Abstract
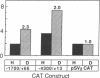
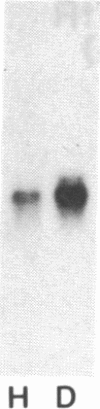
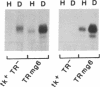
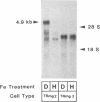
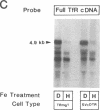
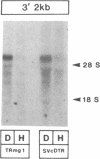
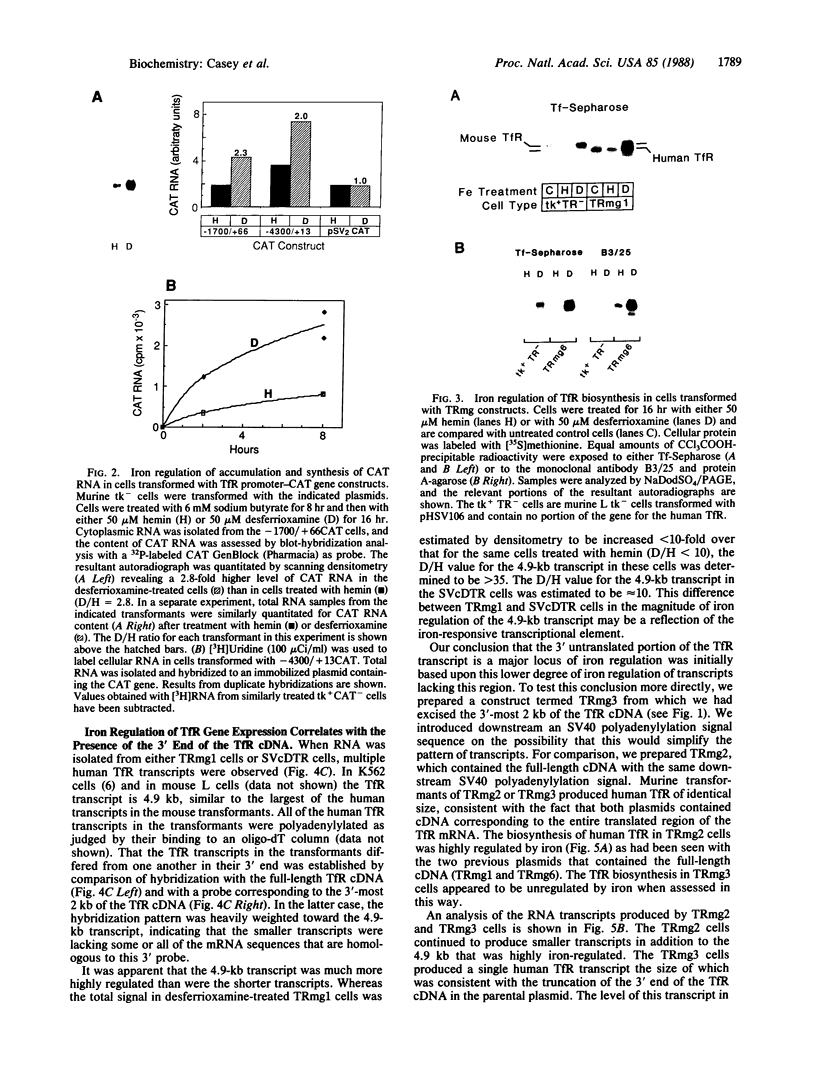
Iron regulation of the human transferrin receptor gene was examined in murine cells transformed with chimeric constructs containing the human transferrin receptor gene's promoter and either the structural gene for bacterial chloramphenicol acetyltransferase or the human transferrin receptor cDNA. The activity of the transferrin receptor gene's promoter with the heterologous indicator gene was found to be approximately equal to 3-fold higher in cells treated with the iron chelator desferrioxamine than in cells treated with the iron source, hemin. A higher degree of iron regulation was seen in the expression of the human transferrin receptor cDNA driven by its own promoter. The receptor cDNA under the control of the simian virus 40 early promoter was also iron-regulated. Several human transferrin receptor transcripts differing in their 3' end were produced in the murine cells regardless of the promoter used, with the shorter transcripts being relatively unregulated by iron. Deletion of cDNA corresponding to most of the 3' untranslated portion of the mRNA for the receptor ablated the iron regulation. We conclude that at least two genetic elements exist for the regulation of the transferrin receptor gene by iron. One has its locus in the DNA upstream of the transferrin receptor gene's transcription start site, and the other is dependent upon the integrity of the sequences in the 3' end of the gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Harford J. An artefact explains the apparent association of the transferrin receptor with a ras gene product. Nature. 1984 Oct 18;311(5987):673–675. doi: 10.1038/311673a0. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- Meyuhas O., Thompson E. A., Jr, Perry R. P. Glucocorticoids selectively inhibit translation of ribosomal protein mRNAs in P1798 lymphosarcoma cells. Mol Cell Biol. 1987 Aug;7(8):2691–2699. doi: 10.1128/mcb.7.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek I., Axel R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol Cell Biol. 1987 Apr;7(4):1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E., Testa U., Louache F., Thomopoulos P., Salvo G., Samoggia P., Peschle C. Expression of transferrin receptors in phytohemagglutinin-stimulated human T-lymphocytes. Evidence for a three-step model. J Biol Chem. 1986 Mar 5;261(7):3036–3042. [PubMed] [Google Scholar]
- Rao K. K., Shapiro D., Mattia E., Bridges K., Klausner R. Effects of alterations in cellular iron on biosynthesis of the transferrin receptor in K562 cells. Mol Cell Biol. 1985 Apr;5(4):595–600. doi: 10.1128/mcb.5.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J. R., Johnson M. L., Rosen J. M. Measurement of mRNA concentration and mRNA half-life as a function of hormonal treatment. Methods Enzymol. 1985;109:572–592. doi: 10.1016/0076-6879(85)09116-9. [DOI] [PubMed] [Google Scholar]
- Schneider C., Kurkinen M., Greaves M. Isolation of cDNA clones for the human transferrin receptor. EMBO J. 1983;2(12):2259–2263. doi: 10.1002/j.1460-2075.1983.tb01732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Trepel J. B., Colamonici O. R., Kelly K., Schwab G., Watt R. A., Sausville E. A., Jaffe E. S., Neckers L. M. Transcriptional inactivation of c-myc and the transferrin receptor in dibutyryl cyclic AMP-treated HL-60 cells. Mol Cell Biol. 1987 Jul;7(7):2644–2648. doi: 10.1128/mcb.7.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Newman R. A., Domingo D. L., Sauvage C. Transferrin receptors: structure and function. Biochem Pharmacol. 1984 Mar 15;33(6):925–932. doi: 10.1016/0006-2952(84)90447-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]