Abstract
The timing of associations between common genetic variants for weight or body mass index (BMI) across the life course may provide insights into the aetiology of obesity. We genotyped variants in FTO (rs9939609) and near MC4R (rs17782313) in 1240 men and 1239 women born in 1946 and participating in the MRC National Survey of Health and Development. Birth weight was recorded and height and weight were measured or self-reported repeatedly at 11 time-points between ages 2 and 53 years. Hierarchical mixed models were used to test whether genetic associations with weight or BMI standard deviation scores (SDS) changed with age during childhood and adolescence (2–20 years) or adulthood (20–53 years). The association between FTO rs9939609 and BMI SDS strengthened during childhood and adolescence (rate of change: 0.007 SDS/A-allele/year; 95% CI: 0.003–0.010, P < 0.001), reached a peak strength at age 20 years (0.13 SDS/A-allele, 0.08–0.19), and then weakened during adulthood (−0.003 SDS/A-allele/year, −0.005 to −0.001, P = 0.001). MC4R rs17782313 showed stronger associations with weight than BMI; its association with weight strengthened during childhood and adolescence (0.005 SDS/C-allele/year; 0.001–0.008, P = 0.006), peaked at age 20 years (0.13 SDS/C-allele, 0.07–0.18), and weakened during adulthood (−0.002 SDS/C-allele/year, −0.004 to 0.000, P = 0.05). In conclusion, genetic variants in FTO and MC4R showed similar biphasic changes in their associations with BMI and weight, respectively, strengthening during childhood up to age 20 years and then weakening with increasing adult age. Studies of the aetiology of obesity spanning different age groups may identify age-specific determinants of weight gain.
INTRODUCTION
Genome-wide association (GWA) studies have recently identified common genetic variants that are robustly associated with higher BMI in adults. A GWA study initially for type 2 diabetes identified a common variant in FTO (rs9939609) which conferred increased risk for diabetes secondary to its associations with greater adult body mass index (BMI) (1). A second GWA study reported a common variant (rs17782313) 200 kb downstream of its nearest gene MC4R to give the next strongest association with adult BMI and, unlike FTO, this variant was also associated with taller adult stature and therefore was more strongly associated with body weight than with BMI (2). The associations between these FTO and MC4R variants with BMI and obesity risk have since been widely replicated in adult populations (3). Their associations with weight and BMI have also been demonstrated in children (1,2); indeed the original report of the MC4R variant described an apparent stronger association with BMI in children than in adults (2).
Rare mutations in MC4R are the leading cause of severe monogenic obesity, and the early onset and hyperphagic phenotypes in affected children is consistent with the biological role of MC4R in the central regulation of satiety (4). The biological action of FTO in humans is yet to be established (5,6), although there is increasing evidence for associations between FTO genotype and differences in eating behaviour, satiety and dietary intake, but not in energy expenditure, in children (7–9). Studies of the associations between these FTO and MC4R variants and body weight or BMI across the life course may provide insights into their mechanisms of action and also into the possible relevance of their related eating behaviour phenotypes in different age populations. Further, variations in the strength of genetic associations by age may contribute to the failure to replicate genetic-association findings (10). However, few studies have been able to investigate the longitudinal pattern of association between these genetic variants and BMI across childhood, adolescence and adulthood.
We therefore genotyped variants in FTO and MC4R in the MRC National Survey of Health and Development, which has measured its participants’ heights and weights repeatedly from age 2 years to age 53 years (11). In particular we assessed whether the genetic associations with body weight and BMI differed with age through childhood and adult life.
RESULTS
Genotype data were available in 1240 men and 1239 women (1233 men and 1233 women for FTO rs9939609; 1235 men and 1234 women for MC4R rs17782313). Of these, 839 (34%) had BMI measured at all 11 ages from 2 to 53 years and 2126 (86%) at eight or more ages. Only 81 (3%) contributed fewer than five measurements. The number of individuals with BMI recorded was lowest at ages 2 (n = 1930), 15 (n = 1903) and 20 (n = 1968) years. At all other ages, over 2000 measurements were available for analysis. Compared with those excluded because of missing genotype data, the included individuals had slightly heavier birth weights (men P = 0.03; women P = 0.04) and included women were slightly taller throughout life (age 4 years P = 0.04; age 11 years P = 0.01; age 15 years P = 0.03; adult height P = 0.01). No differences in any other growth parameters were observed (Table 1).
Table 1.
Mean (SD) for weight, height and BMI at 12 time-points between birth to age 53 years in men and women with genotype data (‘included’)
| Included |
Excluded |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men |
Women |
Men |
Women |
|||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Weight (kg) | ||||||||
| Birth | 1229 | 3.47 (0.52) | 1230 | 3.32 (0.48) | 1568 | 3.42 (0.58) | 1300 | 3.28 (0.55) |
| 2 years | 1037 | 13.2 (1.5) | 1010 | 12.6 (1.5) | 1126 | 13.2 (1.5) | 943 | 12.6 (1.6) |
| 4 years | 1112 | 17.5 (2.1) | 1098 | 17.0 (2.1) | 1184 | 17.5 (2.1) | 995 | 16.9 (2.3) |
| 6 years | 1045 | 20.9 (2.5) | 1046 | 20.4 (2.6) | 1103 | 20.9 (2.5) | 894 | 20.2 (2.9) |
| 7 years | 1021 | 23.0 (2.9) | 1027 | 22.6 (3.1) | 1046 | 23.1 (3.0) | 906 | 22.5 (3.5) |
| 11 years | 1045 | 34.3 (5.9) | 1020 | 35.0 (6.6) | 1020 | 34.3 (6.1) | 875 | 34.8 (7.7) |
| 15 years | 968 | 51.8 (9.4) | 947 | 51.9 (8.1) | 931 | 51.7 (9.7) | 771 | 51.8 (9.7) |
| 20 years | 978 | 70.7 (9.1) | 1013 | 57.9 (8.4) | 884 | 71.1 (9.3) | 746 | 57.6 (8.7) |
| 26 years | 1050 | 73.5 (10.1) | 1076 | 59.1 (8.8) | 773 | 73.7 (9.9) | 706 | 58.8 (9.9) |
| 36 years | 1100 | 76.2 (11.0) | 1125 | 62.1 (10.4) | 539 | 76.8 (12.2) | 533 | 62.2 (12.9) |
| 43 years | 1141 | 78.8 (11.6) | 1167 | 66.3 (12.0) | 476 | 79.5 (13.3) | 453 | 66.4 (14.7) |
| 53 years | 1224 | 83.5 (13.2) | 1221 | 71.5 (13.9) | 227 | 84.3 (15.7) | 281 | 72.0 (16.4) |
| Height (cm) | ||||||||
| 2 years | 1025 | 85.9 (5.2) | 978 | 84.8 (4.5) | 1112 | 85.9 (5.3) | 895 | 84.6 (5.1) |
| 4 years | 1085 | 103.5 (5.1) | 1077 | 102.9 (5.0) | 1162 | 103.6 (5.2) | 960 | 102.4 (5.3) |
| 6 years | 1048 | 114.5 (5.3) | 1041 | 113.8 (5.3) | 1091 | 114.5 (5.2) | 881 | 113.5 (5.6) |
| 7 years | 1064 | 120.4 (5.7) | 1066 | 119.7 (5.4) | 1083 | 120.4 (5.4) | 927 | 119.3 (6.1) |
| 11 years | 1052 | 140.7 (6.8) | 1029 | 141.2 (6.8) | 1030 | 140.8 (6.6) | 884 | 140.8 (6.6) |
| 15 years | 968 | 162.0 (9.1) | 950 | 158.8 (6.1) | 945 | 162.0 (8.7) | 776 | 158.1 (6.5) |
| Adult | 1099 | 175.3 (6.5) | 1125 | 162.6 (5.8) | 537 | 175.3 (6.8) | 530 | 161.8 (6.6) |
| BMI (kg/m2) | ||||||||
| 2 years | 985 | 18.0 (2.5) | 933 | 17.6 (2.4) | 1061 | 18.0 (2.6) | 861 | 17.7 (2.5) |
| 4 years | 1066 | 16.3 (1.6) | 1046 | 16.0 (1.6) | 1132 | 16.3 (1.7) | 940 | 16.1 (1.7) |
| 6 years | 999 | 15.9 (1.3) | 1000 | 15.7 (1.4) | 1051 | 15.9 (1.3) | 841 | 15.7 (1.5) |
| 7 years | 1017 | 15.9 (1.3) | 1022 | 15.7 (1.5) | 1040 | 15.9 (1.3) | 898 | 15.7 (1.7) |
| 11 years | 1038 | 17.3 (2.1) | 1017 | 17.5 (2.5) | 1012 | 17.3 (2.2) | 870 | 17.5 (2.8) |
| 15 years | 953 | 19.6 (2.4) | 939 | 20.6 (2.8) | 928 | 19.6 (2.5) | 761 | 20.7 (3.3) |
| 20 years | 958 | 22.6 (2.4) | 999 | 21.9 (2.9) | 871 | 22.7 (2.5) | 736 | 21.8 (2.9) |
| 26 years | 1050 | 23.4 (2.8) | 1076 | 22.4 (3.1) | 772 | 23.5 (2.9) | 706 | 22.4 (3.5) |
| 36 years | 1096 | 24.8 (3.1) | 1122 | 23.5 (3.7) | 535 | 24.9 (3.5) | 527 | 23.7 (4.7) |
| 43 years | 1141 | 25.7 (3.4) | 1159 | 25.1 (4.5) | 475 | 25.9 (3.9) | 450 | 25.4 (5.5) |
| 53 years | 1224 | 27.4 (3.9) | 1219 | 27.4 (5.2) | 227 | 27.5 (4.6) | 278 | 27.9 (6.4) |
Similar data are also shown for men and women without genotype data (‘excluded’) for comparison.
Associations with FTO rs9939609
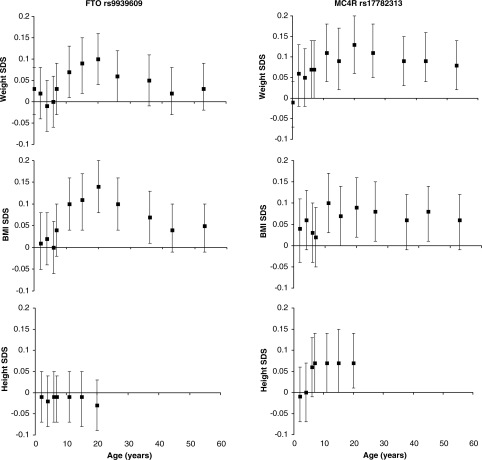
In cross-sectional analyses, the FTO rs9939609 ‘A’ allele was positively associated with BMI from ages 11 to 36 years, with similar trends with BMI also observed at ages 7, 43 and 53 years (Table 2, Fig. 1). At almost all ages, cross-sectional associations with weight SDS were similar to, but weaker than, those with BMI SDS (Fig. 1).
Table 2.
Mean (95% confidence interval) BMI at 11 time-points between ages 2 and 53 years by FTO rs9939609 or MC4R rs17782313 genotype
| N | BMI (kg/m2) | P-value* | |||
|---|---|---|---|---|---|
| FTO rs9939609 | TT | TA | AA | ||
| 2 years | 1918 | 17.8 (17.6,18.0) | 17.8 (17.7,18.0) | 17.8 (17.6,18.1) | 0.7 |
| 4 years | 2112 | 16.2 (16.0,16.3) | 16.2 (16.1,16.3) | 16.2 (16.1,16.4) | 0.5 |
| 6 years | 1999 | 15.8 (15.7,15.9) | 15.8 (15.7,15.9) | 15.8 (15.7,16.0) | 0.9 |
| 7 years | 2039 | 15.7 (15.6,15.8) | 15.8 (15.7,15.9) | 15.8 (15.7,16.0) | 0.2 |
| 11 years | 2055 | 17.2 (17.0,17.3) | 17.4 (17.2,17.5) | 17.7 (17.5,18.0) | 0.001 |
| 15 years | 1892 | 19.9 (19.7,20.1) | 20.0 (19.9,20.2) | 20.6 (20.3,20.9) | 0.001 |
| 20 years | 1957 | 22.0 (21.8,22.2) | 22.2 (19.9,20.2) | 22.9 (22.5,23.2) | <0.001 |
| 26 years | 2126 | 22.7 (22.5,22.9) | 22.9 (22.7,23.0) | 23.4 (23.0,23.8) | 0.001 |
| 36 years | 2218 | 24.0 (23.7,24.2) | 24.1 (23.8,24.3) | 24.6 (24.2,25.0) | 0.02 |
| 43 years | 2300 | 25.3 (25.0,25.6) | 25.3 (25.1,25.5) | 25.7 (25.3,26.1) | 0.1 |
| 53 years | 2443 | 27.2 (26.9,27.5) | 27.4 (27.1,27.6) | 27.7 (27.3,28.2) | 0.07 |
| MC4R rs17782313 | TT | TC | CC | ||
| 2 years | 1924 | 17.8 (17.6,17.9) | 17.8 (17.6,18.0) | 18.3 (17.8,18.8) | 0.3 |
| 4 years | 2115 | 16.1 (16.0,16.2) | 16.2 (16.1,16.3) | 16.4 (16.1,16.7) | 0.09 |
| 6 years | 2003 | 15.8 (15.7,15.9) | 15.8 (15.7,15.9) | 16.0 (15.7,16.2) | 0.4 |
| 7 years | 2043 | 15.8 (15.7,15.9) | 15.8 (15.7,15.9) | 15.8 (15.5,16.2) | 0.6 |
| 11 years | 2058 | 17.2 (17.1,17.4) | 17.5 (17.3,17.7) | 17.7 (17.3,18.2) | 0.005 |
| 15 years | 1897 | 20.0 (19.8,20.1) | 20.2 (20.0,22.5) | 20.4 (19.9,21.0) | 0.05 |
| 20 years | 1961 | 22.1 (21.9,22.2) | 22.3 (22.2,22.5) | 22.7 (22.0,23.3) | 0.01 |
| 26 years | 2131 | 22.8 (22.6,23.0) | 22.9 (22.7,23.1) | 23.5 (22.8,24.2) | 0.02 |
| 36 years | 2222 | 24.0 (23.9,24.2) | 24.2 (23.9,24.5) | 24.5 (23.9,25.2) | 0.09 |
| 43 years | 2302 | 25.2 (25.0,25.4) | 25.5 (25.2,25.8) | 26.0 (25.2,26.7) | 0.02 |
| 53 years | 2446 | 27.3 (27.0,27.5) | 27.4 (27.1,27.7) | 27.8 (27.0,28.5) | 0.09 |
*From additive models adjusted for sex.
Figure 1.
Cross-sectional associations between FTO rs9939609 and MC4R rs17782313 with weight, BMI and height standard deviation scores (SDS) at up to 12 different time-points from birth to age 53 years. Error bars represent regression coefficient and 95% CI from additive genetic models.
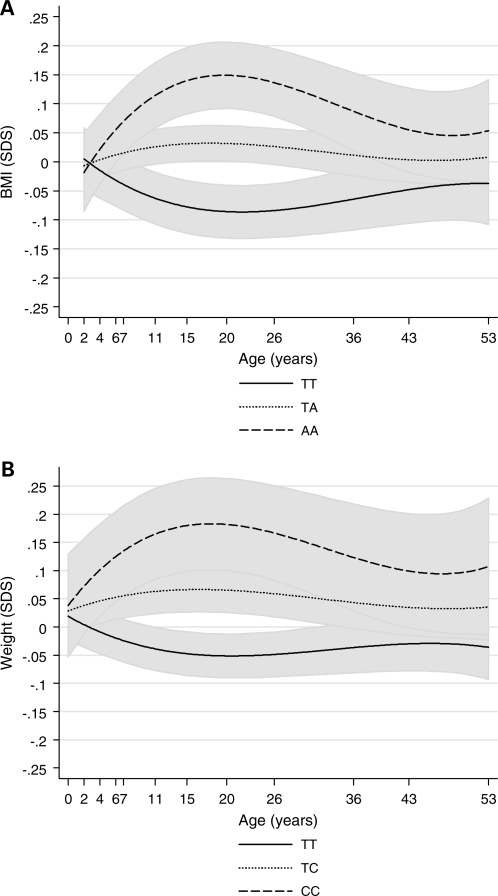
In longitudinal analyses, linear (regression coefficient = 0.019, 95% CI: 0.011–0.026), P < 0.001), quadratic (regression coefficient = −0.00065, 95% CI: −0.0010 to −0.00032, P < 0.001) and cubic (regression coefficient = 0.0000062, 95% CI: 0.0000023–0.000010, P = 0.002) genotype-by-age interaction terms were all significant. Estimation of mean BMI SDS by genotype using the polynomial model indicated that the association between FTO rs9939609 and BMI was greatest at around age 20 years (Fig. 2A). In a linear piecewise model, the association strengthened between ages 2 and 20 years (P < 0.001 for genotype-by-age interaction) at a rate of 0.007 SDS per A-allele per year (95% CI: 0.003–0.010), and reached a peak at age 20 years (0.13 SDS per A-allele, 95% CI: 0.08–0.19) (Table 3). Thereafter, the association with BMI SDS weakened with age from 20 to 53 years (rate of decline: −0.003 SDS per A-allele per year, 95% CI: −0.005 to −0.001, P = 0.001 for genotype-by-age interaction) (Table 3), and by age 53 years there was only a weak association between FTO rs9939609 and BMI SDS (0.05 SDS per A-allele, 95% CI: −0.01 to 0.10). A similar pattern, although with slightly weaker change with age, was observed for weight (Table 3, Fig. 1).
Figure 2.
Linear prediction of mean and 95% prediction interval from additive genetic models for (A) BMI SDS by FTO rs9939609 genotype; (B) Weight SDS by MC4R rs17782313 genotype.
Table 3.
Results from hierarchical mixed models for the longitudinal associations between FTO and MC4R variants with weight, BMI and height SDS
| (a) FTO rs9939609 | N | Mean (95% CI) per A-allele | P-value | |
|---|---|---|---|---|
| BMI SDS | Genotype (age 2 years) | 2466 | 0.01 (−0.04,0.06) | 0.6 |
| Genotype × age (2–20) (change per year 2–20 years) | 0.007 (0.003,0.010) | <0.001 | ||
| Genotype × age (20–53) (change per year 20–53 years) | −0.003 (−0.005, −0.001) | 0.001 | ||
| Weight SDS | Genotype (birth) | 2466 | 0.00 (−0.04,0.05) | 0.9 |
| Genotype × age (birth-20) (change per year 0–20 years) | 0.004 (0.001,0.007) | 0.005 | ||
| Genotype × age (20–53) (change per year 20–53 years) | −0.002 (−0.004, −0.000) | 0.02 | ||
| Height SDS | Genotype (age 2 years) | 2450 | −0.02 (−0.07,0.04) | 0.6 |
| Genotype × age (2–20)* (change per year 2–20 years) | −0.001 (−0.004,0.002) | 0.4 | ||
| (b) MC4R rs17782313 | N | Mean (95% CI) per C-allele | P-value | |
| BMI SDS | Genotype (age 2 years) | 2469 | 0.04 (−0.01,0.10) | 0.2 |
| Genotype × age (2–20) (change per year 2–20 years) | 0.002 (−0.002,0.006) | 0.3 | ||
| Genotype × age (20–53) (change per year 20–53 years) | −0.001 (−0.003,0.001) | 0.3 | ||
| Weight SDS | Genotype (birth) | 2469 | 0.04 (−0.01,0.09) | 0.1 |
| Genotype × age (birth-20) (change per year 0–20 years) | 0.005 (0.001,0.008) | 0.006 | ||
| Genotype × age (20–53) (change per year 20–53 years) | −0.002 (−0.004,0.000) | 0.05 | ||
| Height SDS | Genotype (age 2 years) | 2453 | 0.02 (−0.04,0.08) | 0.5 |
| Genotype × age (2–20)* (change per year 2–20 years) | 0.004 (0.001,0.007) | 0.02 | ||
Regression coefficients presented represent the main effect of genotype and genotype-by-linear age interactions.
*Final adult height (from measure at 36 years) was assumed to have been reached at 20 years.
There were no associations between FTO rs9939609 and childhood height, and only a weak inverse trend with adult height (P = 0.3, Fig. 1).
Associations with MC4R rs17782313
In cross-sectional analyses, the MC4R rs17782313 ‘C’ allele was positively associated with BMI SDS from ages 11 to 43 years (except at age 36 years P = 0.09) (Table 2). Cross-sectional associations with weight SDS were stronger than those with BMI SDS, and were apparent from age 6 years, with similar trends even at ages 2 years (P = 0.1) and 4 years (P = 0.1) (Fig. 1).
In longitudinal analyses, linear (regression coefficient = 0.013, 95% CI: 0.007–0.019, P < 0.001), quadratic (regression coefficient = −0.00049, 95% CI: −0.00077 to −0.00022, P < 0.001) and cubic (regression coefficient = 0.0000050, 95% CI: 0.0000016–0.0000085, P = 0.004) genotype-by-age interaction terms were significant; predictions from these polynomial models of mean weight SDS by genotype indicated that the association between MC4R rs17782313 and weight SDS peaked between ages 15 and 20 years (Fig. 2B). In the linear piecewise model, the association strengthened between birth and age 20 years (P = 0.006 for genotype-by-age interaction) at a rate of 0.005 SDS per C-allele per year (95% CI: 0.001–0.008), so that at age 20 years there was a 0.13 SDS increase in mean weight per C-allele (95% CI: 0.06–0.19) (Table 3). Thereafter, the association with weight SDS weakened with age (rate of decline: −0.002 SDS per C-allele per year, 95% CI: −0.004 to 0.000, P = 0.05 for genotype-by-age interaction) (Table 3), but the association with weight was still apparent at age 53 years (0.08 SDS per C-allele, 95% CI: 0.02, 0.14).
In contrast to the FTO variant, MC4R rs17782313 was positively associated with height from age 7 years onwards (0.07 SDS per C-allele in adulthood, 95% CI: 0.01, 0.14) (Fig. 1). In longitudinal analyses, the association with height SDS strengthened between ages 2 and 20 years (P = 0.02 for genotype-by-age interaction) by 0.004 SDS per C-allele per year (95% CI: 0.001–0.007) (Table 3); although most of this change had appeared by age 7 years, and the association with height SDS appeared to remain constant between age 7 years to adulthood (Fig. 1). In longitudinal polynomial analyses, linear (regression coefficient = 0.016, 95% CI: 0.007–0.025, P < 0.001) and quadratic (regression coefficient = −0.0007, 95% CI: −0.0012 to −0.00002, P < 0.001) genotype-by-age interaction terms were significant for the association between MC4R rs17782313 and height SDS.
DISCUSSION
In a unique study of a longitudinal birth cohort born in 1946 and followed through to age 53 years, we observed that the associations of FTO rs9939609 and MC4R rs17782313 on body size varied with age. The changes with age appeared to be biphasic, in that the associations with both variants strengthened during childhood and adolescence, the associations peaked in early adulthood and thereafter weakened with increasing adult age. Associations with FTO rs9939609 were most apparent with BMI, whereas, MC4R rs17782313 was positively associated with childhood and adult height and therefore showed stronger associations with body weight than with BMI.
The main strength of this study was the availability of longitudinal data on weight and height across childhood, adolescence and adulthood up to age 53 years, which allowed a unique exploration of the life course genetic associations with body size. Weight and height were measured at all ages, except at ages 20 and 26 years, when they were self-reported. Despite the reliance on self-reported measures at those ages, the peak associations between FTO rs9939609 and BMI SDS, and between MC4R rs17782313 and weight SDS, were both seen at age 20 years. Differential mis-reporting of body size by genotype seems unlikely, but if heavier individuals were more likely to under-report their weight than others it is possible that the peak associations and the rates of change in associations with age may be downwardly biased. Our statistical models included individuals without complete outcome data and therefore assumed data to be missing at random. This is a reasonable assumption in the sample studied given that the DNA was collected at the most recent contact with participants and hence the sample represents those still alive and in the study at age 53 years. Those with missing genetic information had similar mean body size measures at most ages compared with those included in the analysis.
There have been only a few other studies exploring the variations in these genetic associations over the life course. In older adults, a study of men in the Health Professionals Follow-Up Study reported that, similar to our findings, associations between the same FTO rs9939609 variant and BMI declined with age. However, in the same report those age effects were not seen in women in the Nurses Health Study (12). Rather, in those women carriers of the MC4R rs17782313 C-allele showed greater gains in adult weight and BMI than non-carriers (13). In both of those populations, heights and weights were self-reported and follow-up only started in adulthood at mean age 56 years in men and 44 years in women (14). In the NSHD we found no evidence of differences between men and women in the genetic associations with body size across the life course. In a study of 1629 Danish men, born between 1943 and 1977, the FTO rs9939609 AA genotype was associated with faster weight gain from birth to age 7 years, but not with further weight gain during childhood and adolescence. Subsequently, in contrast to our findings, the AA genotype was associated with a second increase in BMI z-score, from ages 13 or 20 years up to age 35 years (14). However, in that Danish study, half of the men were selected for the presence of adult obesity (above the 99th percentile), and it is possible that the effect of the FTO rs9939609 variant on BMI or weight gain might be greater in individuals with obesogenic lifestyles, such as sedentary behaviours (15,16). Finally, consistent with our current findings, a study of >7000 UK twins described that the association between the FTO rs9939609 variant and BMI was not apparent at age 4 years, but became increasingly stronger at ages 7–11 years, in parallel with a rise in the heritability of BMI from 48% at age 4 years to 78% at age 11 years (17).
Although our cohort was sampled from a national population, even such representative cohorts will have mean BMI trajectories which differ from cohorts born earlier or later (18). NSHD cohort members were all born during 1 week in 1946 and experienced post-war rationing during the first 8 years of life, which could have diminished the genetic differences in early childhood weight gain and BMI. The prevalence of overweight or obesity in this cohort remained low during childhood and adolescence until the onset of the obesogenic environment in the 1980s when cohort members were aged 30–40 years old (18). Our current observed weakening of the genetic associations with FTO and MC4R variants across mid-life could therefore reflect the increasing impact of environmental influences on physical activity and food consumption coincident with the onset of the obesity epidemic (18). However, in a single study where all the participants were born in the same era it is difficult to disentangle age effects from period effects.
Recent studies have found several further common variants associated with BMI and obesity risk (19–22). Further longitudinal studies of those variants would help to show whether the biphasic changes with age that we identified with both the FTO and MC4R variants are generalizable observations, possibly reflecting age-related trends in the heritability of BMI and obesity risk. Variants in PCSK1, which encodes an enzyme that converts prohormones into key functional regulators of appetite (22,23), demonstrated age-dependent effects on obesity risk in the EPIC-Norfolk Study, being apparent only in younger individuals (aged below the median age 59 years) (24). However, that analysis was based on cross-sectional data where it is not possible to separate age-effects from birth-cohort effects. The importance of longitudinal data in the detection of age-related effects is highlighted by a recent study of the penetrance of rare deleterious mutations in MC4R on the development of monogenic obesity (25). In cross-sectional inter-generational comparisons, the prevalence of obesity among carriers of MC4R mutations reduced with age, from 79% in children, to 60% in 18–52-year-old adults, and 40% in adults aged >52 years, suggesting that these mutations had lower penetrance among older birth cohorts. In contrast, in their longitudinal analysis, the prevalence of obesity increased from 37% at 20 years to 60% at >40 years, indicating that the penetrance of these mutations increased with age (25). Further longitudinal population-based studies spanning a wide range of birth years are therefore required to distinguish between the effects of age and period changes in the obesogenic environment.
In conclusion, genetic variants in FTO and MC4R showed biphasic changes in their associations with BMI and weight, respectively, strengthening with age up to a peak at age 20 years, and then weakening with increasing adult age. We postulate that these changes may reflect the age-dependent influence of satiety and eating behaviour on weight gain and BMI, as weight gain in adult life may be increasingly determined or modified by other hedonic, psycho-social or environmental influences (26). Future studies of the aetiology of obesity spanning different age groups may identify age-specific genetic or other determinants of weight gain.
MATERIALS AND METHODS
The MRC National Survey of Health and Development is a socially stratified birth cohort of 2547 women and 2815 men, who have been followed up since their birth in 1946 with regular data collections (11). The most recent contact was when cohort members were aged 53 years of age and 3035 (1472 men, 1563 women) provided information. The majority (n = 2989) were interviewed and examined in their own homes by trained research nurses with others completing a postal questionnaire (n = 46). Those interviewed at the age of 53 years were, in most respects, representative of the native born population of that age (27). Contact was not attempted for the 1979 individuals who had previously refused to take part, were living abroad, were untraced since last contact at 43 years or had already died. Blood samples were collected from 2756 members. The data collection received MREC approval, and informed consent was given by respondents to each set of questions and measures.
Anthropometry
Birth weight of cohort members to the nearest quarter of a pound was extracted from medical records within a few weeks of delivery and converted into kilograms. Height and weights were measured using standardized protocols at ages 2, 4, 6, 7, 11 and 15 years and at 36, 43 and 53 years. Height and weight were self-reported at ages 20 and 26 years. BMI, defined as weight/height2, was calculated at each age.
Genotyping
DNA was extracted and purified from whole blood using the Puregene DNA Isolation Kit (Flowgen, Leicestershire, UK) according to the manufacturer's protocol. The two SNPs were typed by Source Bioscience PLC using the Applied Biosystems (Foster City, CA, USA) SNPlex technology which is a based on an Oligonucleotide Ligation Assay combined with multiplex PCR amplification and capillary electrophoresis. Genotyping was performed using an ABI 3730xl DNA Analyser and ABI GeneMapper v4.0 software. The integrity of the genotyping was checked by genotyping frequency, concordance of duplicates and Hardy–Weinberg equilibrium (HWE). The call rates for rs9939609 and rs17782313 were 99.5 and 99.6%, respectively, with >95% concordance between duplicate samples and there was no evidence of deviation from HWE (P > 0.05). The minor allele frequency for FTO rs9939609 was 0.41 and for MC4R rs17782313 it was 0.24.
Statistical methods
Sex specific standard deviation scores (SDS) for height, weight and BMI were estimated using internally generated growth charts. The growth charts were constructed using the LMS (L = skewness; M = median; S = coefficient of variation) method (28). This is preferable to the use of an external reference, which can be misleading for historical cohorts (29).
In initial descriptive analyses, cross-sectional associations between each genotype and height, weight and BMI SD scores at each age were performed using linear regression assuming an additive genetic model, with adjustment for sex.
Longitudinal analyses were performed using hierarchical mixed models. These models take account of the correlation between repeated measures on the same individual and allow for incomplete outcome data on the assumption that data are missing at random. To initially test whether the association between genotype and each measure of body size (BMI, weight and height) changed with age, we first fitted linear age and genotype-by-age interaction terms, then added, in turn, quadratic and cubic ages and their interactions with genotype. For simplicity of presentation, and because the findings were very similar to those from the polynomial age models, we then fitted linear piecewise models with a knot at 20 years to allow different slopes for childhood (2–20 years) and adulthood (20–53 years) for BMI. The intercept was allowed to vary according to allele frequency in an additive genetic model. Genotype-by-linear age interaction terms (one for 2–20 years and another for 20–53 years) were then added to the model to assess whether the effect of genotype changed with increasing age. Likelihood ratio tests were used to assess the improvement in fit on addition of each new term in the model. We allowed for a random intercept and random slopes. For the weight trajectories, similar models were fitted except that baseline was taken as birth, due to the availability of birth weight data. For height, baseline was at age 2 years and change in height was modelled only to adulthood using a single slope. Because the first two measures of adult height (at age 20 and 26 years) were self-reported, height at 36 years was used as final attained adult height and was assumed to have been achieved by age 20 years.
Tests for sex interactions were not statistically significant, and therefore all models included both men and women and were adjusted for sex. Analyses were performed using Stata version 10.1.
FUNDING
This work was supported by the Medical Research Council. Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council.
ACKNOWLEDGEMENTS
We are very grateful to the members of this birth cohort for their continuing interest and participation in the study.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R., Elliott K.S., Lango H., Rayner N.W., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loos R.J., Lindgren C.M., Li S., Wheeler E., Zhao J.H., Prokopenko I., Inouye M., Freathy R.M., Attwood A.P., Beckmann J.S., et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loos R.J., Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes. Rev. 2008;9:246–250. doi: 10.1111/j.1467-789X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 5.Fischer J., Koch L., Emmerling C., Vierkotten J., Peters T., Bruning J.C., Ruther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 6.Gerken T., Girard C.A., Tung Y.C., Webby C.J., Saudek V., Hewitson K.S., Yeo G.S., McDonough M.A., Cunliffe S., McNeill L.A., et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecil J.E., Tavendale R., Watt P., Hetherington M.M., Palmer C.N. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 8.Wardle J., Carnell S., Haworth C.M., Farooqi I.S., O'Rahilly S., Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J. Clin. Endocrinol. Metab. 2008;93:3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 9.Timpson N.J., Emmett P.M., Frayling T.M., Rogers I., Hattersley A.T., McCarthy M.I., Davey Smith G. The fat mass- and obesity-associated locus and dietary intake in children. Am. J. Clin. Nutr. 2008;88:971–978. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lasky-Su J., Lyon H.N., Emilsson V., Heid I.M., Molony C., Raby B.A., Lazarus R., Klanderman B., Soto-Quiros M.E., Avila L., et al. On the replication of genetic associations: timing can be everything! Am. J. Hum. Genet. 2008;82:849–858. doi: 10.1016/j.ajhg.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadsworth M., Kuh D., Richards M., Hardy R. Cohort profile: The 1946 National Birth Cohort (MRC National Survey of Health and Development) Int. J. Epidemiol. 2006;35:49–54. doi: 10.1093/ije/dyi201. [DOI] [PubMed] [Google Scholar]
- 12.Qi L., Kang K., Zhang C., van Dam R.M., Kraft P., Hunter D., Lee C.H., Hu F.B. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes. 2008;57:3145–3151. doi: 10.2337/db08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi L., Kraft P., Hunter D.J., Hu F.B. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum. Mol. Genet. 2008;17:3502–3508. doi: 10.1093/hmg/ddn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jess T., Zimmermann E., Kring S.I., Berentzen T., Holst C., Toubro S., Astrup A., Hansen T., Pedersen O., Sorensen T.I. Impact on weight dynamics and general growth of the common FTO rs9939609: a longitudinal Danish cohort study. Int. J. Obes. (Lond.) 2008;32:1388–1394. doi: 10.1038/ijo.2008.110. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen C.H., Stender-Petersen K.L., Mogensen M.S., Torekov S.S., Wegner L., Andersen G., Nielsen A.L., Albrechtsen A., Borch-Johnsen K., Rasmussen S.S., et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 16.Rampersaud E., Mitchell B.D., Pollin T.I., Fu M., Shen H., O'Connell J.R., Ducharme J.L., Hines S., Sack P., Naglieri R., et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch. Intern. Med. 2008;168:1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haworth C.M., Carnell S., Meaburn E.L., Davis O.S., Plomin R., Wardle J. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008;16:2663–2668. doi: 10.1038/oby.2008.434. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Hardy R., Kuh D., Lo Conte R., Power C. Child-to-adult body mass index and height trajectories: a comparison of 2 British birth cohorts. Am. J. Epidemiol. 2008;168:1008–1015. doi: 10.1093/aje/kwn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorleifsson G., Walters G.B., Gudbjartsson D.F., Steinthorsdottir V., Sulem P., Helgadottir A., Styrkarsdottir U., Gretarsdottir S., Thorlacius S., Jonsdottir I., et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 20.Willer C.J., Speliotes E.K., Loos R.J., Li S., Lindgren C.M., Heid I.M., Berndt S.I., Elliott A.L., Jackson A.U., Lamina C., et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benzinou M., Creemers J.W., Choquet H., Lobbens S., Dina C., Durand E., Guerardel A., Boutin P., Jouret B., Heude B., et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat. Genet. 2008;40:943–945. doi: 10.1038/ng.177. [DOI] [PubMed] [Google Scholar]
- 22.Meyre D., Delplanque J., Chevre J.C., Lecoeur C., Lobbens S., Gallina S., Durand E., Vatin V., Degraeve F., Proenca C., et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat. Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 23.Jackson R.S., Creemers J.W., Ohagi S., Raffin-Sanson M.L., Sanders L., Montague C.T., Hutton J.C., O'Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 24.Kilpelainen T.O., Bingham S.A., Khaw K.T., Wareham N.J., Loos R.J. Association of variants in the PCSK1 gene with obesity in the EPIC-Norfolk Study. Hum. Mol. Genet. 2009;18:3496–3501. doi: 10.1093/hmg/ddp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stutzmann F., Tan K., Vatin V., Dina C., Jouret B., Tichet J., Balkau B., Potoczna N., Horber F., O'Rahilly S., et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes. 2008;57:2511–2518. doi: 10.2337/db08-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Rahilly S., Farooqi I.S. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905–2910. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadsworth M.E., Butterworth S.L., Hardy R.J., Kuh D.J., Richards M., Langenberg C., Hilder W.S., Connor M. The life course prospective design: an example of benefits and problems associated with study longevity. Soc. Sci. Med. 2003;57:2193–2205. doi: 10.1016/s0277-9536(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 28.Cole T.J., Green P.J. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat. Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 29.Silverwood R., Leon D., De Stavola B. Long-term trends in BMI: are contemporary childhood BMI growth references appropriate when looking at historical datasets? Longit. Life Course Stud. 2009;1:27–44. [Google Scholar]