Abstract
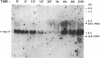
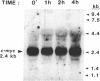
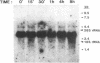
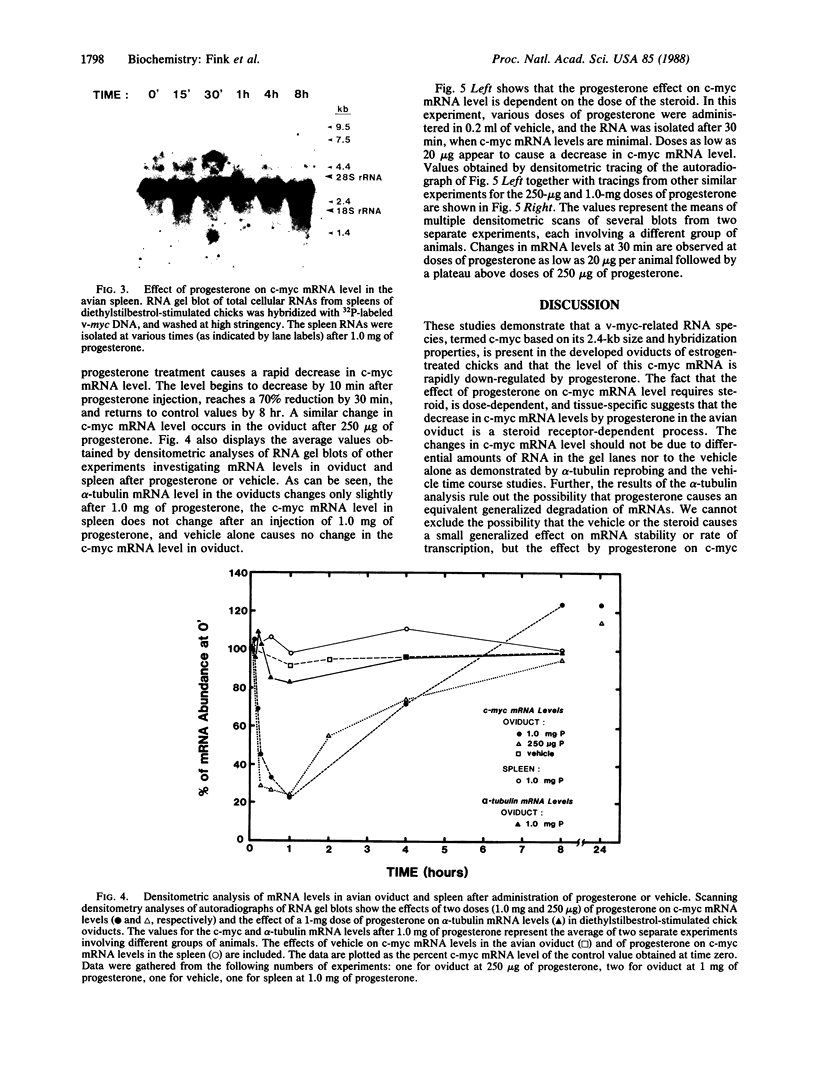
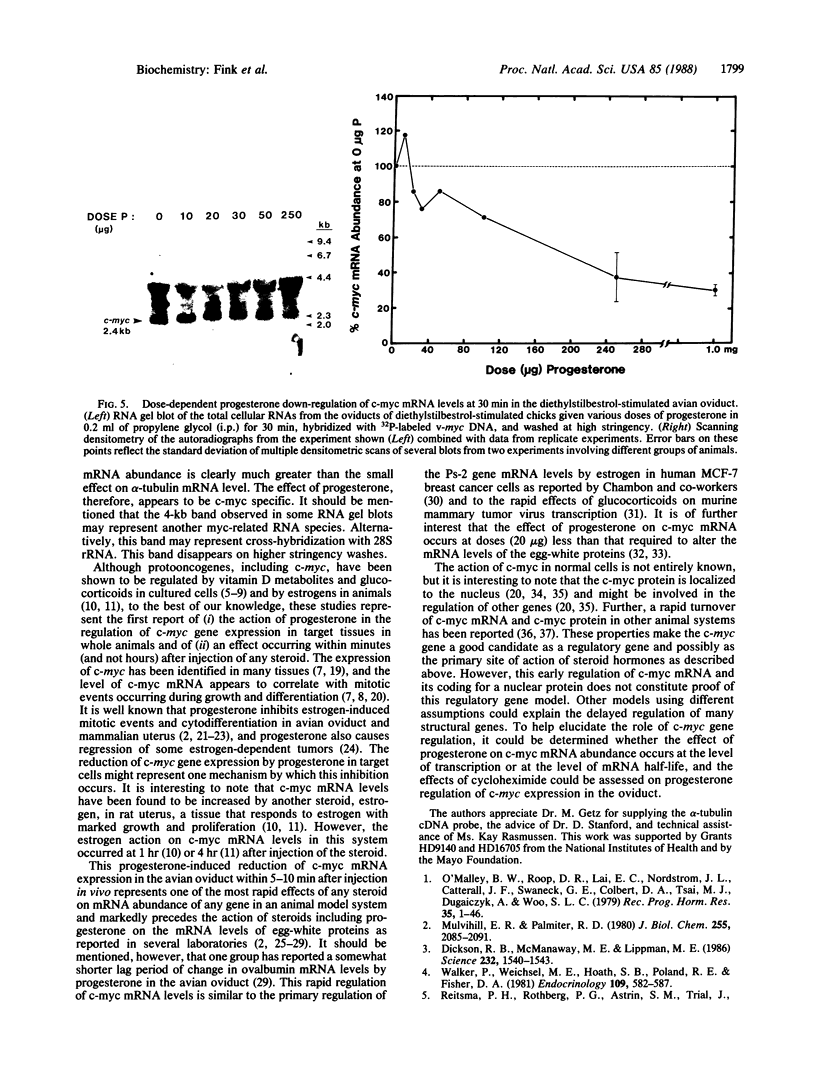
The mRNA levels of genes known to be regulated by sex steroids are not altered until 1 hr or longer after steroid treatment, although the steroid receptor complexes are bound to nuclear acceptor sites within 5 min. In a search for early regulation of gene transcription, total chick oviduct RNA was isolated at various times after injection (i.p.) of progesterone and analyzed for c-myc expression. Levels of c-myc mRNA began to decrease in response to progesterone by 10 min after injection. The mRNA levels continued to decrease, reached a 70% reduction at 30 min, and returned to control values by 8 hr after steroid injection. Changes in alpha-tubulin mRNA levels were markedly less in these same RNA preparations. The effect was dependent on the dose of the steroid and was target-tissue specific. These changes occurred much more rapidly than changes in egg-white protein mRNA levels. Vehicle alone did not alter c-myc mRNA levels. Early regulated genes such as c-myc may represent the initial site of action of steroid receptors in the genome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Ramsay G., Bishop J. M., Pfeifer S. O., Colby W. W., Levinson A. D. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature. 1983 Nov 17;306(5940):274–277. doi: 10.1038/306274a0. [DOI] [PubMed] [Google Scholar]
- Brelvi Z. S., Studzinski G. P. Inhibition of DNA synthesis by an inducer of differentiation of leukemic cells, 1 alpha, 25 dihydroxy vitamin D3, precedes down regulation of the c-myc gene. J Cell Physiol. 1986 Aug;128(2):171–179. doi: 10.1002/jcp.1041280206. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Feigelson P. Cycloheximide inhibition of hormonal induction of alpha 2u-globulin mRNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2669–2673. doi: 10.1073/pnas.76.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. V., Pollard J. W. c-rasH and ornithine decarboxylase are induced by oestradiol-17 beta in the mouse uterine luminal epithelium independently of the proliferative status of the cell. FEBS Lett. 1986 Feb 17;196(2):309–314. doi: 10.1016/0014-5793(86)80269-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., McManaway M. E., Lippman M. E. Estrogen-induced factors of breast cancer cells partially replace estrogen to promote tumor growth. Science. 1986 Jun 20;232(4757):1540–1543. doi: 10.1126/science.3715461. [DOI] [PubMed] [Google Scholar]
- Eastman-Reks S. B., Vedeckis W. V. Glucocorticoid inhibition of c-myc, c-myb, and c-Ki-ras expression in a mouse lymphoma cell line. Cancer Res. 1986 May;46(5):2457–2462. [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Peck E. J., Jr, clark J. H. Progesterone antagonism of the oestrogen receptor and oestrogen-induced uterine growth. Nature. 1975 Mar 27;254(5498):337–339. doi: 10.1038/254337a0. [DOI] [PubMed] [Google Scholar]
- Kelly K., Siebenlist U. The regulation and expression of c-myc in normal and malignant cells. Annu Rev Immunol. 1986;4:317–338. doi: 10.1146/annurev.iy.04.040186.001533. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. R., Palmiter R. D. Relationship of nuclear progesterone receptors to induction of ovalbumin and conalbumin mRNA in chick oviduct. J Biol Chem. 1980 Mar 10;255(5):2085–2091. [PubMed] [Google Scholar]
- Mulvihill E. R., Palmiter R. D. Relationship of nuclear progesterone receptors to induction of ovalbumin and conalbumin mRNA in chick oviduct. J Biol Chem. 1980 Mar 10;255(5):2085–2091. [PubMed] [Google Scholar]
- Murphy L. J., Murphy L. C., Friesen H. G. Estrogen induction of N-myc and c-myc proto-oncogene expression in the rat uterus. Endocrinology. 1987 May;120(5):1882–1888. doi: 10.1210/endo-120-5-1882. [DOI] [PubMed] [Google Scholar]
- Norris J. S., Cornett L. E., Hardin J. W., Kohler P. O., MacLeod S. L., Srivastava A., Syms A. J., Smith R. G. Autocrine regulation of growth: II. Glucocorticoids inhibit transcription of c-sis oncogene-specific RNA transcripts. Biochem Biophys Res Commun. 1984 Jul 18;122(1):124–128. doi: 10.1016/0006-291x(84)90448-0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Roop D. R., Lai E. C., Nordstrom J. L., Catterall J. F., Swaneck G. E., Colbert D. A., Tsai M. J., Dugaiczyk A., Woo S. L. The ovalbumin gene: organization, structure, transcription, and regulation. Recent Prog Horm Res. 1979;35:1–46. doi: 10.1016/b978-0-12-571135-7.50005-9. [DOI] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Progesterone antagonism of estrogen-induced cytodifferentiation in chick oviduct. Science. 1969 Jan 3;163(3862):83–85. doi: 10.1126/science.163.3862.83. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., Shepherd J. H., McKnight G. S. Steroid hormone regulation of ovalbumin and conalbumin gene transcription. A model based upon multiple regulatory sites and intermediary proteins. J Biol Chem. 1981 Aug 10;256(15):7910–7916. [PubMed] [Google Scholar]
- Ramsay G., Evan G. I., Bishop J. M. The protein encoded by the human proto-oncogene c-myc. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7742–7746. doi: 10.1073/pnas.81.24.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver S. S., Van Eys-Fuchs D. C., Hoffmann J. F., Coulson P. B. Ovalbumin messenger ribonucleic acid accumulation in the chick oviduct during secondary stimulation: influence of combinations of steroid hormones and circannual rhythms. Biochemistry. 1980 Apr 1;19(7):1410–1416. doi: 10.1021/bi00548a023. [DOI] [PubMed] [Google Scholar]
- Travers M. T., Knowler J. T. Oestrogen-induced expression of oncogenes in the immature rat uterus. FEBS Lett. 1987 Jan 19;211(1):27–30. doi: 10.1016/0014-5793(87)81267-x. [DOI] [PubMed] [Google Scholar]
- Walker P., Weichsel M. E., Jr, Hoath S. B., Poland R. E., Fisher D. A. Effect of thyroxine, testosterone, and corticosterone on nerve growth factor (NGF) and epidermal growth factor (EGF) concentrations in adult female mouse submaxillary gland: dissociation of NGF and EGF responses. Endocrinology. 1981 Aug;109(2):582–587. doi: 10.1210/endo-109-2-582. [DOI] [PubMed] [Google Scholar]
- Woloschak G. E. Comparison of immunoglobulin heavy chain isotype expression in Peyer's patch and splenic B-cells. Mol Immunol. 1986 Jun;23(6):581–591. doi: 10.1016/0161-5890(86)90094-5. [DOI] [PubMed] [Google Scholar]