallemia spp. At a high salinity, the hyphal compartments in both W. muriae and W. sebi were thicker and shorter compared to what we observed at the low salinity. Such changes of the cell morphology resulted in changed size and shape of the mycelial pellets. The pellets of both W. muriae and W. sebi were small and regularly shaped at the low salinity, whereas at the high salinity, they were larger and variable in shape (Fig. 2). Shortening and thickening of the hyphal compartments and similar changes in pellet morphology have also been seen in less salt-adapted fungi, such as in the halotolerant Aspergillus repens when grown on medium with 12% NaCl (wt/vol) (25). At high salinity, we noted that the mycelial pellets of both W. muriae and W. sebi were less pigmented. In previous studies, a series of unique pyrrolylpolyene pigments of W. sebi were isolated and characterized (2-4), but no studies exist on the effects of high salinities on the pigment expression in Wallemia spp. Although pigmentation can protect cells from various harmful conditions and agents (11, 33), we would assume that the synthesis of these pigments is reduced at the high salinity due to high energy requirements (36).
In contrast to the mycelial growth of W. muriae and W. sebi, W. ichthyophaga grows in multicellular clumps, or sarcinalike structures (49), composed of multiple spherical cells. These clumps were significantly larger at the high salinity, whereas the sizes of cells in the clumps did not change significantly. The ability of microorganisms to organize into multicellular communities, as shown for W. ichthyophaga, can greatly enhance their survival in natural stressful environments as was proposed for yeast populations (37). Similar multicellular structures have also been seen at high salinities in the phylogenetically distant, extremely halotolerant ascomycetous black yeasts, such as Hortaea werneckii (T. Kogej and N. Gunde-Cimerman, unpublished data), Phaeotheca triangularis (15, 48), and Trimmatostroma salinum (27), all of the order Dothideales. The ability to grow meristematically, the common feature of black yeasts at the high salinities, is hypothesized to enhance the ability to survive under conditions of stress (46), such as a high concentration of NaCl (47). Thus, we can conclude that multicellular clumps of W. ichthyophaga could also be a mode of protection at the high salinity.
The surface structures of the multicellular clumps of W. ichthyophaga and the morphological characteristics of their interiors were studied by FIB/SEM. FIB/SEM represents an upgraded imaging approach for biological samples when surface morphology and subsurface structures are being investigated at the same time across a range of magnifications (17, 22). The use of FIB/SEM techniques has revealed that the cells of W. ichthyophaga are densely packed into multicellular clumps, since a dense homogenous structure appeared after sectioning. If the cells were just loosely attached to each other, there should be gaps seen between the cells after FIB/SEM imaging (35). The presence of EPS was also seen by SEM in all three of the Wallemia spp. at the low and high salinities. The ability to produce a certain amount of EPS is also a morphological feature of rock-inhabiting black fungi, in which EPS is involved in the protection against cycles of desiccation, freezing, and thawing (42). It thus appears that EPS might also have a protective function.
TEM shows that in all three Wallemia spp., the cell wall is multilayered, or at least bilayered, at all salinities. It consists of a thick electron-translucent inner layer and a thin electron-dense outer layer. In ascomycetous fungi, the inner layer provides mechanical strength and attachment sites for the proteins that form the outer layer. The data on less-investigated basidiomycetes also suggest that the outer, electron-dense, cell wall layer is proteinaceous (14).
In the present study, increases in cell wall thickness are shown to correlate with successful growth at a low aw, especially in W. ichthyophaga (Table 1; Fig. 3d). It is noteworthy that at the low salinities, the cell walls of cells in the multicellular clumps of W. ichthyophaga were threefold thicker than the cell walls of the hyphae of both W. muriae and W. sebi. At the high salinities, the hyphal cell walls of both W. muriae and W. sebi barely increased in thickness, whereas the thickness of the W. ichthyophaga cell walls increased significantly (0.8 ± 0.2 μm at the high salinity as seen in Fig. 3d). Similarly thick cell walls (0.5 to 1.0 μm) were reported for halophilic black yeasts of the genus Trimmatostroma exposed to high salinities (27) and for the marine fungus Dendryphiella salina (12). Clipson et al. (12) proposed that the thickened cell wall acts as a fungal survival mechanism in extremely saline environments. However, a thickened cell wall is not a general fungal response to salt stress, since certain moderately halotolerant fungi, such as Aspergillus flavus and Penicillium roquefortii, show decreased cell wall thickness under 8% NaCl salt stress, with an increased thickness of the cell membranes seen instead (1). We have also seen a decrease in cell wall thickness with 17% NaCl in the moderately halotolerant black yeast Aureobasidium pullulans, from 0.20 μm in nonsaline growth medium to 0.15 μm (Kogej and Gunde-Cimerman, unpublished). This has led us to speculate that the thickness of the cell wall and the ability to increase the cell wall thickness at higher salinities is an important feature of halophilic fungi but not of halotolerant fungi.
A thickened cell wall is probably related to changes in the molecular composition. It can be a consequence of the branching degree of the polysaccharides (31), an elevated β-1,3-glucan level (31), incorporation of proteins into the cell wall as a result of changed glucan synthesis (43), or elevated chitin levels (24, 26, 31, 38). The mechanisms involved in the cell wall thickening of the Wallemia spp. remain to be elucidated in further studies. However, our preliminary data on the differential expression of W. ichthyophaga genes at low and high salinities might hint at the cross connections between cell wall restructuring processes and fungal osmoadaptation. Our data have shown a pronounced increase in the expression of the cellulase/exo-1,3-β-glucanase gene (i.e., EXG1) that correlate with increasing salinity (C. Gostinčar and N. Gunde-Cimerman, unpublished data). Exo-1,3-β-glucanase is involved in the hydrolysis of glucosidic linkages of cell wall β-1,3-glucans and thus participates in morphogenetic processes. It has also been shown in Saccharomyces cerevisiae that the expression of the EXG1 gene is upregulated when Pbs2, the HOG pathway mitogen-activated protein kinase (MAPK), is overexpressed (23). The partial amino acid sequences of Hog1-like MAPKs from Wallemia spp. have already been determined and compared to the Hog1 protein from S. cerevisiae (30). It remains to be determined, however, whether in W. ichthyophaga there is a pathway analogous to the HOG pathway in S. cerevisiae that regulates the osmoadaptation processes.
In conclusion, the combination of light, FIB/SEM, and TEM has demonstrated various morphological changes at high salinity in three Wallemia spp. At the high salinities, the common morphological feature was the increase in the sizes of both the multicellular clumps of W. ichthyophaga and the mycelial pellets of W. muriae and W. sebi. However, there were marked differences on the cellular level. In both W. muriae and W. sebi, the elongated hyphal cells were shorter and thicker, which resulted in a changed surface-to-volume ratio. The decreased surface-to-volume ratio was mainly a consequence of decreased surface area, since the volume of the hyphal cells remained unchanged. In contrast, the spherical cells of W. ichthyophaga retained their size and thus the surface-to-volume ratio at the high salinity, but the thickening of the cell walls resulted in decreased functional volume of the cells. The thick cell wall might be important as an armor against changes of osmotic pressure, since it provides a mechanical protection against hyposaline stress at dilution conditions. In the natural environment of these fungi, such as solar salterns, changing osmotic conditions as a consequence of change of salinity are a constant threat. In general, the mycelial form, as in W. muriae and W. sebi, enables penetration of solid substrata and colonization of a fixed spatial domain (13). We might speculate that in addition to the growth in multicellular clumps, the spherical form of the cells of W. ichthyophaga has a role in the adaptation to growth in hypersaline water of solar salterns. The sphere has a minimal surface-to-volume ratio, and this cell shape could be one of the important adaptations for growth of W. ichthyophaga in saturated NaCl (i.e., 30% [wt/vol] NaCl). At high salinities, the dominant fungi in the hypersaline waters of the salterns, the halophilic black yeasts H. werneckii, P. triangularis (15, 48), and T. salinum (27), phylogenetically distant from Wallemia spp., all grow meristematically and have spherical cells, a finding which supports this conclusion.
The overall morphological changes seen in Wallemia spp. correspond to the phylogenetics based on the ITS ribosomal DNA gene (49). W. ichthyophaga clearly shows its halophilic character, which might represent an evolutionary “cul de sac” of this ecologically very specialized and unique species. Due to its character, we believe that W. ichthyophaga is a particularly suitable model organism for the study of halophily in eukaryotes. Intensified studies of halophilic eukaryotic microorganisms can give us clues toward our understanding of stress responses and of the targets, processes, and networks involved in the complex field of salt tolerance.
Acknowledgments
We thank Janja Zajc and Živa Pipan Tkalec (both from the University of Ljubljana, Ljubljana, Slovenia) for assistance with sample preparation and TEM. We are also grateful to Tonica Bončina (University of Maribor, Maribor, Slovenia), Marziale Milani (University of Milan, Milan, Italy), and Francesco Tatti (FEI Company, Italy) for their expert help and assistance with the FIB/SEM.
This study was supported by the Ministry of Higher Education, Science, and Technology of the Republic of Slovenia; by a Young Researcher grant to M.K.K.; and by the Slovenian Research Agency (grant J4-1019).
Footnotes
Published ahead of print on 6 November 2009.
REFERENCES
- 1.Abu-Seidah, A. A. 2007. Effect of salt stress on amino acids, organic acids and ultrastructure of Aspergillus flavus and Penicillium roquefortii. Int. J. Agric. Biol. 3:419-425. [Google Scholar]
- 2.Ahmed, F. R., M. J. Buckingham, G. E. Hawkes, and T. P. Toube. 1984. Pyrrolylpolyenes. 5. Revision of the structures of the principal pigments of Wallemia sebi. A nuclear Overhauser enhancement study. J. Chem. Res. 6:178-179. [Google Scholar]
- 3.Ahmed, F. R., and T. P. Toube. 1983. Some aspects of the electron impact induced fragmentation of pyrrolylpolyenes. Int. J. Mass Spectrom. Ion Phys. 47:427-430. [Google Scholar]
- 4.Ahmed, F. R., and T. P. Toube. 1984. Pyrrolylpolyenes. 6. Synthesis of wallemia A and wallemia E. J. Chem. Soc. Perkin Trans. 1:1577-1579. [Google Scholar]
- 5.Almagro, A., C. Prista, B. Benito, M. C. Loureiro-Dias, and J. Ramos. 2001. Cloning and expression of two genes coding for sodium pumps in the salt-tolerant yeast Debaryomyces hansenii. J. Bacteriol. 183:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomberg, A., and L. Adler. 1992. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 33:145-212. [DOI] [PubMed] [Google Scholar]
- 7.Bowman, S. M., and S. J. Free. 2006. The structure and synthesis of the fungal cell wall. Bioessays 28:799-808. [DOI] [PubMed] [Google Scholar]
- 8.Butinar, L., S. Santos, I. Spencer-Martins, A. Oren, and N. Gunde-Cimerman. 2005. Yeast diversity in hypersaline habitats. FEMS Microbiol. Lett. 244:229-234. [DOI] [PubMed] [Google Scholar]
- 9.Butinar, L., S. Sonjak, P. Zalar, A. Plemenitaš, and N. Gunde-Cimerman. 2005. Melanized halophilic fungi are eukaryotic members of microbial communities in hypersaline waters of solar salterns. Bot. Mar. 48:73-79. [Google Scholar]
- 10.Butinar, L., P. Zalar, J. C. Frisvad, and N. Gunde-Cimerman. 2005. The genus Eurotium: members of indigenous fungal community in hypersaline waters of salterns. FEMS Microbiol. Ecol. 51:155-166. [DOI] [PubMed] [Google Scholar]
- 11.Chung, Y. S., J. Kim, D. Han, K. Chae, and K. Jahng. 2003. Ultrastructure of the cell wall of a null pigmentation mutant, npgA1, in Aspergillus nidulans. J. Microbiol. 41:224-231. [Google Scholar]
- 12.Clipson, N., D. Jennings, and J. Smith. 1989. The response to salinity at the microscopic level of the marine fungus Dendryphiella salina Nicot and Pugh as investigated stereologically. New Phytol. 113:121-127. [Google Scholar]
- 13.Cooke, R. C., and J. M. Whipps. 1993. Growth dynamics and transformations, p. 59-84. In R. C. Cooke (ed.), Ecophysiology in fungi. Blackwell Scientific Publication, Oxford, United Kingdom.
- 14.De Groot, P. W., A. F. Ram, and F. M. Klis. 2005. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 42:657-675. [DOI] [PubMed] [Google Scholar]
- 15.de Hoog, G. S., H. Beguin, and W. H. Batenburg-van de Vegte. 1997. Phaeotheca triangularis, a new meristematic black yeast from a humidifier. Antonie van Leeuwenhoek 71:289-295. [DOI] [PubMed] [Google Scholar]
- 16.de Hoog, G. S., P. Zalar, B. G. van den Ende, and N. Gunde-Cimerman. 2005. Relation of halotolerance to human-pathogenicity in the fungal tree of life: an overview of ecology and evolution under stress, p. 373-395. In N. Gunde-Cimerman, A. Oren, and A. Plemenitaš (ed.), Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya, vol. 9. Springer, Dordrecht, the Netherlands. [Google Scholar]
- 17.Drobne, D., M. Milani, V. Leser, and F. Tatti. 2007. Surface damage induced by FIB milling and imaging of biological samples is controllable. Microsc. Res. Tech. 70:895-903. [DOI] [PubMed] [Google Scholar]
- 18.Galinski, E. A. 1995. Osmoadaptation in bacteria. Adv. Microb. Physiol. 37:272-328. [PubMed] [Google Scholar]
- 19.Gams, W., E. S. Hoekstra, and A. Aptroot. 1998. CBS course of mycology. Centraalbureau voor Schimmelcultures, Baarn, the Netherlands.
- 20.Gunde-Cimerman, N., P. Zalar, S. de Hoog, and A. Plemenitaš. 2000. Hypersaline waters in salterns: natural ecological niches for halophilic black yeasts. FEMS Microbiol. Ecol. 32:235-240. [DOI] [PubMed] [Google Scholar]
- 21.Hohmann, S. 2002. Osmotic adaptation in yeast: control of the yeast osmolyte system. Int. Rev. Cytol. 215:149-187. [DOI] [PubMed] [Google Scholar]
- 22.Ishitani, T., H. Hirose, and H. Tsuboi. 1995. Focused-ion-beam digging of biological specimens. J. Electron Microsc. 44:110-114. [PubMed] [Google Scholar]
- 23.Jiang, B., A. F. Ram, J. Sheraton, F. M. Klis, and H. Bussey. 1995. Regulation of cell wall beta-glucan assembly: PTC1 negatively affects PBS2 action in a pathway that includes modulation of EXG1 transcription. Mol. Gen. Genet. 248:260-269. [DOI] [PubMed] [Google Scholar]
- 24.Kapteyn, J. C., H. van den Ende, and F. M. Klis. 1999. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1426:373-383. [DOI] [PubMed] [Google Scholar]
- 25.Kelavakar, U., K. S. Rao, and H. S. Chhatpar. 1993. Sodium chloride stress induced morphological and ultrastructural changes in Aspergillus repens. Ind. J. Exp. Biol. 31:511-515. [PubMed] [Google Scholar]
- 26.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 27.Kogej, T., A. A. Gorbushina, and N. Gunde-Cimerman. 2006. Hypersaline conditions induce changes in cell-wall melanization and colony structure in a halophilic and a xerophilic black yeast species of the genus Trimmatostroma. Mycol. Res. 110:713-724. [DOI] [PubMed] [Google Scholar]
- 28.Kogej, T., C. Gostinčar, M. Volkmann, A. A. Gorbushina, and N. Gunde-Cimerman. 2006. Mycosporines in extremophilic fungi: novel complementary osmolytes? Environ. Chem. 3:105-110. [Google Scholar]
- 29.Latgé, J. P. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66:279-290. [DOI] [PubMed] [Google Scholar]
- 30.Lenassi, M., T. Vaupotič, N. Gunde-Cimerman, and A. Plemenitaš. 2007. The MAP kinase HwHog1 from the halophilic black yeast Hortaea werneckii: coping with stresses in solar salterns. Saline Systems 3:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesage, G., and H. Bussey. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:317-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mager, W. H., and M. Siderius. 2002. Novel insights into the osmotic stress response of yeast. FEMS Yeast Res. 2:251-257. [DOI] [PubMed] [Google Scholar]
- 33.Mandal, P., T. S. Roy, T. K. Das, U. Banerjee, I. Xess, and J. D. Nosanchuk. 2007. Differences in the cell wall architecture of melanin lacking and melanin producing Cryptococcus neoformans clinical isolates from India: an electron microscopic study. Braz. J. Microbiol. 38:662-666. [Google Scholar]
- 34.Martin, D. D., R. A. Ciulla, and M. F. Roberts. 1999. Osmoadaptation in Archaea. Appl. Environ. Microbiol. 65:1815-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milani, M., and D. Drobne. 2006. Focused ion beam manipulation and ultramicroscopy of unprepared cells. Scanning 28:148-154. [DOI] [PubMed] [Google Scholar]
- 36.Oren, A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palková, Z., and L. Váchová. 2006. Life within a community: benefit to yeast long-term survival. FEMS Microbiol. Rev. 30:806-824. [DOI] [PubMed] [Google Scholar]
- 38.Pessoni, R. A. B., G. Freshour, R. C. L. Figueiredo-Ribeiro, M. G. Hanh, and M. R. Braga. 2005. Cell-wall structure and composition of Penicilluim janczewskii as affected by inulin. Mycologia 97:304-311. [DOI] [PubMed] [Google Scholar]
- 39.Pitt, J. I., and A. D. Hocking. 1997. Fungi and food spoilage. Blackie Academic & Professional, London, England.
- 40.Roberts, M. F. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samson, R. A., E. S. Hoekstra, J. C. Frisvad, and O. Filtenborg (ed.). 2002. Introduction to food- and airborne fungi, 6th ed. Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands.
- 42.Selbmann, L., G. S. de Hoog, A. Mazzaglia, E. I. Friedmann, and S. Onofri. 2005. Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 51:1-32. [Google Scholar]
- 43.Smits, G. J., H. van den Ende, and F. M. Klis. 2001. Differential regulation of cell wall biogenesis during growth and development in yeast. Microbiology 147:781-794. [DOI] [PubMed] [Google Scholar]
- 44.Turk, M., L. Méjanelle, M. Šentjurc, J. O. Grimalt, N. Gunde-Cimerman, and A. Plemenitaš. 2004. Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi. Extremophiles 8:53-61. [DOI] [PubMed] [Google Scholar]
- 45.Wasser, S. P., I. Grishkan, N. Gunde-Cimerman, A. S. Buchalo, T. Kis-Papo, P. A. Volz, P. Zalar, and E. Nevo. 2003. Species diversity of the Dead Sea, p. 203-270. In E. Nevo, A. Oren, and S. P. Wasser (ed.), Fungal life in the Dead Sea. Gantner, Ruggell, Liechtenstein, Königstein, Germany.
- 46.Wollenzien, U., G. S. de Hoog, W. E. Krumbein, and C. Urzì. 1995. On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Sci. Total Environ. 167:287-294. [Google Scholar]
- 47.Zalar, P., G. S. de Hoog, and N. Gunde-Cimerman. 1999. Ecology of halotolerant dothideaceous black yeasts. Stud. Mycol. 43:38-48. [Google Scholar]
- 48.Zalar, P., G. S. de Hoog, and N. Gunde-Cimerman. 1999. Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Stud. Mycol. 43:49-56. [Google Scholar]
- 49.Zalar, P., G. Sybren de Hoog, H. J. Schroers, J. M. Frank, and N. Gunde-Cimerman. 2005. Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie van Leeuwenhoek 87:311-328. [DOI] [PubMed] [Google Scholar]