Abstract
Voltage-dependent sodium channels are the central players in the excitability of neurons, cardiac muscle, and skeletal muscle. Hundreds of mutations in sodium channels have been associated with human disease, particularly genetic forms of epilepsy, arrhythmias, myotonia, and periodic paralysis. In this issue of the JCI, Jarecki and colleagues present evidence suggesting that many such mutations alter the gating of sodium channels to produce resurgent sodium current, an unusual form of gating in which sodium channels reopen following an action potential, thus promoting the firing of another action potential (see the related article beginning on page 369). The results of this study suggest a widespread pathophysiological role for this mechanism, previously described to occur normally in only a few types of neurons.
Our understanding of channelopathies — human disorders arising from mutations of ion channel genes — has gone through several waves of discovery. First, there was the implication that ion channels may play a causal role in disease pathology from the observation of abnormal ionic conductances in muscle biopsied from individuals with myotonia or periodic paralysis, studied using microelectrode recording (1, 2). Then came identification of mutations in ion channel genes, made possible by discovery of ion channel gene superfamilies; disease-associated mutations were identified by genome-wide linkage studies or by a candidate gene approach guided by the discovery of aberrant conductances in affected cells (3). This approach enabled the identification of numerous channelopathies in heart, brain, and peripheral nerve (4). The third wave of discovery involved the functional characterization of mutant ion channels (5, 6), usually by expression of mutant channels in heterologous expression systems, or, more rarely, by study of mutant channels in native tissue. The study by Jarecki and colleagues in this issue of the JCI (7) uses a clever twist for examining functional effects of ion channel mutations, by expressing cloned sodium channels in native neurons instead of a standard expression system — a procedure that allowed functional characterization of a particular kind of channel gating that has previously been impossible to capture in expression systems derived from nonexcitable cells.
By recording ionic currents from mutant channels heterologously expressed in common expression systems, such as frog oocytes or fibroblast cell lines, much progress has been made in our understanding of how mutations affect channel function. Surprisingly, many disease-associated mutations result in the formation of functional channels in these systems, but with abnormal characteristics. The vast majority of functional defects involve channel gating — the process by which channels open or close in response to membrane potential, ligand binding, stretch, or heat. A broad common theme has emerged: the consequence of mutant channel activity is often depolarization of the cell, through either gain-of-function changes (too much ionic current) for sodium or Ca2+ channels or loss-of-function changes for K+ or Cl– channels (5). However, this principle is not absolute, since a number of loss-of-function changes in sodium channels are also known (6). These biophysical measurements of channel gating have been complemented by computational models of cellular excitability that demonstrate, for example, how an observed defect in channel gating is sufficient to trigger abnormal bursts of sustained discharges or to render a cell inexcitable as a result of a persistently depolarized state of refractoriness.
Sodium channel gating
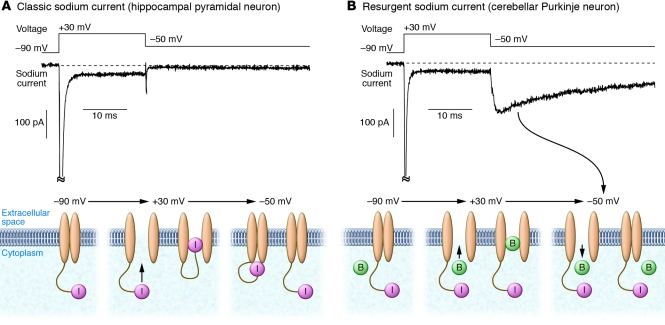
Voltage-gated sodium channels (VGSCs) carry inward sodium current that underlies action potentials in mammalian neurons, skeletal muscle, and cardiac muscle. VGSCs are closed at normal resting potentials. When the cell membrane is depolarized (made less negatively charged), VGSCs first open (i.e., activate) and then inactivate, closing to a state that cannot be opened by further depolarization. Before being available again for activation, the channels must recover from inactivation. Recovery occurs when the membrane is repolarized to the resting potential. In most cells, recovery from inactivation is electrically silent — no sodium current flows (Figure 1A and ref. 8). However, in some neurons, sodium channels open transiently during recovery from inactivation, generating a resurgent sodium current that flows after the membrane is repolarized (Figure 1B and ref. 9). This surge of sodium current provides a depolarizing influence after an action potential, producing a kind of anti-refractory behavior. The mechanism by which resurgent current is generated likely involves an intracellular, positively charged particle that binds to open sodium channels in a voltage-dependent manner, blocking strongly at depolarized voltages but exiting at hyperpolarized voltages (9, 10); the exit corresponds to flow of resurgent current at these voltages (Figure 1B). Binding of the particle seems competitive with normal inactivation, which occurs when an intracellular loop of the main channel subunit binds to and occludes the intracellular mouth of the pore (11). In cells with resurgent sodium current, depolarizing current flows as channels recover from inactivation, thereby promoting the firing of a second action potential, and such cells tend to fire bursts of closely spaced action potentials. A small, but growing, list of neurons is known to express resurgent sodium current, including cerebellar Purkinje neurons (9), subthalamic nucleus neurons (12), deep cerebellar nuclei neurons (13), cerebellar granule neurons (13–15), mesencephalic trigeminal motor neurons (16), vestibular nucleus neurons (17), and a subpopulation of primary sensory neurons in dorsal root ganglia (DRG; ref. 18). Resurgent sodium current has not been described in cardiac muscle or skeletal muscle.
Figure 1. Conceptual models of sodium channel gating during flow of classic and resurgent sodium current.
(A) Conventional sodium current kinetics. Top: Current recorded from a hippocampal CA1 pyramidal neuron in response to indicated voltage sequence. Bottom: Interpretation of channel gating. Channels are closed at –90 mV. Upon depolarization to +30 mV, channels activate, carrying inward sodium current. After a few milliseconds, channels inactivate, corresponding to block of each channel by an intracellular loop of the main channel subunit (particle labeled “I,” tethered to channel). Upon repolarization to –50 mV, channels partially recover from inactivation (back to non-inactivated closed state), but no current flows. (B) Sodium current kinetics for sodium channels carrying resurgent current. Top: Current recorded from a cerebellar Purkinje neuron in response to the same voltage sequence shown in A. Bottom: Interpretation of channel gating. In addition to normal inactivation, there is a competing process behaving as if a blocking particle (B), different from the intracellular loop of the main channel subunit, can enter and occlude the channel when it is open, preventing normal inactivation. Upon repolarization to –50 mV, channels occupied by the blocking particle transiently open as the blocking particle exits the channel. The transient opening produces current at –50 mV (resurgent current). The diagram is oversimplified; in actuality, some channels inactivate normally and do not result in resurgent current. Also, when the blocking particle exits at –50 mV, channels can either return to closed state or enter the normal inactivated state. Current records redrawn with permission from Journal of Neuroscience (19). Schematics of the changing conformation of the channels modified with permission from Journal of Neuroscience (20).
Testing for sodium channel resurgent properties must be performed in excitable cells
The exact molecular machinery underlying sodium channel resurgent current is unknown. Grieco and colleagues (10) hypothesized that the blocking particle may be part of a particular accessory subunit, β4, but this is still unproven, partly because it has thus far been impossible to reconstitute resurgent gating behavior in heterologous expression systems using cloned channels. In their current study, Jarecki and colleagues overcame this problem by using primary cultures of rat DRG neurons as an expression system for mutant sodium channels (7). From previous work, they knew that a subpopulation of DRG neurons expresses resurgent sodium current normally (18), and thus must possess the necessary intracellular milieu and/or accessory subunits, even if these are not yet well defined. To isolate the sodium current from exogenous sodium channels expressed in the neurons, the authors engineered the channels to lack the binding site for tetrodotoxin (TTX) and used TTX to block current from native sodium channels. With this system, the authors could convincingly identify TTX-resistant resurgent current from the heterologously expressed channels. A limitation of the system is that only approximately 60% of DRG neurons express resurgent current either normally or after transfection with exogenous channels (presumably reflecting heterogeneity in the cell population), making it necessary to perform many experiments with each mutation to obtain appropriate statistics.
Jarecki and colleagues astutely reasoned that since many disease-associated VGSC mutations slow the process of channel inactivation (5), and the resurgent mechanism appears to compete with normal inactivation (9), such mutations might induce or enhance resurgent current. Their data convincingly demonstrate a proof of principle for this hypothesis (7). They found that mutations associated with painful neuropathy (i.e., in the peripheral neuronal sodium channel Nav1.7 isoform), cardiac arrhythmia (i.e., in the cardiac myocyte sodium channel Nav1.5), or myotonia of skeletal muscle (i.e., in the skeletal muscle sodium channel Nav1.4) all resulted in slowed inactivation and exaggerated resurgent sodium currents when expressed in DRG neurons. The enhancement of resurgent current can be expected to promote high-frequency, repetitive firing in a way that slowing inactivation alone may not; indeed, computer simulations performed as part of their study demonstrate that enhanced resurgent current can, in principle, cause repetitive firing of neurons. Their finding that multiple mutations causing diminished inactivation in multiple sodium channel subtypes can enhance resurgent current is especially exciting because a great many other sodium channelopathies are known to diminish channel inactivation, which suggests that enhancement of resurgent current might occur in many other cases.
Functional consequences in skeletal muscle of altered sodium channels with exaggerated resurgent currents
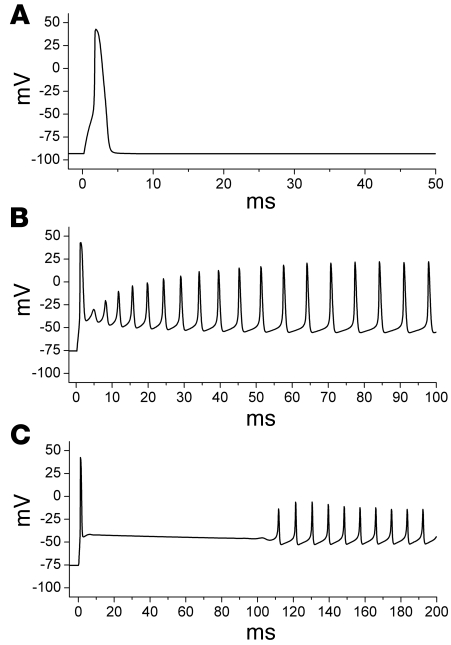
How the special features of resurgent sodium current might influence the disease phenotype can be illustrated by modeling of the excitability of skeletal muscle. As pointed out by Jarecki and colleagues (7), an abnormally enhanced resurgent sodium current is expected to promote self-sustained after-discharges in affected muscle fibers, which would manifest clinically as myotonic stiffness caused by involuntary after-contraction. Because the resurgent sodium current is present only transiently upon repolarization, lasting for about 30 or 40 ms, the prediction is that an abnormal resurgent current cannot produce the sustained depolarized shift in the muscle resting potential that underlies an attack of periodic paralysis, such as that predicted by incomplete inactivation without resurgence (5). Figure 2 shows the membrane voltage response of a model muscle cell to a brief current stimulus pulse. Normally, a suprathreshold stimulus elicits a single action potential, and the fiber repolarizes to the resting potential (Figure 2A). Simulation of a paramyotonia congenita (PMC) mutant VGSC — for which the kinetics of inactivation are slowed 5-fold compared with normal sodium channels, and which has a resurgent sodium current of 3% of the peak current — produces a train of myotonic after-discharges (Figure 2B). If the resurgent component is artificially increased by a 5-fold increase in the open to open/blocked rate constant, then the concomitant increase of inward resurgent sodium current does produce a plateau after-depolarization (Figure 2C). But because the resurgent sodium current is transient, the membrane potential eventually repolarizes and breaks into a train of repetitive after-discharges. This simulation demonstrates that a resurgent sodium current in skeletal muscle will promote myotonia, but is not predicted to cause paralysis, because a transient resurgent current, no matter how large, cannot produce a steady-state depolarized shift in the resting potential.
Figure 2. Resurgent sodium current effects on action potential firing in a simulated skeletal muscle fiber.
Membrane voltage response to a brief suprathreshold stimulus is shown for 3 different sodium channel configurations. (A) With normal sodium channels, a single action potential is elicited. (B) Simulation of the sodium channel defect in PMC (5-fold slower rate of inactivation compared with wild-type channels, plus 3% resurgent current), which produces a sustained burst of myotonic after-discharges. (C) Artificial enhancement of the resurgent properties beyond the 3% used to simulate PMC results in a brief depolarized plateau, which decays to a myotonic burst. Sustained depolarization with action potential failure, as occurs in periodic paralysis, cannot be generated by enhancement of the resurgent sodium current alone.
An important question that remains unanswered is whether mutations that can produce resurgent current in a cell type that allows it (like the DRG neurons used as the expression system) do produce it in the cell types in which the channels are actually present in vivo. For the Nav1.7 channel mutations associated with paroxysmal extreme pain disorder, a rare condition with severe jaw, eye, and rectal pain as well as flushing, the faulty channels are expressed endogenously in DRG neurons, so it seems very likely that the enhanced resurgent channel behavior observed by Jarecki et al. (7) does occur in vivo. However, it is still uncertain whether this is true for mutant cardiac and skeletal muscle channels (Nav1.5 and Nav1.4, respectively), because resurgent sodium current has not been reported in native cardiac or skeletal muscle (although it is also not clear whether appropriate voltage pulse protocols and/or maneuvers to reduce normal inactivation have been used to look for it). It will be crucial to determine whether the cellular milieu in skeletal muscle or heart can support resurgent sodium currents before concluding that resurgent current is a likely disease mechanism for VGSC mutations in these tissues. For mutations in neuronal sodium channels, the expectation is that the mutations will enhance resurgent current only in cell types capable of producing resurgent current under normal circumstances. The full extent of such cell types is not yet known.
Clinically used sodium channel inhibitors, including antiarrhythmics and anti-convulsants, all act by interacting with the gating machinery of sodium channels. Because the molecular machinery of resurgent current is different from that of normal gating, it may well be possible to design or identify drugs capable of selectively reducing resurgent sodium current. If enhanced resurgent current turns out to be a common mechanism for many channelopathies, as suggested by the work by Jarecki and colleagues (7), elucidation of the molecular machinery of this unusual gating mechanism could take on new clinical significance.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 120:80–83 (2010). doi:10.1172/JCI41340
See the related article beginning on page 369.
References
- 1.Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974;240(2):505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehmann-Horn F, Rüdel R, Ricker K, Lorkovic H, Dengler R, Hopf HC. Two cases of adynamia episodica hereditaria: in vitro investigation of muscle cell membrane and contraction parameters. Muscle Nerve. 1983;6(2):113–121. doi: 10.1002/mus.880060206. [DOI] [PubMed] [Google Scholar]
- 3.George AL., Jr Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115(8):1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79(4):1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- 5.Cannon SC. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu Rev Neurosci. 2006;29:387–415. doi: 10.1146/annurev.neuro.29.051605.112815. [DOI] [PubMed] [Google Scholar]
- 6.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28(46):11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarecki BW, Piekarz AD, Jackson JO, II, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. . J Clin Invest. 2010;120(1):369–378. doi: 10.1172/JCI40801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo C, Bean BP. Sodium channels must deactivate to recover from inactivation. Neuron. 1994;12(4):819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 9.Raman IM, Bean BP. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys J. 2001;80(2):729–737. doi: 10.1016/S0006-3495(01)76052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 2005;45(2):233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 11.West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+ -channel inactivation. . Proc Natl Acad Sci U S A. 1992;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do MT, Bean BP. Sodium currents in subthalamic nucleus neurons from Nav1.6-null mice. J Neurophysiol. 2004;92(2):726–733. doi: 10.1152/jn.00186.2004. [DOI] [PubMed] [Google Scholar]
- 13.Afshari FS, et al. Resurgent Na currents in four classes of neurons of the cerebellum. J Neurophysiol. 2004;92(5):2831–2843. doi: 10.1152/jn.00261.2004. [DOI] [PubMed] [Google Scholar]
- 14.Castelli L, Nigro MJ, Magistretti J. Analysis of resurgent sodium-current expression in rat parahippocampal cortices and hippocampal formation. Brain Res. 2007;1163:44–55. doi: 10.1016/j.brainres.2007.05.065. [DOI] [PubMed] [Google Scholar]
- 15.Magistretti J, Castelli L, Forti L, D’Angelo E. Kinetic and functional analysis of transient, persistent and resurgent sodium currents in rat cerebellar granule cells in situ: an electrophysiological and modelling study. J Physiol. 2006;573(Pt 1):83–106. doi: 10.1113/jphysiol.2006.106682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enomoto A, Han JM, Hsiao CF, Chandler SH. Sodium currents in mesencephalic trigeminal neurons from Nav1.6 null mice. J Neurophysiol. 2007;98(2):710–719. doi: 10.1152/jn.00292.2007. [DOI] [PubMed] [Google Scholar]
- 17.Gittis AH, du Lac S. Similar properties of transient, persistent, and resurgent Na currents in GABAergic and non-GABAergic vestibular nucleus neurons. . J Neurophysiol. 2008;99(5):2060–2065. doi: 10.1152/jn.01389.2007. [DOI] [PubMed] [Google Scholar]
- 18.Cummins TR, Dib-Hajj SD, Herzog RI, Waxman SG. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 2005;579(10):2166–2170. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17(12):4517–5426. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grieco TM, Afshari FS, Raman IM. A role for phosporylation in the maintenance of resurgent sodium current in cerebellar Purkinje neurons. J Neurosci. 2002;22(8):3100–3107. doi: 10.1523/JNEUROSCI.22-08-03100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]