Abstract
The biodegradation of the most abundant atmospheric organic C1 to C4 compounds (formate, acetate, lactate, succinate) by five selected representative microbial strains (three Pseudomonas strains, one Sphingomonas strain, and one yeast strain) isolated from cloud water at the puy de Dôme has been studied. Experiments were first conducted under model conditions and consisted of a pure strain incubated in the presence of a single organic compound. Kinetics showed the ability of the isolates to degrade atmospheric compounds at temperatures representative of low-altitude clouds (5°C and 17°C). Then, to provide data that can be extrapolated to real situations, microcosm experiments were developed. A solution that chemically mimicked the composition of cloud water was used as an incubation medium for microbial strains. Under these conditions, we determined that microbial activity would significantly contribute to the degradation of formate, acetate, and succinate in cloud water at 5°C and 17°C, with lifetimes of 0.4 to 69.1 days. Compared with the reactivity involving free radicals, our results suggest that biological activity drives the oxidation of carbonaceous compounds during the night (90 to 99%), while its contribution accounts for 2 to 37% of the reactivity during the day, competing with photochemistry.
The chemistry of organic compounds in the atmosphere is believed to be essentially driven by free radicals and oxidants generated by photochemical processes (e.g., see references 9 and 37). Recent investigations have raised the possibility that unexpected actors, i.e., living microbes, could also be involved (3, 5, 7-8, 13, 15).
Clouds play a major role in the transformation of atmospheric compounds (27) and influence the composition of the atmosphere through liquid-gas exchanges (26, 32). They are also considered atmospheric niches for microbial life. Cloud water is a complex mixture of organic and inorganic compounds originating from both the gas and the solid phases of the atmosphere. Organic species in the atmosphere either originate from direct sources such as automobile exhaust or are produced within the atmosphere by oxidation of hydrocarbons. Their concentrations are controlled by long-range transport and photochemical production (23, 30). The dissolved organic carbon concentration in cloud water generally ranges from 1 to 20 mg liter−1 (35, 38). Low-weight carboxylic acids and aldehydes dominate the organic fraction, with formate, acetate, and formaldehyde being the most abundant (20, 29, 34, 41). The chemistry of these soluble organic compounds play a crucial role in the budget of volatile organic compounds in the troposphere and in the budget of secondary organic aerosol particles, which is a major uncertainty in the assessment of the role of aerosol particles in climate change (21). Indeed, a number of studies have shown that chemical mechanisms in clouds contribute significantly to the formation of secondary organic aerosol particles (22). More specifically, a substantial fraction of organic species such as oxalic, formic, and acetic acids originates from aqueous phase oxidation processes, mainly by OH., NO3., and HO2. (17, 31).
Cloud droplets also host living bacteria (103 to 105 cells ml−1) (1, 6, 10, 40); the total bacterial C production at 0°C in clouds has been estimated to be 1 to 10 pg C year−1 (40). Heterotrophic microorganisms can sustain growth in cloud water under laboratory conditions by using dissolved organic compounds as substrates, the concentration of which is presumably the limiting nutritive factor for cell multiplication in these environments (5). This activity toward organic compounds likely participates in carbon chemistry in clouds. In addition, nitrifying bacteria have been detected in clouds and they could be involved in the transformation of atmospheric nitrogenous species (25). One key objective now is to quantify the importance of the biological oxidation pathways compared to that of chemical and photochemical processes.
In previous work, we provided a description of the microbial content of low-altitude clouds (puy de Dôme summit, 1,465 m above sea level) and provided an overview of the capability of isolated bacteria and yeast to degrade atmospheric organic compounds under laboratory conditions (3-6). Here we present rates of biodegradation of atmospheric organic C1 to C4 compounds by five selected representative microbial strains isolated from cloud water. Experiments were performed with pure strains incubated in the presence of a single organic compound. We focused first on determining biodegradation rates at two different incubation temperatures (5°C and 17°C). The colder (5°C) corresponds to the mean annual temperature measured at the puy de Dôme summit, while 17°C is approximately the maximal temperature observed there when a cloud forms (see http://wwwobs.univ-bpclermont.fr/SO/beam/data.php).
Then, with the objective to provide data that can be extrapolated to real situations, microcosm experiments in which a solution that chemically mimicked the composition of cloud water was used as an incubation medium were developed. Based on the results obtained under microcosm conditions, in-cloud lifetimes of mono- and dicarboxylic acids (formate, acetate, and succinate) were estimated in relation to their biotransformation by microorganisms at 5°C and 17°C. These lifetimes were finally compared to reactions with the free radicals OH. and NO3. in the liquid phase, showing that microbial activity represents a real alternative route for the oxidation of organic species in cloud water.
MATERIALS AND METHODS
Strains.
Four strains of bacteria and one yeast strain isolated from cloud water samples collected at the puy de Dôme summit were used. The bacterial strains, Pseudomonas sp. strain PDD-14b-2 (DQ512788), Pseudomonas viridiflava strain PDD-14b-14 (DQ512797), Pseudomonas graminis strain PDD-13b-3 (DQ512786), and Sphingomonas sp. strain PDD-7b-13 (DQ512776), have been described elsewhere (4); the yeast strain, PDD-14b-1, is still unidentified. They were selected for their representation in cloud water; for instance, Pseudomonas and Sphingomonas species were detected in 100% and 75%, respectively, of the samples collected at the puy de Dôme between December 2003 and September 2004. Pseudomonas strains have also been found in cloud water in Scotland (1) and in the Italian Pô valley (19).
Biodegradation test conditions. (i) Cell preparation.
The investigated strain was grown aerobically (200 rpm) at 17°C or 27°C in tryptic soy (TS) or R2A (39) broth. Cells were harvested after 24 to 48 h of growth by centrifugation (4,000 × g, 15 min, 4°C), rinsed twice with 0.8% NaCl, and finally resuspended in the incubation medium. The cell concentration in the test medium was adjusted close to 109, 108, 107, or 106 bacteria ml−1 and around 107 yeast cells ml−1 after measurement of the optical density at 575 nm. Serial dilutions of the suspension were also plated on R2A or tryptic soy agar (TSA) and incubated at 17°C or 27°C for exact CFU counts. The exact cell count was used to calculate the biodegradation rates expressed as mol h−1 cell−1 (see the description of calculations of biodegradation rates and lifetimes below). The experimental blank consisted of cells suspended in the test medium. At defined time points (t = 0, 1, 2, 3, 4, 5, 6, 7, 8, 24, 48, and 72 h), samples were taken for measurement of the concentration of substrate; cells were pelleted (12,000 × g, 3 min) from 1 ml of the incubation medium, and the supernatants were kept frozen (−25°C) until analysis.
(ii) Incubation with a unique substrate.
The test medium was composed as follows: 25 ml of 0.1 M phosphate buffer at pH 7.0 containing about 20 mM sodium formate (Aldrich), lactic acid (l and d isomers, mixing ratio of approximately 2.5:1; Touzart and Matignon), or sodium succinate (Aldrich). The cell concentrations were around 109 bacteria ml−1 and 107 yeast cells ml−1. Incubations were performed at 5°C and 17°C. In the specific case of P. graminis, an additional experiment was performed at 17°C using substrate/cell ratios of 20 mM/108 cells ml−1, 2 mM/107 cells ml−1, and 0.2 mM/106 cells ml−1; these were obtained by diluting the solution containing the highest concentrations. Supernatant samples were subjected to 1H nuclear magnetic resonance (NMR) analysis.
(iii) Incubation in a microcosm.
The artificial cloud water medium was obtained by diluting by a factor of 1,000 in ultrapure water a solution containing 0.2 M acetic acid (Acros), 0.145 M formic acid (Aldrich), 0.03 M oxalic acid (Fluka), 0.015 M succinic acid (Fluka), 0.1 M MgCl2, 6H2O (Fluka), 0.4 M CaCl2, 2H2O (Aldrich) 0.05 M K2SO4 (Fluka), 2.2 M NaCl (Aldrich), 2.0 M NO3NH4 (Fluka), 0.3 M NaOH (Merck), and 0.45 M H2SO4 (Acros) (see Table 2 for the final composition). This solution was sterilized by autoclave and incubated with 106 cells ml−1 at 5°C or 17°C. Supernatant samples were analyzed by ion chromatography.
TABLE 2.
Chemical composition and pH of the artificial cloud water solution that mimics the natural cloud water collected at the puy de Dôme station between 2001 and 2008a
| Compound or parameter | Concn (μM) |
||
|---|---|---|---|
| Artificial cloud | Natural cloud water |
||
| Min | Max | ||
| Acetate | 20 | 0.31 | 47.8 |
| Formate | 14.5 | 0.25 | 69.8 |
| Succinate | 1.5 | 0.02 | 4.1 |
| Oxalate | 3 | 0.10 | 16.9 |
| Cl− | 320 | 0.49 | 1,948.9 |
| NO3− | 200 | 0.80 | 766.7 |
| SO42− | 50 | 1.94 | 369.5 |
| Na+ | 251 | 0.37 | 678.5 |
| NH4+ | 200 | 6.28 | 1,801.7 |
| K+ | 10 | 0.13 | 124.1 |
| Mg2+ | 10 | 0.03 | 47.9 |
| Ca2+ | 40 | 0.30 | 74.8 |
| pH | 5-5.3 | 3.9 | 7.6 |
Sample analyses. (i) Measurements by 1H NMR.
A volume of 450 μl of supernatant from the samples collected at different time points was mixed with 50 μl of sodium tetradeuterated trimethylsilylpropionate (Eurisotop) in solution in D2O (2 mM final concentration). Analyses by 1H NMR were made by following a protocol similar to that of Amato et al. (3) and using a Bruker Avance 400 spectrometer functioning at 400.13 MHz and 21°C.
(ii) Analyses by ion chromatography.
All of the vials were rinsed three times with ultrapure water; experiments were performed under a hood and while wearing gloves to avoid any chemical contamination. Fifty-microliter samples were diluted by a factor of 100 in ultrapure water before analysis. Ions were measured by ionic chromatography (Dionex DX320, column AS11 for anions, eluant KOH; Dionex ICS1500, column CS16 for cations, eluant hydroxymethanesulfonate).
Calculations of biodegradation rates and lifetimes.
The biodegradation rate (Kx) of a compound, x, in mol h−1 cell−1, was determined by the linear regression equation Kx = (C0 − Ct)/(t × Ncells), with C0 and Ct the concentrations of x in mol liter−1 after 0 and t minutes of incubation, respectively; t is the incubation time in minutes, and Ncells is the concentration, in cells liter−1, of the cells participating in biodegradation.
Lifetimes (T) linked to biological activity were calculated by assuming no variation of the biodegradation rates with the concentration of substrates but direct proportionality to the number of cells involved. For a compound, x, the lifetime in days is given by the equation Tx = Cx/Ncell × Kx × 24, with Cx the concentration of compound x in cloud water in mol liter−1 and Ncell the concentration of cells in cloud water in cells liter−1.
RESULTS
Biodegradation of atmospheric carboxylic acids as unique carbon sources.
The five microbial strains isolated from cloud water were incubated in the presence of formate, acetate, l- and d-lactate, and succinate at 5°C and 17°C in 0.1 M phosphate buffer at pH 7.0. Each reaction mixture consisted of a pure culture in the presence of a single organic compound at a given temperature. For technical reasons (low sensitivity of 1H NMR), the experimental conditions involved cells and substrates at concentrations higher than those actually found in cloud water, i.e., 109 versus ∼105 cells ml−1 (6) and 20 mM versus ∼2 μM (35), respectively; however, the ratio of cell to substrate concentrations was realistic compared to that in real clouds.
The measured rates of biodegradation of each compound by each isolate are reported in Table 1. For bacteria, they ranged from 2.56 × 10−18 mol h−1 cell−1 for d-lactate (P. viridiflava) to 1.09 × 10−15 mol h−1 cell−1 for acetate (P. graminis) at 17°C and from 5.89 × 10−19 mol h−1 cell−1 for acetate (Sphingomonas sp.) to 3.80 × 10−16 mol h−1 cell−1 for l-lactate (P. graminis) at 5°C. The highest rates of carboxylic acid transformation were generally measured for P. graminis at both 5°C and 17°C, while Sphingomonas sp. was often the least efficient. At 5°C, the average biodegradation rates ranged from 5. 12 × 10−17 mol h−1 cell−1 (d-lactate) to 1.71 × 10−16 mol h−1 cell−1 (l-lactate). At 17°C, the average rate ranges are of the same of order magnitude, 1.02 × 10−16 to 3.71 × 10−16 mol h−1 cell−1 for all of the substrates, except that for d-lactate, which is more difficult to degrade, the average rate is 1 order of magnitude lower (around 5 × 10−17 mol h−1 cell−1). This lower degradation efficiency of d-lactate was shown before for a large number of strains when comparing the percentages of l- and d-lactate degradation after 24 h of incubation (5). This phenomenon is related to the different enzymes involved in this pathway. The highest rates of degradation were found for the yeast strain; it rapidly degraded the proposed substrates, particularly l-lactate at 5°C (1.56 × 10−14 mol h−1 cell−1) and acetate at 17°C (3.24 × 10−14 mol h−1 cell−1). The differences in biodegradation rate between bacteria and yeasts are probably due to the fact that yeast cells are about 10 times bigger than bacterial cells, resulting in higher rates in yeasts on a per-cell basis. In addition, they are eukaryotes and their metabolic pathways and regulations are probably different from those of bacteria.
TABLE 1.
Rates of biodegradation of carboxylic acids by pure strains at 5°C and 17°Ca
| Organism or parameter | Rate of biodegradation (mol h−1 cell−1) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formate |
Acetate |
l-Lactate |
d-Lactate |
Succinate |
||||||
| 5°C | 17°C | 5°C | 17°C | 5°C | 17°C | 5°C | 17°C | 5°C | 17°C | |
| Sphingomonas sp. | 1.12 × 10−16 | 3.32 × 10−17 | 5.89 × 10−19 | 9.84 × 10−17 | NDb | 2.76 × 10−17 | 1.05 × 10−17 | 8.61 × 10−18 | 3.91 × 10−17 | 9.37 × 10−17 |
| Pseudomonas graminis | 3.44 × 10−16 | 4.58 × 10−16 | 1.07 × 10−16 | 1.09 × 10−15 | 3.80 × 10−16 | 2.96 × 10−16 | 1.42 × 10−16 | 1.37 × 10−16 | 3.54 × 10−16 | 3.77 × 10−16 |
| Pseudomonas sp. | 3.10 × 10−17 | 1.65 × 10−16 | 6.34 × 10−17 | 9.40 × 10−17 | 8.44 × 10−17 | 1.74 × 10−17 | 4.06 × 10−17 | ND | 6.67 × 10−17 | 5.08 × 10−17 |
| Pseudomonas viridiflava | 1.68 × 10−16 | 5.86 × 10−16 | 4.29 × 10−17 | 2.02 × 10−16 | 4.83 × 10−17 | 6.73 × 10−17 | 1.13 × 10−17 | 2.56 × 10−18 | 2.20 × 10−16 | 1.05 × 10−16 |
| Avg bacterial rate | 1.64 (± 1.14) × 10−16 | 3.10 (± 2.21) × 10−16 | 5.35 (± 3.83) × 10−17 | 3.71 (± 4.17) × 10−16 | 1.71 (± 1.71) × 10−16 | 1.02 (± 1.13) × 10−16 | 5.12 (± 5.39) × 10−17 | 4.94 (± 7.15) × 10−17 | 1.70 (± 1.26) × 10−16 | 1.47 (± 1.11) × 10−16 |
| Yeast | 3.06 × 10−15 | 4.73 × 10−15 | 6.80 × 10−15 | 3.24 × 10−14 | 1.56 × 10−14 | 7.38 × 10−15 | 6.90 × 10−15 | 1.87 × 10−15 | 2.38 × 10−15 | 4.73 × 10−15 |
The incubation medium consisted of 3.14 (± 0.20)×109 bacteria ml−1 or 2.69 (± 0.67)×107 yeast cells ml−1 in the stationary phase of growth in 0.1 M phosphate buffer (pH 7.0) containing the tested compound at 20 mM (l- and d-lactate were tested simultaneously at a mixing ratio of approximately 2.5:1). Rates were calculated at the first degree approximation and were assumed to be linear over time. Average bacterial rates correspond to means ( ± standard deviations) of the biodegradation rates measured with the four bacterial strains. The uncertainty for measurements is 5 to 10%.
ND, not determined.
Biodegradation of atmospheric carboxylic acids under microcosm conditions.
Cloud water is generally an acidic complex mixture of organic and inorganic compounds, and its chemical composition may affect enzymatic reactions. To investigate the influence of the presence of major chemicals dissolved in cloud water on biodegradation kinetics, microcosm experiments were set up. The incubation medium was composed by a solution containing ions such as nitrate, chloride, sodium, and ammonium and some organic species like formate, acetate, succinate, and oxalate; this was not buffered, and the final medium was acidic (pH ∼5) (Table 2). This mixture of organic and inorganic compounds was designed to have chemical characteristics that mimicked the typical composition of cloud water, in accordance with the measurements of samples collected at the puy de Dôme station (35, 38; M. Parazols et al., unpublished data). The solution was inoculated with P. graminis at a concentration of 106 cells ml−1, i.e., about 1 order of magnitude higher than the actual concentration of bacteria in cloud water (6). The concentrations of all of the organic and inorganic species in the incubation medium were also 10 times higher than in real cloud water. To check the possible influence of these shifts in concentration on the resulting rates of biodegradation, the linearity of the degradation rates was first tested. Suspensions of P. graminis at concentrations of 106, 107, and 108 cells ml−1 were incubated in 0.1 M phosphate buffer in the presence of 0.2, 2.0, and 20 mM carboxylic acid, respectively. Table 3 shows the biodegradation rates measured under these conditions; the results attested that there was no effect of the absolute cell and acid concentrations for a given ratio and the reaction rates were thus considered linear. Table 4 shows the reaction rates and inferred lifetimes of formate, acetate, and succinate in cloud water in relation to their transformation by either biological or chemical activity at 5°C and 17°C (see Table 4, footnote a, for details). Degradation rates were increased by 1 order of magnitude under microcosm conditions compared to simple conditions. Note that oxalate was not degraded by P. graminis under either microcosm conditions or during incubation with oxalate as the sole substrate at pH 7.0 and at 5°C or 17°C, suggesting that this strain lacks the necessary enzyme(s).
TABLE 3.
Biodegradation rates of formate, acetate, lactate, and succinate as single carbon sources at 17°C by P. graminisa
| Cell concn (cells ml−1) | Compound concn (M) | Rate of biodegradation (mol h−1 cell−1) |
|||
|---|---|---|---|---|---|
| Formate | Acetate | Lactate | Succinate | ||
| 1 × 10−8 | 2 × 10−2 | 4.9 × 10−14 | 6.9 × 10−15 | 5.2 × 10−15 | 2.3 × 10−15 |
| 1 × 10−7 | 2 × 10−3 | 4.1 × 10−14 | 7.2 × 10−15 | 7.0 × 10−15 | 4.8 × 10−15 |
| 1 × 10−6 | 2 × 10−4 | 3.8 × 10−14 | 8.6 × 10−15 | 3.9 × 10−15 | 2.2 × 10−15 |
TABLE 4.
Inferred lifetimes in cloud water of formate, acetate, and succinate at 5°C and 17°C and rates of degradation by P. graminis in microcosm and by free radicals OH· and NO3·a
| Organism or compound (temp [°C]) | Formate |
Acetate |
Succinate |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Reaction rateb | Rate in cloud water (10−7 mol liter−1 h−1) | Inferred lifetime (days) | Reaction rateb | Rate in cloud water (10−7 mol liter−1 h−1) | Inferred lifetime (days) | Reaction rateb | Rate in cloud water (10−7 mol liter−1 h−1) | Inferred lifetime (days) | |
| P. graminis (5) | 3.8 (± 1.3) × 10−15 | 3.1 | 2.0 | 1.5 (± 0.2) × 10−16 | 0.12 | 69.1 | 5.2 (± 2.6) × 10−16 | 0.415 | 1.5 |
| P. graminis (17) | 1.4 (± 0.4) × 10−14 | 11.3 | 0.5 | 2.4 (± 1.4) × 10−15 | 1.88 | 4.4 | 2.1 (± 0.6) × 10−15 | 1.71 | 0.4 |
| OH· (5) | 2.55 × 109 | 159.0 | 0.04 | 4.50 × 107 | 3.89 | 2.1 | 3.65 × 108 | 2.37 | 0.3 |
| OH· (17) | 3.04 × 109 | 190.6 | 0.03 | 5.90 × 107 | 5.10 | 1.6 | 4.43 × 108 | 2.87 | 0.2 |
| NO3· (5) | 3.00 × 107 | 0.324 | 18.7 | 1.16 × 106 | 0.013 | 665.2 | 5.50 × 107 | 0.045 | 14.0 |
| NO3· (17) | 4.20 × 107 | 0.329 | 18.4 | 2.04 × 106 | 0.022 | 378.2 | 5.50 × 107 | 0.045 | 14.0 |
Lifetimes were calculated by using the following concentrations: [bacteria] = 8.4 × 104 cells ml−1 (6), [OH·] = 1.2 × 10−13 mol liter−1, [NO3·] = 1.5 × 10−14 mol liter−1, [formate] = 14.5 × 10−6 mol liter−1, [acetate] = 20 × 10−6 mol liter−1, and [succinate] = 1.5 × 10−6 mol liter−1. Kinetic constants and concentrations of OH· and NO3· were taken from Leriche et al. (33) and Herrmann et al. (24), respectively; organic acid concentrations are from Table 2.
Values for organisms are in mol h−1 cell−1, and values for compounds are in liters mol−1 s−1. Reaction rates measured with P. graminis are the mean values of 3 replicates at 5°C and 5 replicates at 17°C.
Implication in atmospheric chemistry.
Microcosm experiments do not reflect the physical characteristic of clouds, i.e., the distribution of water in droplets which influences the exchanges at the air-water interface. However, this is thought not to affect biodegradation rates, which are probably more dependent on the chemical composition. From our measurements realized under microcosm conditions, we calculated lifetimes for the main organic species in cloud water, i.e., formate, acetate, and succinate (Table 4). In a cloud with typical biological and chemical contents and assuming a cell population that is as active under natural conditions as in the microcosm, the lifetimes of formate, acetate, and succinate due to biological activity are 2.0, 69.1, and 1.5 days at 5°C and 0.5, 4.4, and 1.5 days at 17°C, respectively. Oxidation processes in the atmosphere are largely catalyzed by free radicals, of which the principals ones are OH. and NO3.. Hydroxyl radicals are generated by photochemical pathways and are implicated in the oxidation processes that occur during the day, while nitrate radicals play a role during the night (18). At 5°C (the average temperature in warm clouds), lifetimes related to biological activity were 5 to 50 times longer than lifetimes linked to oxidation by OH. but about 10 times shorter than the lifetimes due to oxidation by NO3., with variations depending on the chemical species. At 17°C (the upper temperature limit in summer) during daytime, the lifetimes of acetate and succinate are of the same order of magnitude for microbial activity and for OH. activity (4.4 versus 1.6 and 0.4 versus 0.2, respectively) and only 17 times higher for OH. reactivity in the case of formate (Table 4). At this temperature, P. graminis was far more active than NO3., with lifetimes 35 to 86 shorter, showing that biological activity could predominate at night.
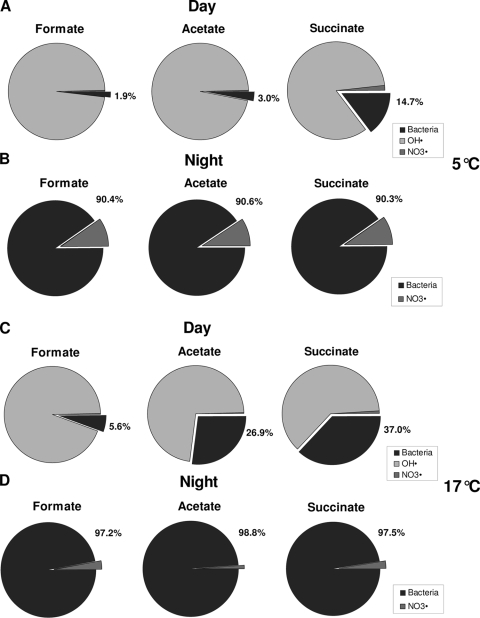
Figure 1 shows the relative participation of P. graminis and free radicals in catalyzing the oxidation of formate, acetate, and succinate during the day and during the night. At 5°C in the presence of sunlight, OH. radicals are present and bacterial activity would account for 2 to 15% of the degradation of organic acids (Fig. 1A); during the night, biological activity only competes with NO3. radicals and its participation in the oxidation of carboxylic acids would reach 90% (Fig. 1B). At 17°C, the bacterial relative contribution to the oxidation of organic species would vary from 6 to 37% (Fig. 1C) of the total during daytime and from 97 to 99% during nighttime. These results suggest that biochemical pathways would have a relatively limited influence on the degradation of organic compounds during the day at 5°C but would become nonnegligible at the highest temperature. At both temperatures, microbial activity would be the dominant phenomenon during the night, at least for low-altitude clouds.
FIG. 1.
Estimated relative influence of bacterial activity (in black) and free radicals (hydroxyl [OH.] and nitrate [NO3.]) on the degradation of formate, acetate, and succinate in cloud water at 5°C during the day (A) and night (B) and at 17°C during the day (C) and night (D). During nighttime (i.e., in the absence of photochemical reactivity), OH. radicals are considered not to be present. The following concentrations were used for calculations: [bacteria], 8.4 × 104 cell ml−1 (6); [OH.], 1.2 × 10−13 mol liter−1 (daytime); [NO3.], 1.5 × 10−14 mol liter−1; [formate], 14.5 × 10−6 mol liter−1; [acetate], 20 × 10−6 mol liter−1; [succinate], 1.5 × 10−6 mol liter−1. Kinetic constants and concentrations of OH. and NO3. were taken from Leriche et al. (33) and Herrmann et al. (24), respectively; organic acid concentrations are from Table 2.
DISCUSSION
The biodegradation of formate, acetate, and succinate by strains isolated from cloud water at puy de Dôme had been studied under optimal temperature conditions (3, 5). Here we provide evidence that biodegradation is possible under cold conditions such as those encountered in low-altitude clouds. This is consistent with their ability to grow at 5°C, which can thus be supported by atmospheric compounds, thanks to the presence of cold-tolerant enzymes (4). Furthermore, a number of studies have also demonstrated the existence of microbial activity under extreme cold conditions, including subzero temperatures (2, 11-12, 28, 40). Hence, the biodegradation of organic species likely also occurs in supercooled clouds. Moreover, the activity of microorganisms in degrading organic acids under conditions that mimic those found in clouds (acidic pH, mixture of organic and inorganic compounds, and low temperature) was also demonstrated.
Interestingly, the metabolic pathways of degradation of the organic compounds studied can be similar to those catalyzed by photochemical processes. As an example, photochemistry, through the production of OH. radicals, is involved in the progressive oxidation of hydrocarbons and others organic compounds to CO2. The final step is constituted by the oxidization of formate (14, 36), and this reaction is also possibly catalyzed by microbial metabolism (16). So, a part of the chemical transformations occurring in the atmosphere and attributed to photochemistry could, in fact, involve microbiological activity. To clarify this hypothesis, an attempted extrapolation of the biodegradation rates previously determined for organic acids to cloud water was proposed and biological lifetimes were compared to typical lifetimes calculated for both daytime (OH.) and nighttime (NO3.) chemistry. The time scales estimated for the degradation of organic compounds of cloud water, falling in the range of a few days, indicate that microbial activity could contribute to degrade organic compounds in the atmosphere, at least in low-altitude clouds. These time scales are consistent with those reported earlier by Ariya and Amyot (7) for the degradation of dicarboxylic acids as unique substrates by a fungal strain isolated from air (Geotrichum sp.). Lifetimes of 2 to 10 days were proposed for malonic, succinic, glutaric, adipic, pimelic, and pinic acids. Our results showed that biotransformation processes could even be the main sink for organic acids during nighttime, when the free radical load reaches its lowest concentration.
The implication of such results is clearly that the biological transformation of organic material in clouds needs to be accounted for to quantify the formation of secondary material in the atmosphere. Clearly, the calculated lifetimes are much longer than the typical lifetime of a single cloud event; they are comparable to the lifetime of airborne material in the temperate troposphere (up to several days). It is not unusual during the course of their atmospheric transport that atmospheric particles (including bacteria, fungi, and viruses) undergo several condensation-evaporation cycles, spending a significant fraction of their time in clouds. Quantifying the impact of biological activity on the global cycling of organic matter is beyond the scope of the present paper and would require advanced chemical transport modeling.
In clouds at a temperature of 5°C, which is the most representative temperature for low-altitude clouds, and assuming that 1% of the volume of the troposphere is occupied by clouds and that they carry a biomass as active as the cell populations used in microcosms, cloud-borne bacteria would be responsible for the degradation of nearly 6.5 million tons of organic compounds composed of carbon per year. Considering that these low-weight organic compounds are completely oxidized into carbon dioxide, this corresponds to an annual production of about 24 millions tons of CO2 by microbial activity in cloud water.
Clouds are very complex multiphase systems that cannot be reproduced in laboratories working under bulk conditions. Our long-term strategy is to create mathematical process models of both biological and radical reactions and use different parameters, such as degradation kinetic constants (such as those determined in this paper), number and type of cells, chemical composition, temperature, pH, light flux, etc., to simulate more realistic clouds which are in constant evolution. These cloud chemistry models take into account the exchanges existing between the interstitial phases (gases and particles) and cloud droplets and crystals (15). Modeling is the only way that will help us to know and quantify precisely the real contribution of microbiological processes, relative to photochemical processes, to cloud chemistry, depending on different scenarios. The way is still long; however, experimental data, especially biodegradation rate constants, collected by us and by other groups on microbial activity in cloud water will help to reach this goal.
Acknowledgments
This research was funded by the CNRS and the French Ministry of Research under the LEFE-CHAT and ORE-BEAM programs. Pierre Amato and Mickael Vaïtilingom acknowledge Ph.D. scholarships from the French Ministry of Research.
Footnotes
Published ahead of print on 23 October 2009.
REFERENCES
- 1.Ahern, H., K. A. Walsh, T. C. J. Hill, and B. F. Moffett. 2007. Fluorescent pseudomonads isolated from Hebridean cloud and rain water produce biosurfactants but do not cause ice nucleation. Biogeosciences 4:115-124. [Google Scholar]
- 2.Amato, P., and B. C. Christner. 2009. Energy metabolism response to low temperature and frozen conditions in Psychrobacter cryohalolentis. Appl. Environ. Microbiol. 75:711-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amato, P., M. Ménager, M. Sancelme, P. Laj, G. Mailhot, and A.-M. Delort. 2005. Microbial population in cloud water at the Puy de Dôme: implications for the chemistry of clouds. Atmos. Environ. 39:4143-4153. [Google Scholar]
- 4.Amato, P., M. Parazols, M. Sancelme, P. Laj, G. Mailhot, and A.-M. Delort. 2007. Microorganisms isolated from the water phase of tropospheric clouds at the puy de Dôme: major groups and growth abilities at low temperature. FEMS Microbiol. Ecol. 59:242-254. [DOI] [PubMed] [Google Scholar]
- 5.Amato, P., F. Demeer, A. Melaouhi, S. Fontanella, A.-S. Martin-Biesse, M. Sancelme, P. Laj, and A.-M. Delort. 2007. A fate of organic acids, formaldehyde and methanol in cloud water: their biotransformation by micro-organisms. Atmos. Chem. Phys. 7:4159-4169. [Google Scholar]
- 6.Amato, P., M. Parazols, M. Sancelme, P. Laj, G. Mailhot, and A.-M. Delort. 2007. An important oceanic source of microorganisms for cloud water at the puy de Dôme (France). Atmos. Environ. 41:8253-8263. [Google Scholar]
- 7.Ariya, P. A., and M. Amyot. 2004. New directions: the role of bioaerosols in atmospheric chemistry and physics. Atmos. Environ. 38:1231-1232. [Google Scholar]
- 8.Ariya, P. A., O. Nepotchatykh, O. Ignatova, and M. Amyot. 2002. Microbiological degradation of atmospheric organic compounds. Geophys. Res. Lett. 29:2077-2080. [Google Scholar]
- 9.Barth, M. C. 2006. The importance of cloud drop representation on cloud photochemistry. Atmos. Res. 82:294-309. [Google Scholar]
- 10.Bauer, H., A. Kasper-Giebl, M. Löflund, H. Giebl, R. Hitzenberger, F. Zibuschka, and H. Puxbaum. 2002. The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmos. Res. 64:109-119. [Google Scholar]
- 11.Carpenter, E., S. Lin, and D. G. Capone. 2000. Bacterial activity in South Pole. Appl. Environ. Microbiol. 66:4514-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christner, B. C. 2002. Incorporation of DNA and protein precursors into macromolecules by bacteria at −15°C. Appl. Environ. Microbiol. 68:6435-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Côté, V., G. Kos, R. Mortazavi, and P. A. Ariya. 2008. Microbial and “de novo” transformation of dicarboxylic acids by three airborne fungi. Sci. Total Environ. 390:530-537. [DOI] [PubMed] [Google Scholar]
- 14.Deguillaume, L., M. Leriche, and N. Chaumerliac. 2005. Impact of radical versus non-radical pathway in the Fenton chemistry on the iron redox cycle in clouds. Chemosphere 60:718-724. [DOI] [PubMed] [Google Scholar]
- 15.Deguillaume, L., M. Leriche, P. Amato, P. A. Ariya, A.-M. Delort, U. Pöschl, N. Chaumerliac, H. Bauer, A. I. Flossmann, and C. E. Morris. 2008. Microbiology and atmospheric processes: chemical interactions of primary biological aerosols. Biogeosciences 5:1073-1084. [Google Scholar]
- 16.Delort, A.-M. 2006. Chapter 9. Use of NMR to study in situ bioconversion of gaseous compounds, p. 117-131. In P. Lens (ed.), Gas resources for resource recovery. IWA Publishing, London, United Kingdom.
- 17.Ervens, B., G. Feingold, G. J. Frost, and M. Kreidenweiss. 2004. A modeling study of aqueous production of dicarboxylic acids: 1. Chemical pathways and speciated organic mass production. J. Geophys. Res. 109:D15205.1-D15205.20. [Google Scholar]
- 18.Finlayson-Pitts, B., and J. J. R. Pitts. 1997. Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 276:1045-1051. [DOI] [PubMed] [Google Scholar]
- 19.Fuzzi, S., P. Mandrioli, and A. Perfetto. 1997. Fog droplets—an atmospheric source of secondary biological aerosol particles. Atmos. Environ. 31:287-290. [Google Scholar]
- 20.Fuzzi, S., M. C. Facchini, S. Decesari, E. Matta, and M. Mircea. 2002. Soluble organic compounds in fog and cloud droplets: what have we learned over the past few years? Atmos. Res. 64:89-98. [Google Scholar]
- 21.Fuzzi, S., M. O. Andreae, B. J. Huebert, M. Kulmala, T. C. Bond, M. Boy, S. J. Doherty, A. Guenther, M. Kanakidou, K. Kawamura, V.-M. Kerminen, U. Lohmann, L. M. Russell, and U. Pöschl. 2006. Critical assessment of the current state of scientific knowledge, terminology, and research needs concerning the role of organic aerosols in the atmosphere, climate, and global change. Atmos. Chem. Phys. 6:2017-2038. [Google Scholar]
- 22.Gelencsér, A., and Z. Varga. 2005. Evaluation of the atmospheric significance of multiphase reactions in atmospheric secondary organic aerosol formation. Atmos. Chem. Phys. 5:2823-2831. [Google Scholar]
- 23.Granby, K., C. S. Christensen, and C. Lohse. 1997. Urban and semi-rural observations of carboxylic acids and carbonyls. Atmos. Environ. 31:1403-1415. [Google Scholar]
- 24.Herrmann, H., A. Tilgner, P. Barzaghi, Z. Majdik, S. Gligorovski, L. Poulain, and A. Monod. 2005. Towards a more detailed description of tropospheric aqueous phase organic chemistry: CAPRAM 3.0. Atmos. Environ. 39:4351-4363. [Google Scholar]
- 25.Hill, K. A., P. B. Shepson, E. S. Galdavy, C. Anastasio, P. S. Kourtev, A. Konopka, and B. H. Stirm. 2007. Processing of atmospheric nitrogen by clouds above forest environment. J. Geophys. Res. 112:D11301. doi: 10.1029/2006JD008002. [DOI] [Google Scholar]
- 26.Jacob, D. J. 1986. Chemistry of OH in remote clouds and its role in the production of formic acid and peroxymonosulfate. J. Geophys. Res. 91:9807-9826. [Google Scholar]
- 27.Jonson, J. E., and I. S. A. Isaksen. 1993. Tropospheric ozone chemistry. The impact of cloud chemistry. J. Atmos. Chem. 16:99-122. [Google Scholar]
- 28.Junge, K., H. Eicken, and J. W. Deming. 2004. Bacterial activity at −2 to −20°C in Arctic wintertime sea ice. Appl. Environ. Microbiol. 70:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamura, K., S. Ng, L. Steinberg, and I. R. Kaplan. 2001. Wet deposition of low molecular weight mono- and di-carboxylic acids, aldehydes and inorganic species in Los Angeles. Atmos. Environ. 35:3917-3926. [Google Scholar]
- 30.Kawamura, K., Y. Imai, and L. A. Barrie. 2005. Photochemical production and loss of organic acids in high Arctic aerosols during long-range transport and polar sunrise ozone depletion events. Atmos. Environ. 39:599-614. [Google Scholar]
- 31.Legrand, M., S. Preunkert, T. Oliveira, C. A. Pio, S. Hammer, A. Gelencsér, A. Kasper-Giebl, and P. Laj. 2007. Origin of C2-C5 dicarboxylic acids in the European atmosphere inferred from year-round aerosol study conducted at a west-east transect. J. Geophy. Res. 112:D23S07. doi: 10.1029/2006JD008019. [DOI]
- 32.Lelieveld, J., and P. J. Crutzen. 1991. The role of clouds in tropospheric photochemistry. J. Atmos. Chem. 12:229-267. [Google Scholar]
- 33.Leriche, M., L. Deguillaume, and N. Chaumerliac. 2003. Modeling study of strong acids formation and partitioning in a polluted cloud during wintertime. J. Geophys. Res. 108:AAC14.1-AAC14.11. doi: 10.1029/2002JD002950. [DOI] [Google Scholar]
- 34.Löflund, M., A. Kasper-Giebl, B. Schuster, H. Giebl, R. Hitzenberger, and H. Puxbaum. 2002. Formic, acetic oxalic and succinic acid concentrations and their contribution to organic carbon in cloud water. Atmos. Environ. 36:1553-1558. [Google Scholar]
- 35.Marinoni, A., P. Laj, K. Sellegri, and G. Mailhot. 2004. Cloud chemistry at the puy de Dôme: variability and relationships with environmental factors. Atmos. Chem. Phys. 4:715-728. [Google Scholar]
- 36.Monod, A., A. Chebbi, R. Durand-Jolibois, and P. Carlier. 2000. Oxidation of methanol by hydroxyl radicals in aqueous solution under simulated cloud droplet conditions. Atmos. Environ. 34:5283-5294. [Google Scholar]
- 37.Neu, J. L., M. J. Prather, and J. E. Penner. 2007. Global atmospheric chemistry: integrating over fractional cloud cover. J. Geophys. Res. 112:D11306. [Google Scholar]
- 38.Parazols, M., A. Marinoni, P. Amato, O. Abida, P. Laj, and G. Mailhot. 2006. Speciation and role of iron in cloud droplets at the puy de Dôme station. J. Atmos. Chem. 54:267-281. [Google Scholar]
- 39.Reasoner, D. J., and E. E. Geldreich. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattler, B., H. Puxbaum, and R. Psenner. 2001. Bacterial growth in supercooled cloud droplets. Geophys. Res. Lett. 28:239-242. [Google Scholar]
- 41.van Pinxteren, D., A. Plewka, D. Hofmann, K. Müller, H. Kramberger, B. Svrcina, K. Bächmann, W. Jaeschke, S. Mertes, J. L. Collett, Jr., and H. Herrmann. 2005. Schmücke hill cap cloud and valley stations aerosol characterization during FEBUKO (II): organic compounds. Atmos. Environ. 39:4305-4320. [Google Scholar]