Abstract
Several members of the chemokine family play an important role in reparative fibrosis and are involved in the pathogenesis of remodeling following myocardial infarction. Chemokines may regulate the fibrotic process through recruitment and activation of mononuclear cell subsets and fibroblast progenitors (fibrocytes), by exerting direct effects on resident fibroblasts, and by modulating angiogenesis. Monocyte Chemoattractant Protein (MCP)-1/CCL2 is the best studied chemokine in cardiac fibrosis. Disruption of the MCP-1 axis reduces fibrosis attenuating dilation of the infarcted ventricle. In addition, MCP-1 signaling is activated in response to insults that do not cause cardiomyocyte death, such as brief ischemia or pressure overload and regulates fibrous tissue deposition in experimental models of fibrotic non-infarctive cardiomyopathy. Understanding the role of chemokine-mediated interactions in the development of cardiac fibrosis may identify novel therapeutic targets for treatment of patients with heart failure.
Keywords: chemokines, TGF-beta, myocardial infarction, cardiac fibrosis, MCP-1, myofibroblast, cardiomyopathy, heart failure, extracellular matrix, MIP-1alpha
2. CARDIAC FIBROSIS
Cardiac fibrosis is characterized by net accumulation of extracellular matrix in the myocardium and is an integral component of most cardiac pathologic conditions. Fibrotic remodeling of the myocardium is the end-result of most types of cardiac injury. Insults resulting in cardiomyocyte death, such as myocardial infarction, trigger a reparative response that ultimately leads to replacement of dead cardiomyocytes with a collagen-based scar. However, cardiac fibrosis also develops in response to injurious stimuli that do not cause extensive cardiomyocyte loss. Interstitial and perivascular deposition of collagen is often found in dysfunctional myocardial segments from patients with ischemic cardiomyopathy in the absence of completed infarction, and correlates inversely with contractile reserve (1). Moreover, myocardial fibrosis is associated with a wide variety of cardiac conditions due to hemodynamic, toxic, metabolic and immunologic disturbances. Pressure overload induced by hypertension or aortic stenosis results in extensive cardiac fibrosis, associated initially with increased stiffness and diastolic dysfunction, that frequently progresses to ventricular dilation and combined diastolic and systolic heart failure (2). Volume overload due to valvular regurgitant lesions also results in activation of cardiac fibroblasts leading to fibrosis of the heart (3). Hypertrophic cardiomyopathy is associated with fibrous tissue deposition in the cardiac interstitium, accompanied by alterations in the extracellular matrix scaffold, that may contribute to cardiomyocyte disarray (4). Hearts with dilated cardiomyopathy often exhibit progressive fibrosis characterized by increased interstitial cellularity and accumulation of extracellular matrix proteins (5). Finally, cardiac fibrosis is a hallmark of the cardiomyopathic processes associated with metabolic disturbances such as diabetes (6), (7) and obesity (8).
3. THE EXTRACELLULAR MATRIX NETWORK IN THE NORMAL HEART
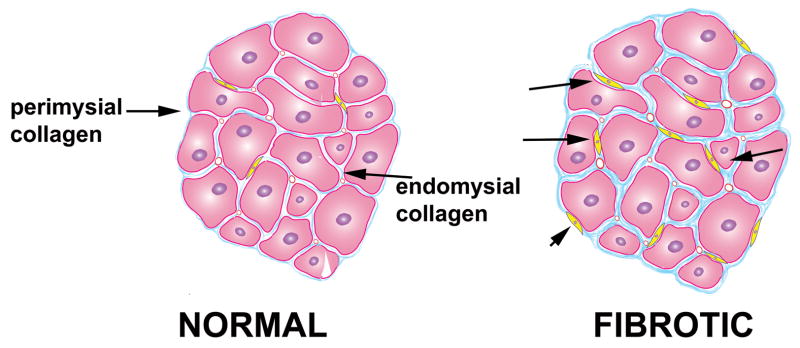
In the normal heart the extracellular matrix network provides structural support to the cellular elements and contributes to pump function by transmitting cardiomyocyte-generated force. Transmission of a systolic force is facilitated by a network comprised primarily of type I collagen with smaller amounts of type III collagen, the main constituents of the cardiac extracellular matrix (2). Based on morphological characteristics the cardiac matrix network can be subdivided into three constituents: the epi-, peri- and endomysium (Figure 1) (9). The epimysium envelops the entire cardiac muscle and is located on the endocardial and epicardial surfaces providing support for endothelial and mesothelial cells. The perimysium arises from the epimysium and surrounds groups of muscle fibers. The endomysium is the final arborization of the perimysium and enwraps individual muscle fibers. Endomysial struts tether muscle fibers together and to their nutrient microvasculature and function as the sites for connections to cardiomyocyte cytoskeletal proteins across the plasma membrane (2), (10). In the fibrotic heart there is increased deposition of perimysial collagen resulting in interstitial fibrosis, while perivascular fibrosis also develops involving the intramural coronary arterial vasculature (Figure 1).
Figure 1. Interstitial fibrosis is found in a wide variety of cardiac conditions.
In the normal myocardium extracellular matrix proteins provides structural support and contributes to transmission of contractile activity. In the fibrotic heart there is myofibroblast infiltration (arrows) and increased deposition of extracellular matrix proteins in the endomysium and perimysium.
4. THE FUNCTIONAL CONSEQUENCES OF CARDIAC FIBROSIS
Death of a significant number of functional cardiomyocytes following infarction is followed by their replacement by fibrous tissue and results in both systolic and diastolic dysfunction. However, cardiac fibrosis has functional consequences even in the absence of significant cardiomyocyte loss. Homeostatic control of the cardiac extracellular matrix involves ongoing synthesis and degradation of extracellular matrix proteins (11), (12) by resident cardiac fibroblasts. Disturbance of the balance between the synthetic and degradative processes results in both structural and functional abnormalities of the heart. It is widely accepted that interstitial fibrosis is initially associated with a stiffer ventricle and diastolic dysfunction. However, increased deposition of extracellular matrix in the cardiac interstitium is accompanied by activation of matrix metalloproteinases (MMPs). Thus, in the fibrotic ventricle matrix synthesis is associated with concurrent degradation of matrix proteins that ultimately leads to disruption of myocardial excitation-contraction coupling in both systole and diastole (13). These alterations result in development of ventricular dilation and systolic failure (14). Disturbances of the collagen network in the fibrotic heart may cause systolic dysfunction through several distinct mechanisms:
Loss of fibrillar collagen may impair transduction of cardiomyocyte contraction into myocardial force development resulting in uncoordinated contraction of cardiomyocyte bundles (15).
Interactions between interstitial proteins (such as laminin and collagen) and their receptors may play an important role in cardiomyocyte homeostasis (16).
Fibrosis may result in sliding displacement (slippage) of cardiomyocytes leading to a decrease in the number of muscular layers in the ventricular wall and subsequent left ventricular dilation (17), (18), (19).
In addition to its profound effects on ventricular function, cardiac fibrosis also promotes generation of arrhythmias through impaired anisotropic conduction and subsequent formation of reentry circuits (20).
5. INFLAMMATION AND CARDIAC FIBROSIS
Fibrosis typically results from activation of inflammatory and reparative pathways in response to a persistent injurious stimulus (21). Most types of cardiac injury trigger a local inflammatory reaction, inducing upregulation of cytokines, and fibrogenic growth factors and promoting activation of proteases (22). Myocardial infarction results in cardiomyocyte necrosis and induces an intense inflammatory response; subsequent activation of pro-fibrotic pathways is an important component of the reparative process, but also plays a role in the pathogenesis of adverse remodeling. Although the close association between the inflammatory and fibrotic response following myocardial infarction is well-established, evidence suggests that inflammatory and fibrogenic pathways are also triggered by brief or sublethal insults that do not result in significant cardiomyocyte loss. Pressure overload, volume overload and brief repetitive ischemic events trigger a transient inflammatory reaction in the myocardium followed by the development of perivascular and interstitial fibrosis. These pathologic changes ultimately lead to diastolic and systolic ventricular dysfunction.
6. THE CHEMOKINE SUPERFAMILY
The chemokines (23), constitute a superfamily of small basic proteins, with molecular weights in the range of ~8–15kDa, that share structural similarities (24). Chemokines are subdivided into four families (CC, CXC, XC or CX3C) based on the number of amino acids between their aminoterminal cysteine residues. CXC chemokines are further classified into two groups (ELR + and ELR−) according to the presence of the tripeptide motif Glu-Leu-Arg (ELR motif) in the aminoterminal region.
Chemokines are critically involved in basal and inflammatory leukocyte locomotion and trafficking (25, 26) and their principal targets are hematopoietic cells. Inflammatory chemokines are upregulated upon tissue injury and in order to induce a chemotactic response in vivo they must be immobilized on cell or extracellular matrix surfaces through interactions with glycosaminoglycans (27). Interactions between chemokines bound to the surface of activated endothelial cells and their receptors expressed by rolling leukocytes play an essential role in extravasation of leukocytes in inflamed areas. In addition to effects on cell locomotion, chemokines are capable of eliciting a variety of other responses affecting leukocyte activation and degranulation, mitogenesis, and apoptosis. Moreover, certain chemokines appear to exert a wide range of effects on cell types beyond the immune system, including endothelial cells (resulting in angiogenic or angiostatic actions) (28), smooth muscle cells, fibroblasts, neurons, epithelial cells and cardiomyocytes.
7. CHEMOKINES AND TISSUE FIBROSIS
Through their effects on inflammatory cells, fibroblasts and endothelial cells, chemokines appear to be essential regulators of fibroproliferative processes in a variety of tissues (21). Extensive evidence implicates CC chemokines in the pathogenesis of pulmonary (29), renal (30) and hepatic (31) fibrosis. Macrophage inflammatory protein (MIP)-1alpha neutralization reduced bleomycin-induced pulmonary fibrosis (32), whereas MCP-1 inhibition attenuated renal (33), pulmonary (34) and hepatic (35) fibrosis in a variety of experimental models. Studies using genetically targeted mice further supported the fibrogenic effects of CC chemokines. Mice with targeted deletion of CCR1, the main receptor for MIP-1alpha and RANTES (regulated upon activation, normal T cell expressed and secreted), had reduced renal fibrosis (36), whereas animals lacking the MCP-1 receptor CCR2 exhibited attenuated bleomycin-induced pulmonary fibrosis (37). CXC chemokines (38) and the CX3C chemokine fractalkine (39) are also involved in the pathogenesis of fibrotic diseases. Several distinct mechanisms may be responsible for the effects of chemokines in tissue fibrosis. First, chemokine-mediated recruitment of leukocytes may create a fibrogenic environment through the secretion of a wide variety of pro-fibrotic factors. Second, several chemokines are involved in chemotactic recruitment of fibroblast progenitors. In mice, interactions involving the chemokine receptors CXCR4, CCR7 (40), CCR2 (41) and CCR5 (42) have been shown to regulate fibrocyte recruitment to the lung. Third, chemokines may exert direct effects on cardiac fibroblasts. MCP-1 modulates dermal fibroblast phenotype by enhancing MMP synthesis (43) and stimulates collagen synthesis by lung fibroblasts (44). On the other hand, interferon-gamma-inducible protein (IP)-10 exerts anti-fibrotic effects by suppressing growth factor-mediated fibroblast motility (45). Fourth, several members of the chemokine family may regulate tissue fibrosis by modulating angiogenesis. Formation of neovessels is essential in the pathogenesis of fibroproliferative diseases (38).
Thus, the cellular effects of the chemokines and their established role in fibrosis of the lung, skin and kidney support the hypothesis that chemokine-mediated interactions may also modulate fibrotic remodeling in the heart. Whether these effects play an essential role in the pathogenesis of reparative and interstitial cardiac fibrosis is an area of active investigation.
8. CHEMOKINES AND REPARATIVE FIBROSIS FOLLOWING MYOCARDIAL INFARCTION
The reparative response following myocardial infarction ultimately results in formation of a scar and can be divided into three overlapping phases: the inflammatory phase, the proliferative phase and the maturation phase (46), (47). During the inflammatory phase induction of chemokines, cytokines, and adhesion molecules results in recruitment of leukocytes into the infarcted area (48), (49). Neutrophils and macrophages clear the infarct from dead cells and matrix debris (50). As neutrophils become apoptotic, macrophages release growth factors (such as transforming growth factor (TGF)-beta) leading to suppression of inflammation and formation of granulation tissue. At this stage chemokine synthesis is suppressed (51), while fibroblasts and endothelial cells proliferate. During the proliferative phase of healing, activated myofibroblasts, deposit extracellular matrix proteins (52–54), (55) and angiogenic endothelial cells form an extensive microvascular network. Maturation of the scar follows: fibroblasts undergo apoptosis, the infarct vasculature matures, and a collagen-based scar is formed (56), (57). The reparative response in the infarcted heart is closely intertwined with adverse ventricular remodeling (58), a complex process associated with chamber dilation, cardiac hypertrophy, and geometric alterations of the ventricle resulting in increased sphericity and accentuated systolic and diastolic dysfunction (59). Post-infarction remodeling involves both the infarcted and non-infarcted heart and results in increased interstitial fibrosis in the spared peri-infarct area (60), (61).
Chemokine-mediated pathways are primarily activated during the inflammatory phase of infarct healing. Cardiomyocyte death results in activation of several overlapping pro-inflammatory pathways that induce chemokine expression in the infarcted heart. Localized generation of C5a, formation of reactive oxygen species and activation Toll-Like Receptor (TLR)-mediated pathways induce nuclear factor (NF)-kappaB activation and upregulate chemokine synthesis by endothelial cells in the infarcted myocardium. Both CC and CXC chemokines are markedly and consistently induced in experimental models of myocardial infarction.
9. THE ROLE OF THE CC CHEMOKINES IN HEALING MYOCARDIAL INFARCTION
Monocyte Chemoattractant Protein (MCP)-1/CCL2, the best studied CC chemokine in myocardial infarction, plays an essential role in mononuclear cell recruitment and activation (62, 63). Binding of MCP-1 to its receptor CCR2 induces chemotaxis and activates β1 integrin-dependent adhesion of monocytic cells through distinct signaling pathways (64). MCP-1-mediated chemotaxis is mediated via p38 Mitogen Activated Protein Kinase (MAPK) and Rho kinase signaling cascades, whereas beta1 integrin-dependent adhesion of monocytic cells to Vascular Cell Adhesion Molecule (VCAM)-1 and fibronectin requires extracellular signal-regulated kinase (ERK) signaling (64). Disruption of MAPK, Rho kinase, or Phosphoinositide 3-kinase (PI-3K), is sufficient to prevent chemotaxis in response to MCP-1, suggesting that activation of several independent pathways is required to produce a full chemotactic response (65), (64).
In addition to its critical role in mononuclear cell recruitment, MCP-1 exerts important actions on non-hematopoietic cells, inducing angiogenic and arteriogenic effects (66) and modulating fibroblast phenotype and activity by increasing collagen expression and by regulating MMP synthesis (44). MCP-1 upregulation has been demonstrated in canine (67), rat (68, 69) and mouse models (70) of experimental myocardial infarction. Studies from our laboratory demonstrated that MCP-1 −/− mice had decreased and delayed macrophage infiltration in the healing infarct and exhibited delayed replacement of injured cardiomyocytes with granulation tissue. MCP-1 −/− infarcts had decreased mRNA expression of the pro-inflammatory cytokines tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and IL-6, and showed defective macrophage differentiation (48). However, the consequences of MCP-1 deficiency extend beyond the inflammatory phase of infarct healing. MCP-1 null animals exhibited reduced expression of the inhibitory cytokines TGF-beta, and IL-10 in the infarcted heart. MCP-1 deficiency diminished myofibroblast accumulation without significantly affecting infarct angiogenesis. Despite showing delayed phagocytotic removal of dead cardiomyocytes, MCP-1 −/− mice had attenuated left ventricular remodeling, but similar infarct size when compared with wildtype animals.
Further experiments suggested that the role of MCP-1 in myocardial infarction extends beyond its monocyte chemoattractant effects: MCP-1 inhibition with a neutralizing antibody resulted in defects comparable with the pathological findings noted in infarcted MCP-1 −/− animals, in the absence of impairment in monocyte recruitment (48).
Thus, absence of MCP-1 appears to result in attenuation of adverse remodeling at the expense of a prolonged inflammatory phase and delayed replacement of injured cardiomyocytes with granulation tissue (71). Experiments using mice with targeted deletion of the MCP-1 receptor, CCR2, also suggested that disruption of the MCP-1 axis has protective effects in post-infarction remodeling (72). CCR2 null mice had decreased infiltration with macrophages and exhibited attenuated ventricular dilation following myocardial infarction. CCR2 absence was associated with markedly decreased MMP expression and lower gelatinolytic activity in the infarcted ventricle. Attenuated matrix degradation may explain, at least in part, the protection from the development of adverse remodeling noted in CCR2 null animals.
10. HOW DOES MCP-1 MODULATE REPARATIVE FIBROSIS FOLLOWING MYOCARDIAL INFARCTION?
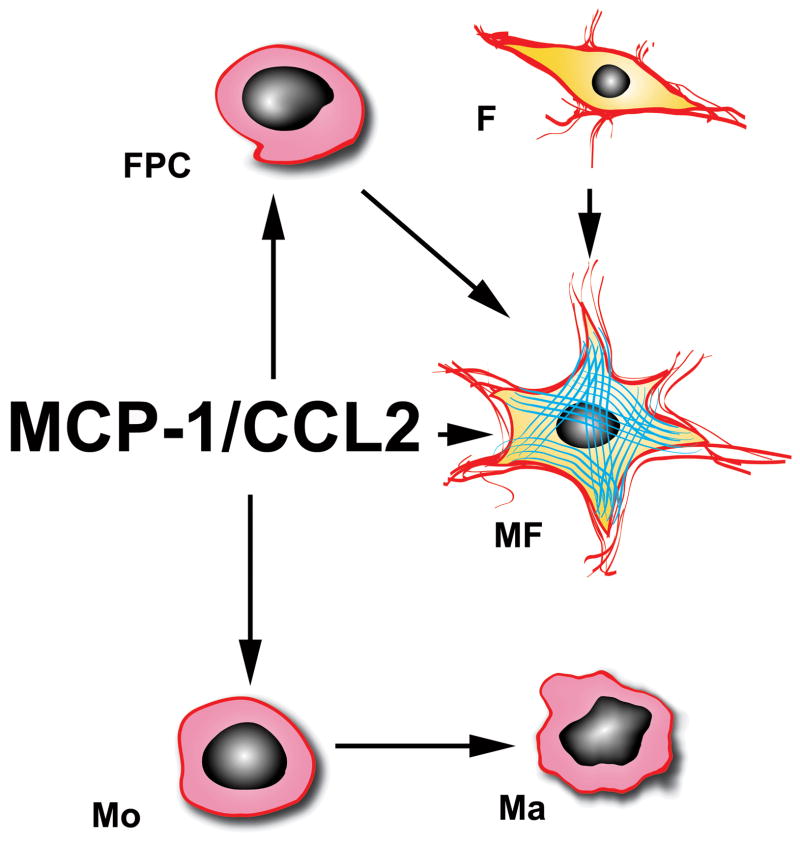
The effects of MCP-1 on the healing infarct may be mediated through several distinct pathways (Figure 2).
Figure 2. MCP-1/CCL2 modulates the reparative fibrotic response following myocardial infarction.
MCP-1 is critically involved in infiltration of the infarct with mononuclear cells (Mo) and may selectively recruit CCR2 positive monocytes. MCP-1 may also promote macrophage (Ma) activation and differentiation. Activated myofibroblasts (MF) in the infarct originate from resident fibroblast (F) proliferation or from recruitment of fibroblast progenitor cells (FPC). MCP-1 may mediate FPC recruitment and may directly modulate myofibroblast phenotype and function.
Recruitment of monocytes in the ischemic myocardium is regulated by MCP-1. Emerging evidence suggests that peripheral blood monocytes are a heterogeneous population. In mice, two distinct subpopulations have been identified that circulate in approximately equal numbers (73): a subset that expresses the MCP-1 receptor, CCR2, but has low expression of the fractalkine receptor CX3CR1 (CX3CR1 lo) and is preferentially recruited in inflammatory processes, and a CCR2 negative subpopulation comprised of cells with intense CX3CR1 expression (CX3CR1 hi), that home to normal tissues and become resident macrophages. MCP-1 induction in the healing infarct may result in enhanced recruitment of CCR2 positive cells. In contrast, disruption of the MCP-1 axis may result in decreased infiltration of the infarct with CCR2+ monocytes and high representation of the CX3CR1 hi subset. CX3CR1 hi monocytes may exhibit decreased phagocytic activity and reduced capability to express cytokines upon stimulation with pro-inflammatory mediators. The significance of distinct monocyte subpopulations in infarct healing was demonstrated in a recent investigation by Nahrendorf and co-workers (74) showing that infarcted mouse hearts sequentially recruit Ly-6C hi and Ly-6C lo monocytes through CCR2 and CX3CR1 respectively. The Ly-6C hi subset appears to exhibit phagocytic, proteolytic and pro-inflammatory functions, whereas Ly-6C lo cells promote healing and have profibrotic and angiogenic properties (74). These concepts may also be highly relevant in human pathobiology. Subsets of human peripheral blood monocytes with distinct chemokine receptor profiles have been identified. CD14+CD16+ monocytes express lower CCR2 but higher CCR5 levels; in contrast CD14+ monocytes exhibit high CCR2 and low CCR5 expression (75). Polarized CCR2 expression is accompanied by differential chemotactic responsiveness to MCP-1 (75).
MCP-1 may also directly modulate macrophage differentiation, phagocytic activity and cytokine expression. Previous investigations indicated that MCP-1 induces monocyte IL-1 (76) and IL-6 synthesis (77) and that it may be involved in differentiation of monocytes into foam macrophages (78).
The reduced myofibroblast density in MCP-1 null infarcts may result from decreased proliferative activity of resident fibroblasts or impaired recruitment of fibroblast progenitor cells, capable of differentiating into fibroblasts. The significance of these cells in the infarcted myocardium remains unknown.
11. THE ROLE OF OTHER MEMBERS OF THE CC CHEMOKINE FAMILY IN MYOCARDIAL INFARCTION
Whether other CC chemokines are critically involved in reparative cardiac fibrosis remains unknown. MCP-3, a potent mononuclear cell chemoattractant, is transiently induced in mouse myocardial infarcts (79). Transplantation of MCP-3-expressing cardiac fibroblasts into the infarct border zone one month after coronary ligation resulted in enhanced homing of injected mesenchymal stem cells in the infarcted myocardium (79). However, the exact role of endogenous MCP-3 in cardiac repair remains unknown. In addition, a robust induction of MIP-1alpha and MIP-1beta is noted in murine infarcts (80); however, their importance in myocardial injury and repair has not been investigated. A small clinical study suggested that increased serum levels of RANTES, a CC chemokine that induces chemotaxis of eosinophils and specific subsets of mononuclear cells, are found in patients with acute myocardial infarction (81). Whether these findings reflect upregulation of RANTES expression in the infarct is not known.
A recently published investigation demonstrated reduced functional impairment and attenuated structural remodeling after myocardial infarction in mice with deletion of CCR1, a promiscuous CC chemokine receptor primarily activated by MIP-1alpha and RANTES (82). Protection of the infarcted heart in CCR1 null mice was associated with reduced early recruitment of neutrophils and improved infarct healing. Translation of these findings into human infarction is hampered by species differences in CCR1 biology. The CCR1 ligand MIP-1alpha is a potent chemoattractant for mouse, but not for human neutrophils (83).
12. CXC CHEMOKINES AND REPARATIVE FIBROSIS FOLLOWING MYOCARDIAL INFARCTION
ELR-positive CXC chemokines, such as IL-8, epithelial neutrophil-activating peptide (ENA)-78, growth-regulated oncogene (GRO)-alpha, GRO-beta, GRO-gamma and neutrophil-activating peptide (NAP)-2, induce neutrophil chemotaxis and activation (24, 84) and have angiogenic properties. In contrast, ELR-negative CXC chemokines (such as platelet factor 4 (PF4/CXCL4), IP-10/CXCL10, and monokine induced by gamma-interferon (MIG/CXCL9)) do not stimulate neutrophil chemotaxis and not only fail to induce angiogenesis, but exert potent angiostatic effects in the presence of either ELR-CXC chemokines or other angiogenic factors, (such as basic fibroblast growth factor) (28, 85).
IL-8/CXCL8 is the prototypical ELR-positive CXC chemokine. IL-8 upregulation has been documented in canine (86) and rabbit (87) models of experimental myocardial infarction. Elucidation of the role of IL-8 in cardiac injury has been hampered by the absence of an IL-8 homologue in mice. IL-8, and possibly other ELR-positive CXC chemokines, synthesized by endothelial cells and leukocytes, may play an important role in neutrophil recruitment in the infarcted myocardium. In addition, IL-8 may activate infiltrating granulocytes by inducing the neutrophil respiratory burst and granule release, and by enhancing cellular adhesion. In addition, IL-8 may also have effects beyond its neutrophil chemotactic properties (88). IL-8 neutralization significantly reduces the degree of necrosis in a rabbit model of myocardial ischemia-reperfusion injury without affecting neutrophil infiltration (89). In rodents, several ELR positive CXC chemokines, including GRO-alpha/KC, MIP-2 and lipopolysaccharide-induced CXC chemokine (LIX), induce neutrophil chemotaxis and activation through binding to their main receptor, CXCR2. Deficiency of CXCR2 resulted in significantly decreased inflammatory leukocyte recruitment in murine infarcts, suggesting a crucial role for these chemokines in inflammatory cell infiltration (90). Experiments in a rat model of infarction suggested that, although KC and MIP-2 are upregulated in the injured heart, neutrophil recruitment in reperfused rat infarcts appeared to be mainly due to expression of LIX (91). The relative significance of ELR-positive CXC chemokines in regulating the post-infarction inflammatory response in infarcted rodent hearts remains unclear; however, because of species-specific actions this information would probably be of limited significance for understanding the pathobiology of human infarction. CXCR2 signaling may exert actions beyond neutrophil chemotaxis. Experiments using a Langendorff preparation indicated protective effects of CXCR2 signaling on myocardial viability (90). The molecular basis for the presumed direct effects of CXCR2 signaling on cardiomyocytes remains unclear.
The role of the ELR-negative CXC chemokines in the healing infarct is an area of active investigation. We have demonstrated a marked transient upregulation of IP-10/CXCL10 in reperfused canine myocardial infarcts (92). IP-10 mRNA expression is downregulated following 24 h of reperfusion, whereas IL-8 message levels remain high. The exact role of IP-10 upregulation in the infarcted myocardium remains unclear. IP-10 is critically involved in effector T cell trafficking (93), has angiostatic effects (94) and may exert direct antifibrotic actions (45), (95). It is tempting to hypothesize that the early transient induction of IP-10 in the ischemic myocardium may serve to prevent premature wound angiogenesis and fibrous tissue deposition in the infarct, until the injured myocardium has been cleared from dead cells and debris by infiltrating phagocytes and a fibrin-rich provisional matrix is formed in order to support ingrowth of granulation tissue. Subsequent suppression of IP-10 through TGF-beta-mediated actions may allow unopposed angiogenic and fibrogenic activity facilitating the reparative process. Thus, IP-10 may orchestrate infarct healing through its effects on angiogenesis and fibrous tissue deposition.
Stromal cell-derived factor (SDF)-1/CXCL12 is a non-ELR containing CXC chemokine with chemotactic effects for CD34+ progenitors (96) and primitive hematopoietic cells (97). SDF-1alpha is induced in non-reperfused rat infarcts (98); however, its role in regulating the post-infarction inflammatory and reparative response is unknown. Endogenous SDF-1 may regulate the recruitment, maturation and function of CXCR4-positive progenitor cells in ischemic tissues (99), (100). Experiments using a rat model of coronary occlusion demonstrated that transplantation of cells engineered to express SDF-1 into the peri-infarct zone resulted in attenuated adverse remodeling (99). The beneficial effects of SDF-1 may be due to therapeutic stem cell homing into the injured myocardium resulting in neovascularization and enhanced preservation of cardiomyocytes (101). Recent evidence suggested that the SDF-1/CXCR4 biological axis may be particularly important for the trafficking and extravasation of fibrocytes in the lung (102). Whether SDF-1 modulates the fibrotic response following myocardial infarction remains unknown.
13. THE ROLE OF THE CHEMOKINES IN CARDIAC FIBROSIS IN THE ABSENCE OF MYOCARDIAL INFARCTION
Although extensive experimental evidence suggests that the inflammatory response plays an important role in reparative fibrosis following myocardial infarction, our knowledge on the possible involvement of inflammatory pathways in fibrotic cardiac remodeling in the absence of cardiomyocyte death remains limited. Several challenges hamper understanding of the role of inflammation in non-infarctive cardiomyopathic processes. First, interstitial cardiac fibrosis is not a single clinical entity. Several distinct injurious pathways may be activated in the cardiac interstitium in response to sublethal cardiac injury; the mechanisms responsible for fibrotic remodeling may depend on the type of the initial insult. Second, in the clinical context many patients have co-existing conditions (such as diabetes, hypertension, obesity, etc.) that may independently contribute to the development of cardiac fibrosis. Third, development of animal models that simulate the complexity of the clinical conditions associated with perivascular and interstitial fibrosis of the heart is difficult.
Because of the challenges in dissecting the mechanisms involved in the pathogenesis of interstitial cardiac fibrosis, evidence suggesting a role for chemokine-mediated pathways in non-infarctive fibrotic remodeling of the ventricle is scarce.
14. MCP-1 IS INVOLVED IN THE PATHOGENESIS OF EXPERIMENTAL ISCHEMIC CARDIOMYOPATHY
CCL2/MCP-1 is the best-studied chemokine in experimental models of non-infarctive fibrosis. Experiments using a canine model demonstrated that a single brief episode of coronary occlusion followed by reperfusion is sufficient to induce MCP-1 upregulation in the heart, despite the absence of cardiomyocyte death (103). MCP-1 induction following a single brief ischemic insult is mediated through reactive oxygen generation (103). MCP-1 upregulation was also found in a murine model of fibrotic cardiomyopathy due to brief repetitive myocardial ischemia and reperfusion (104). After three days of brief (15 min) daily episodes of coronary occlusion followed by reperfusion, mice exhibited an interstitial inflammatory response in the ischemic myocardium associated with marked induction of MCP-1. Chemokine upregulation was followed by development of interstitial fibrosis in the absence of significant cardiomyocyte loss. As fibrosis developed, the inflammatory response was suppressed (104).
Experiments from our laboratory using genetically targeted mice and antibody neutralization strategies demonstrated that reactive oxygen-mediated induction of MCP-1 plays an essential role in fibrotic remodeling of the ventricle following brief repetitive ischemia and reperfusion. Both MCP-1 gene disruption and inhibition with a neutralizing antibody protected the myocardium from the development of interstitial fibrosis and attenuated left ventricular dysfunction (105).
In the cardiomyopathic heart MCP-1 may mediate its pro-fibrotic effects through several distinct mechanisms:
MCP-1 may act through recruitment and activation of mononuclear cells. Monocytes chemotactically attracted through MCP-1/CCR2 signaling may be an important source of fibrogenic mediators, such as TGF-beta and Fibroblast Growth Factors. In addition to its chemotactic properties, MCP-1 may also enhance the fibrogenic potential of mature macrophages by inducing TGF-beta1 and by stimulating collagen synthesis (106).
MCP-1 may directly modulate fibroblast phenotype and activity. In vitro studies have demonstrated that MCP-1 enhances portal fibroblast proliferation and myofibroblast differentiation (107), upregulates collagen and TGF-β1 expression by rat pulmonary fibroblasts (44), and stimulates production of MMP-1 and tissue inhibitor of metalloproteinases (TIMP)-1 by human skin fibroblasts (43). In pulmonary fibrosis, the fibrogenic actions of MCP-1 may be mediated at least in part through inhibition of prostaglandin E2 (PGE2), a potent suppressor of fibroblast proliferation and activation (108). Although our experiments failed to demonstrate direct effects of MCP-1 on murine cardiac fibroblast collagen and MMP synthesis (105), important modulatory actions of MCP-1 on fibroblast phenotype cannot be excluded.
MCP-1 may be an essential mediator in the recruitment of fibrocytes, a circulating population of cells that share leukocyte and mesenchymal markers, and are capable of myofibroblast differentiation (109), (110). Recent investigations indicated that MCP-1/CCR2 signaling is important for recruitment of fibroblast progenitors to the alveolar space after fibrotic injury in the lung (41).
Our investigation demonstrated that MCP-1 gene disruption significantly decreased macrophage infiltration following brief repetitive ischemia and reperfusion and resulted in reduced expression of osteopontin (105) a matricellular protein markedly induced during monocyte to macrophage differentiation (111). Furthermore, myofibroblasts isolated from wildtype hearts after 5 days of brief repetitive I/R exhibited much higher proliferative capacity than myofibroblasts derived from MCP-1 null hearts undergoing repetitive coronary occlusion and reperfusion protocols. These findings suggested that the pro-fibrotic actions of MCP-1 may depend on paracrine signals released by leukocytes recruited in the myocardium through MCP-1/CCR2 interactions.
15. MCP-1 EXPRESSION IN PATIENTS WITH ISCHEMIC FIBROTIC CARDIOMYOPATHY
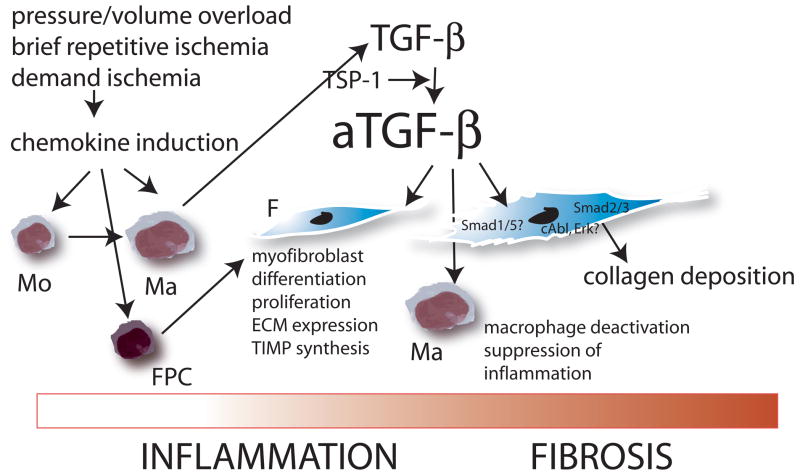
Patients with advanced ischemic cardiomyopathy exhibit extensive collagen deposition in the form of reparative or diffuse interstitial fibrosis (17). Severe coronary disease is often associated with frequent brief episodes of demand ischemia that do not lead to myocardial infarction but may induce reactive oxygen-mediated chemokine upregulation. Myocardial induction of CC chemokines, such as MCP-1, may stimulate mononuclear cell recruitment creating a fibrogenic milieu that induces extracellular matrix deposition and regional myocardial dysfunction. This concept is supported by our investigations using samples obtained from dysfunctional myocardial segments from patients with ischemic cardiomyopathy undergoing aortocoronary bypass surgery. Myocardial segments with significant dysfunction in the absence of infarction showed increased MCP-1 expression and local leukocyte infiltration (112). Furthermore, dysfunctional segments with recovery of function after surgical revascularization exhibited higher MCP-1 levels and enhanced inflammatory activity in comparison to segments with irreversible dysfunction. In contrast, persistently dysfunctional myocardium exhibited decreased inflammatory activity and enhanced collagen deposition (113). These findings suggested that the development of ischemic cardiomyopathy is a continuous process: at an early stage brief episodes of ischemia may induce an inflammatory response activating fibrogenic pathways. However, prolonged activation of ischemia-induced inflammation may trigger inhibitory mechanisms (such as TGF-beta activation) that suppress pro-inflammatory mediator synthesis, while inducing synthesis of genes associated with fibrosis (Figure 3) (114), (115).
Figure 3. The role of chemokine-mediated inflammation in the development of cardiac fibrosis in the absence of myocardial infarction.
Insults that do not cause cardiomyocyte death, such as brief repetitive ischemia and reperfusion, demand ischemia, pressure or volume overload activate inflammatory pathways through generation of reactive oxygen species or via angiotensin II. Chemokine induction results in recruitment of mononuclear cells (Mo) in the myocardium; subsequent activation of inhibitory pathways (such as TGF-beta) suppress inflammation while inducing fibrosis. Symbols: Ma, macrophage; F, fibroblast; FPC, fibroblast progenitor cells.
16. THE ROLE OF MCP-1 IN CARDIAC FIBROSIS DUE TO PRESSURE AND VOLUME OVERLOAD
Cardiac pressure overload induces cardiac hypertrophy and fibrosis in a variety of clinical conditions, including aortic stenosis and hypertension. Several investigations have demonstrated that experimental pressure overload triggers an inflammatory reaction (116). However, whether members of the chemokine family play an essential role in fibrotic remodeling of the pressure-overloaded heart, or their induction simply represents an epiphenomenon resulting from activation of angiotensin II signaling, remains unclear. Pressure overload due to suprarenal aortic constriction in a rat model, induced myocardial MCP-1 mRNA expression followed by macrophage accumulation, reactive fibrosis and cardiomyocyte hypertrophy (117). Treatment with the angiotensin II type I (AT-1) receptor antagonist candesartan significantly attenuated MCP-1 upregulation suggesting that AT-1 signaling may play a key role in MCP-1 induction in the pressure overloaded heart (118). Chronic treatment with a monoclonal neutralizing anti-MCP-1 antibody inhibited, not only macrophage accumulation, but also fibroblast proliferation and TGF-beta induction. Furthermore, MCP-1 inhibition attenuated myocardial fibrosis, but not cardiomyocyte hypertrophy, and ameliorated diastolic dysfunction without affecting blood pressure and systolic function (119). The significance of angiotensin-II-mediated activation of the MCP-1 axis was supported by experiments demonstrating that CCR2 expression in monocytes plays an essential role in vascular inflammation and remodeling following infusion of angiotensin II (120).
The role of the chemokines in fibrosis due to volume overload has not been systematically explored. Enhanced myocardial MCP-1 expression was noted in a rat model of volume overload due to formation of an aortocaval fistula (121). MCP-1 upregulation in this model correlated with the severity of heart failure and was associated with accumulation of leukocytes and interstitial fibroblasts. However, the potential involvement of chemokines in the pathogenesis of fibrosis in volume-overloaded hearts has not been studied.
17. CHEMOKINES, TGF-BETA, AND THE TRANSITION FROM INFLAMMATION TO FIBROSIS
Chemokine-mediated inflammation in the injured heart is a transient event, followed by resolution of the inflammatory infiltrate and development of fibrosis (116), (104). Evidence suggests that mediators involved in repression of chemokine synthesis also induce fibrous tissue deposition, and may play an important role in the pathogenesis of cardiac fibrosis (51), (122), (115), (50). Through its pleiotropic effects, TGF-beta is ideally suited as a key mediator in the transition from inflammation to fibrosis. TGF-beta mediates anti-inflammatory actions suppressing cytokine and chemokine expression by stimulated mononuclear and endothelial cells (92). On the other hand TGF-beta exerts potent pro-fibrotic effects by inducing myofibroblast activation, by stimulating the synthesis of various extracellular matrix proteins (123) and by suppressing matrix degradation through decreased expression and activity of proteases (124), (125). Thus, following cardiac injury TGF-beta may play a dual role suppressing inflammatory chemokine synthesis and leukocyte extravasation, while promoting extracellular matrix deposition. Extensive evidence demonstrates TGF-beta induction in the fibrotic heart in both experimental models of cardiac fibrosis and human patients with cardiomyopathy (126). TGF-beta and beta2 mRNA is abundantly expressed in hearts from patients with dilated (127) and hypertrophic cardiomyopathy (128) and is associated with increased collagen deposition (127). Furthermore, two different approaches generating mice with cardiac overexpression of TGF-beta1 suggested pro-fibrotic effects of TGF-beta in the heart. Rosenkranz and co-workers demonstrated that cardiac TGF-beta1 overexpression resulted in ventricular fibrosis associated with increased collagen deposition and inhibition of interstitial collagenases (129), (130). Nakajima and co-workers on the other hand (131) showed that transgenic mice with a mutation that blocks covalent tethering of the TGF-beta1 latent complex to the extracellular matrix had a large proportion of constitutively active TGF-beta1 in the heart. Despite showing similar levels of TGF-beta in both atria and ventricles, these animals exhibited only atrial and not ventricular fibrosis (131). Other investigations demonstrated that decreased TGF-beta activity prevented fibrotic remodeling of the ventricle in several distinct experimental models of cardiac fibrosis. Heterozygous TGF-beta1 +/− deficient mice exhibited less age-associated fibrosis and improved left ventricular compliance than controls (132). In addition, TGF-beta blockade prevented myocardial fibrosis in pressure overloaded rats (133).
Chemokine-mediated inflammation may provide the environment necessary for induction and activation of TGF-beta signaling in the injured heart. Activated mononuclear cells, recruited through chemokine-mediated pathways are a major source of TGF-beta following cardiac injury. Inflammatory cells secrete proteases that degrade the extracellular matrix facilitating liberation of stored TGF-beta. Infiltrating macrophages and lymphocytes produce mediators essential for TGF-beta activation. Activation of TGF-beta signaling pathways suppresses the inflammatory reaction following cardiac injury; however, TGF-beta-mediated repression of chemokine synthesis and subsequent resolution of inflammation come at a high cost resulting in cardiac fibrosis and dysfunction (114), (115), (61). Thus, the chemokines may represent the key initial stimulus that activates profibrotic TGF-beta-mediated cascades in the injured heart (Figure 3).
18. CONCLUSIONS
Chemokine-mediated inflammation plays a central role in reparative cardiac fibrosis and may be involved in the pathogenesis of interstitial fibrotic remodeling induced by insults that do not cause cardiomyocyte death. Through its effects on mononuclear cells and fibroblasts, MCP-1 appears to be an essential mediator in the development of cardiac fibrosis. Several investigations suggested that disruption of the MCP-1 axis attenuates adverse cardiac remodeling, reducing fibrosis in experimental models of myocardial infarction and ischemic cardiomyopathy. MCP-1 may be a promising therapeutic target in post-infarction remodeling; current reperfusion strategies may provide a unique opportunity for delivery of an MCP-1 inhibitor into the infarcted myocardium. In contrast, chronic chemokine inhibition to attenuate cardiac fibrosis in fibrotic cardiomyopathic conditions is more problematic and would likely be complicated by impaired responses to injury and reduced angiogenesis. Dissecting the role of chemokine-mediated pathways in both reparative and interstitial cardiac fibrosis may identify novel therapeutic strategies for patients with heart failure.
Acknowledgments
Dr Frangogiannis’ laboratory is supported by NIH R01 HL-76246 and R01 HL-85440.
Abbreviations
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- MIP
macrophage inflammatory protein
- RANTES
regulated upon activation, normal T cell expressed and secreted
- IP
interferon-gamma inducible protein
- TGF
transforming growth factor
- TLR
toll-like receptor
- NF
nuclear factor
- MAPK
Mitogen-activated protein kinase
- VCAM
vascular cell adhesion molecule
- ERK
extracellular signal-regulated kinase
- PI-3K
Phosphoinositide 3-kinase
- TNF
tumor necrosis factor
- IL
interleukin
- ENA
epithelial neutrophil-activating peptide
- GRO
growth-regulated oncogene
- NAP
neutrophil-activating peptide
- PF
platelet factor
- MIG
monokine induced by gamma-interferon
- LIX
lipopolysaccharide- induced CXC chemokine
- SDF
stromal cell-derived factor
- TIMP
tissue inhibitor of metalloproteinases
- PGE2
prostaglandin E2
- AT-1
angiotensin II type I
References
- 1.Nagueh SF, Mikati I, Weilbaecher D, Reardon MJ, Al-Zaghrini GJ, Cacela D, He ZX, Letsou G, Noon G, Howell JF, Espada R, Verani MS, Zoghbi WA. Relation of the contractile reserve of hibernating myocardium to myocardial structure in humans. Circulation. 1999;100:490–496. doi: 10.1161/01.cir.100.5.490. [DOI] [PubMed] [Google Scholar]
- 2.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borer JS, Truter S, Herrold EM, Falcone DJ, Pena M, Carter JN, Dumlao TF, Lee JA, Supino PG. Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation. 2002;105:1837–1842. doi: 10.1161/01.cir.0000014419.71706.85. [DOI] [PubMed] [Google Scholar]
- 4.Lombardi R, Betocchi S, Losi MA, Tocchetti CG, Aversa M, Miranda M, D’Alessandro G, Cacace A, Ciampi Q, Chiariello M. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation. 2003;108:1455–1460. doi: 10.1161/01.CIR.0000090687.97972.10. [DOI] [PubMed] [Google Scholar]
- 5.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- 6.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Asbun J, Manso AM, Villarreal FJ. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: effects of losartan and vitamin E. Am J Physiol Heart Circ Physiol. 2005;288:H227–234. doi: 10.1152/ajpheart.00340.2004. [DOI] [PubMed] [Google Scholar]
- 8.Bharati S, Lev M. Cardiac conduction system involvement in sudden death of obese young people. Am Heart J. 1995;129:273–281. doi: 10.1016/0002-8703(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 9.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 10.Shirwany A, Weber KT. Extracellular matrix remodeling in hypertensive heart disease. J Am Coll Cardiol. 2006;48:97–98. doi: 10.1016/j.jacc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 12.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 13.Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8:S319–325. doi: 10.1054/jcaf.2002.129260. [DOI] [PubMed] [Google Scholar]
- 14.Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39:1384–1391. doi: 10.1016/s0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- 15.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–132. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, Hultenby K, Ruiz-Lozano P, Ross J, Jr, Tryggvason K, Chien KR. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- 17.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Sonnenblick EH, Olivetti G, Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27:291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 19.Francis GS, McDonald K, Chu C, Cohn JN. Pathophysiologic aspects of end-stage heart failure. Am J Cardiol. 1995;75:11A–16A. doi: 10.1016/s0002-9149(99)80378-6. [DOI] [PubMed] [Google Scholar]
- 20.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 23.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 24.Clark-Lewis I, Kim KS, Rajarathnam K, Gong JH, Dewald B, Moser B, Baggiolini M, Sykes BD. Structure-activity relationships of chemokines. J Leukoc Biol. 1995;57:703–711. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 25.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 26.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 27.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 28.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 29.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol. 1995;57:782–787. doi: 10.1002/jlb.57.5.782. [DOI] [PubMed] [Google Scholar]
- 30.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 31.Kershenobich Stalnikowitz D, Weissbrod AB. Liver fibrosis and inflammation. A review. Ann Hepatol. 2003;2:159–163. [PubMed] [Google Scholar]
- 32.Smith RE, Strieter RM, Phan SH, Lukacs NW, Huffnagle GB, Wilke CA, Burdick MD, Lincoln P, Evanoff H, Kunkel SL. Production and function of murine macrophage inflammatory protein-1 alpha in bleomycin-induced lung injury. J Immunol. 1994;153:4704–4712. [PubMed] [Google Scholar]
- 33.Lloyd CM, Dorf ME, Proudfoot A, Salant DJ, Gutierrez-Ramos JC. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol. 1997;62:676–680. doi: 10.1002/jlb.62.5.676. [DOI] [PubMed] [Google Scholar]
- 34.Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, Takeshita A, Kitamoto S, Egashira K, Hara N. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1038–1044. doi: 10.1152/ajplung.00167.2003. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruta S, Nakamuta M, Enjoji M, Kotoh K, Hiasa K, Egashira K, Nawata H. Anti-monocyte chemoattractant protein-1 gene therapy prevents dimethylnitrosamine-induced hepatic fibrosis in rats. Int J Mol Med. 2004;14:837–842. [PubMed] [Google Scholar]
- 36.Eis V, Luckow B, Vielhauer V, Siveke JT, Linde Y, Segerer S, Perez De Lema G, Cohen CD, Kretzler M, Mack M, Horuk R, Murphy PM, Gao JL, Hudkins KL, Alpers CE, Grone HJ, Schlondorff D, Anders HJ. Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:337–347. doi: 10.1097/01.asn.0000111246.87175.32. [DOI] [PubMed] [Google Scholar]
- 37.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204:594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 38.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuichi K, Gao JL, Murphy PM. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am J Pathol. 2006;169:372–387. doi: 10.2353/ajpath.2006.060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, Matsushima K, Mukaida N. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007;170:843–854. doi: 10.2353/ajpath.2007.051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol. 2000;164:6174–6179. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 44.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 45.Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–254. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 48.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 49.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 51.Zymek P, Nah DY, Bujak M, Ren G, Koerting A, Leucker T, Huebener P, Taffet G, Entman M, Frangogiannis NG. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc Res. 2007;74:313–322. doi: 10.1016/j.cardiores.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber KT, Sun Y, Katwa LC, Cleutjens JP, Zhou G. Connective tissue and repair in the heart. Potential regulatory mechanisms. Ann N Y Acad Sci. 1995;752:286–299. doi: 10.1111/j.1749-6632.1995.tb17438.x. [DOI] [PubMed] [Google Scholar]
- 53.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 54.Weber KT, Sun Y, Cleutjens JP. Structural remodeling of the infarcted rat heart. Exs. 1996;76:489–499. doi: 10.1007/978-3-0348-8988-9_30. [DOI] [PubMed] [Google Scholar]
- 55.Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, Mains IM, Mingoia JT, Flack EC, Lindsey ML. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 57.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 58.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 59.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 60.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 61.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 62.Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- 63.Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7–29. doi: 10.1159/000058723. [DOI] [PubMed] [Google Scholar]
- 64.Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–16560. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- 65.Yen H, Zhang Y, Penfold S, Rollins BJ. MCP-1-mediated chemotaxis requires activation of non-overlapping signal transduction pathways. J Leukoc Biol. 1997;61:529–532. doi: 10.1002/jlb.61.4.529. [DOI] [PubMed] [Google Scholar]
- 66.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 67.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, Hawkins HK, Birdsall HH, MacKay CR, LaRosa GJ, Rossen RD, Smith CW, Entman ML. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation. 1997;95:693–700. doi: 10.1161/01.cir.95.3.693. [DOI] [PubMed] [Google Scholar]
- 68.Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, Matsushima K, Sasayama S. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79:195–203. [PubMed] [Google Scholar]
- 69.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest. 2000;80:1127–1136. doi: 10.1038/labinvest.3780119. [DOI] [PubMed] [Google Scholar]
- 70.Tarzami ST, Cheng R, Miao W, Kitsis RN, Berman JW. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J Mol Cell Cardiol. 2002;34:209–221. doi: 10.1006/jmcc.2001.1503. [DOI] [PubMed] [Google Scholar]
- 71.Xia Y, Frangogiannis NG. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm Allergy Drug Targets. 2007;6:101–107. doi: 10.2174/187152807780832265. [DOI] [PubMed] [Google Scholar]
- 72.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 74.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 76.Gavrilin MA, Deucher MF, Boeckman F, Kolattukudy PE. Monocyte chemotactic protein 1 upregulates IL-1beta expression in human monocytes. Biochem Biophys Res Commun. 2000;277:37–42. doi: 10.1006/bbrc.2000.3619. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 78.Tabata T, Mine S, Kawahara C, Okada Y, Tanaka Y. Monocyte chemoattractant protein-1 induces scavenger receptor expression and monocyte differentiation into foam cells. Biochem Biophys Res Commun. 2003;305:380–385. doi: 10.1016/s0006-291x(03)00771-x. [DOI] [PubMed] [Google Scholar]
- 79.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 80.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parissis JT, Adamopoulos S, Venetsanou KF, Mentzikof DG, Karas SM, Kremastinos DT. Serum profiles of C-C chemokines in acute myocardial infarction: possible implication in postinfarction left ventricular remodeling. J Interferon Cytokine Res. 2002;22:223–229. doi: 10.1089/107999002753536194. [DOI] [PubMed] [Google Scholar]
- 82.Liehn EA, Merx MW, Postea O, Becher S, Djalali-Talab Y, Shagdarsuren E, Kelm M, Zernecke A, Weber C. Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. J Cell Mol Med. 2008;12:496–506. doi: 10.1111/j.1582-4934.2007.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark-Lewis I, Schumacher C, Baggiolini M, Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem. 1991;266:23128–23134. [PubMed] [Google Scholar]
- 85.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 86.Kukielka GL, Smith CW, LaRosa GJ, Manning AM, Mendoza LH, Daly TJ, Hughes BJ, Youker KA, Hawkins HK, Michael LH, et al. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest. 1995;95:89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ. Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J Clin Invest. 1995;95:2720–2728. doi: 10.1172/JCI117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takami M, Terry V, Petruzzelli L. Signaling pathways involved in IL-8-dependent activation of adhesion through Mac-1. J Immunol. 2002;168:4559–4566. doi: 10.4049/jimmunol.168.9.4559. [DOI] [PubMed] [Google Scholar]
- 89.Boyle EM, Jr, Kovacich JC, Hebert CA, Canty TG, Jr, Chi E, Morgan EN, Pohlman TH, Verrier ED. Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 1998;116:114–121. doi: 10.1016/S0022-5223(98)70249-1. [DOI] [PubMed] [Google Scholar]
- 90.Tarzami ST, Miao W, Mani K, Lopez L, Factor SM, Berman JW, Kitsis RN. Opposing effects mediated by the chemokine receptor CXCR2 on myocardial ischemia-reperfusion injury: recruitment of potentially damaging neutrophils and direct myocardial protection. Circulation. 2003;108:2387–2392. doi: 10.1161/01.CIR.0000093192.72099.9A. [DOI] [PubMed] [Google Scholar]
- 91.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 92.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–1430. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 93.Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, Luster AD. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 94.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 96.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 99.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 100.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 101.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 102.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, Entman ML. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001;159:1301–1311. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 106.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H, Kaneko S. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555–563. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 107.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G765–771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 108.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, 3rd, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284:L342–349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 109.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 110.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krause SW, Rehli M, Kreutz M, Schwarzfischer L, Paulauskis JD, Andreesen R. Differential screening identifies genetic markers of monocyte to macrophage maturation. J Leukoc Biol. 1996;60:540–545. doi: 10.1002/jlb.60.4.540. [DOI] [PubMed] [Google Scholar]
- 112.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Shan K, Aggeli C, Reardon MJ, Letsou GV, Espada R, Ramchandani M, Entman ML, Zoghbi WA. Evidence for an active inflammatory process in the hibernating human myocardium. Am J Pathol. 2002;160:1425–1433. doi: 10.1016/S0002-9440(10)62568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Dewald O, Gersch C, Shan K, Aggeli C, Reardon M, Letsou GV, Espada R, Ramchandani M, Entman ML, Zoghbi WA. Active interstitial remodeling: an important process in the hibernating human myocardium. J Am Coll Cardiol. 2002;39:1468–1474. doi: 10.1016/s0735-1097(02)01792-8. [DOI] [PubMed] [Google Scholar]
- 114.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 115.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baumgarten G, Knuefermann P, Kalra D, Gao F, Taffet GE, Michael L, Blackshear PJ, Carballo E, Sivasubramanian N, Mann DL. Load-dependent and -independent regulation of proinflammatory cytokine and cytokine receptor gene expression in the adult mammalian heart. Circulation. 2002;105:2192–2197. doi: 10.1161/01.cir.0000015608.37608.18. [DOI] [PubMed] [Google Scholar]
- 117.Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, Kudo H, Sugi Y, Fukui D, Yasukawa H, Kuwahara F, Imaizumi T. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens Res. 2006;29:711–718. doi: 10.1291/hypres.29.711. [DOI] [PubMed] [Google Scholar]
- 118.Tokuda K, Kai H, Kuwahara F, Yasukawa H, Tahara N, Kudo H, Takemiya K, Koga M, Yamamoto T, Imaizumi T. Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension. 2004;43:499–503. doi: 10.1161/01.HYP.0000111831.50834.93. [DOI] [PubMed] [Google Scholar]
- 119.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 120.Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 121.Behr TM, Wang X, Aiyar N, Coatney RW, Li X, Koster P, Angermann CE, Ohlstein E, Feuerstein GZ, Winaver J. Monocyte chemoattractant protein-1 is upregulated in rats with volume-overload congestive heart failure. Circulation. 2000;102:1315–1322. doi: 10.1161/01.cir.102.11.1315. [DOI] [PubMed] [Google Scholar]
- 122.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 123.Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263:3039–3045. [PubMed] [Google Scholar]
- 124.Laiho M, Saksela O, Andreasen PA, Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986;103:2403–2410. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. Embo J. 1987;6:1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 127.Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kuhl U, Schultheiss HP. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 128.Li RK, Li G, Mickle DA, Weisel RD, Merante F, Luss H, Rao V, Christakis GT, Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–881. doi: 10.1161/01.cir.96.3.874. [DOI] [PubMed] [Google Scholar]
- 129.Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, Bohm M. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 130.Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schluter KD, Bohm M. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1) Am J Physiol Heart Circ Physiol. 2002;283:H1253–1262. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- 131.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 132.Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- 133.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]