Abstract
The constitutive androstane receptor (CAR) is a xenosensing nuclear receptor and regulator of cytochrome P450s (CYPs). However, the role of CAR as a basal regulator of CYP expression nor its role in sexually dimorphic responses have been thoroughly studied. We investigated basal regulation and sexually dimorphic regulation and induction by the potent CAR activator TCPOBOP and the moderate CAR activator Nonylphenol (NP). NP is an environmental estrogen and one of the most commonly found environmental toxicants in Europe and the United States. Previous studies have demonstrated that NP induces several CYPs in a sexually dimorphic manner, however the role of CAR in regulating NP-mediated sexually dimorphic P450 expression and induction has not been elucidated. Therefore, wild-type and CAR-null male and female mice were treated with honey as a carrier, NP, or TCPOBOP and CYP expression monitored by QPCR and Western blotting. CAR basally regulates the expression of Cyp2c29, Cyp2b13, and potentially Cyp2b10 as demonstrated by QPCR. Furthermore, we observed a shift in the testosterone 6α/15α-hydroxylase ratio in untreated CAR-null female mice to the male pattern, which indicates an alteration in androgen status and suggests a role for androgens as CAR inverse agonists. Xenobiotic-treatments with NP and TCPOBOP induced Cyp2b10, Cyp2c29, and Cyp3a11 in a CAR-mediated fashion; however NP only induced these CYPs in females and TCPOBOP induced these CYPs in both males and females. Interestingly, Cyp2a4, was only induced in wild-type male mice by TCPOBOP suggesting Cyp2a4 induction is not sensitive to CAR-mediated induction in females. Overall, TCPOBOP and NP show similar CYP induction profiles in females, but widely different profiles in males potentially related to lower sensitivity of males to either indirect or moderate CAR activators such as NP. In summary, CAR regulates the basal and chemically-inducible expression of several sexually dimorphic xenobiotic metabolizing P450s in a manner that varies depending on the ligand.
Keywords: constitutive androstane receptor (CAR), nonylphenol, cytochrome P450 (CYP), sexually dimorphic, liver
Introduction
The constitutive androstane receptor (CAR) is a nuclear receptor that mediates the hepatic regulation and expression of a wide variety of genes involved in endobiotic and xenobiotic clearance (Kretschmer and Baldwin 2005). CAR regulates Phase I genes, such as CYP2B6, CYP2C8/9 and CYP3A4, phase II conjugation enzymes such as UDP-glucoronysyltrasnferase, and phase III transporters such as multidrug resistance-associated proteins 2 and 3 (Goodwin et al. 2002; Kats et al. 2002; Sueyoshi et al. 1999; Sugatani et al. 2001; Xiong et al. 2002). In addition, CAR is involved in the regulation of gluconeogenesis, fatty acid oxidation and the metabolism of steroid hormones, bile acids, and bilirubin (Huang et al. 2003; Sugatani et al. 2001; Ueda et al. 2002; Wei et al. 2000)
In contrast to other nuclear receptors that contain five domains, CAR contains only three: a highly conserved DNA-binding domain; a hinge region; and a divergent ligand binding / dimerization/ transcriptional activation domain (Pascussi et al. 2003). This in part may explain some of the unique features of CAR including its constitutive activity (Suino et al. 2004). Inside the cell, CAR is retained in the cytoplasm forming a complex with heat shock proteins (Hsp90), immunophilins, P-23, and cytoplasmic CAR retention protein (CCRP), a bifunctional linker protein (Kobayashi et al. 2003; Yoshinori et al. 2003). Upon activation CAR is translocated to the nucleus in response to stress by the recruitment of protein phophatase 2A (PP2A) leading to dephosphorylation of a Ser-202 near the C terminus of the ligand binding domain (Hosseinpour et al. 2006; Yoshinori et al. 2003). Interestingly, it has been hypothesized that the majority of CAR activators work through an indirect dephosphorylation pathway similar to phenobarbital instead of binding directly to CAR (Shindo et al. 2007).
CAR and its relative, the pregnane X receptor (PXR), cross talk by sharing response elements and showing overlapping affinities for some ligands (Handschin and Meyer 2003); providing each other a backup system for responding to toxicants, but also increasing nuclear receptor interactions and making it difficult to interpret some data. The heterodimerization of CAR or PXR with RXR (Baes et al. 1994; Kliewer et al. 1998) and subsequent interaction with the phenobarbital responsive enhancer module (PBREM) or xenobiotic responsive enhancer module (XREM) induces the expression of classical biomarkers such as Cyp2b10 (CAR) and Cyp3a11 (PXR) as well as other CYP genes involved in detoxification (Ferguson et al. 2002; Jackson et al. 2006; Wang et al. 2003). The primary CYP families involved in detoxification of foreign chemicals are found in families 1–3, and several of these are inducible by CAR or PXR (Kretschmer and Baldwin 2005).
Many of the xenobiotic detoxifying P450s are gender specific or gender predominant (Hernandez et al. 2006; Wiwi et al. 2004). Male specific or male predominant liver P450s include Cyp2d9 and 4a12 in the mouse (Noshiro and Negishi 1986; Wiwi et al. 2004). Female predominant liver P450s include Cyps 2a4, 2b9, 3a41 and 3a44 in the mouse (Burkhart et al. 1985; Noshiro and Negishi 1986; Sakuma et al. 2002; Wiwi et al. 2004). Gender predominance has been primarily attributed to the frequency of growth hormone (GH) pulse secreted by the pituitary with pulses more frequent in female rats and episodic bursts in male rats (Waxman et al. 1991). However, other factors including the nuclear receptors RXRα and HNF4α may in part play a role in the gender predominant expression of several P450s in the CYP2-4 families (Cai et al. 2003; Wiwi et al. 2004).
CAR demonstrates greater activity in females than males. For example, it has been reported that TCPOBOP increases liver proliferation in females more than males (Ledda-Columbano et al. 2003). Estrogens activate CAR and this may increase CAR activity in females relative to males (Kawamoto et al. 2000). Furthermore, androgens also inhibit CAR activity in mice (Forman et al. 1998), and this may reduce CAR activity in males relative to females. Lastly, CAR demonstrates female predominant mRNA expression (Petrick and Klaassen 2007). Taken together, this data indicates that CAR may have greater activity in female mice and therefore help control and maintain basal and inductive expression of several sexually dimorphic CYPs.
Nonylphenol (NP) is a plasticizer and common environmental estrogen (Acevedo et al. 2005; Soto et al. 1991) that is also a moderately potent activator of CAR (Hernandez et al. 2007). Previous research has demonstrated sexually dimorphic regulation of Cyp2a, 2b, and 3a-subfamily members by nonylphenol (Hernandez et al. 2006; Laurenzana et al. 2002). Therefore, we hypothesized that nonylphenol may mediate its sexually dimorphic CYP induction through CAR. In addition, the CAR activators phenobarbital and TCPOBOP demonstrate gender predominant induction, as they induce the female-predominant P450, Cyp2a4, only in males (Wei et al. 2002). Therefore, we decided to compare the sexually dimorphic induction of CYPs by TCPOBOP and NP using wild-type and CAR-null mice to determine the role of CAR in mediating gender predominant induction of CYPs.
Lastly, there is limited research regarding the possible regulatory role of CAR in basal CYP expression. Currently no data has directly monitored or linked CAR to basal CYP expression or gender predominant CYP expression. Therefore, this study will also examine the basal expression of CYPs in male and female wild-type and CAR-null mice in order to assess the role of CAR in regulating basal CYP expression. Gender differences in CYP expression mediated by CAR may cause gender-specific outcomes following exposure to pharmaceuticals or environmental chemicals. The overall purpose of this study is to determine whether CAR regulates basal CYP expression in a sexually dimorphic manner, and test whether the moderate (NP) and potent (TCPOBOP) CAR activators demonstrate significant differences in CYP induction in a gender predominant fashion in wild-type or CAR-null adult mice.
Materials and Methods
Chemicals
Technical grade 4-NP (~85% para-isomers) was obtained from Fluka Chemical Co. (Seelze, Germany). Absolute ethanol, zoxazolamine (2-amino-5-chlorobenzoxazole 97% purity), 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), and ketamine HCl/xylazine HCl (800mg/120mg) were obtained from Sigma Aldrich (St. Louis, MO).
Mice
All studies were carried out according to NIH guidelines for the humane use of research animals and were pre-approved by the Baylor College of Medicine, University of Texas at El Paso, or Clemson University Animal Care and Use Committee. CAR-null mice, on a B6129 background, were previously described (Wei et al. 2000), and are housed at both the Baylor College of Medicine and the University of Texas at El Paso. Strain (B6129PF1/J), obtained from The Jackson Laboratory (Bar Harbor, ME) at 3–5 weeks old, was used as a control (wild-type;WT) to provide an approximate genetic match to the CAR-null mice. WT mice were held for 5 weeks prior to treatment, so that all treatments were performed with 8–10 week old mice. Mice were provided water, and fed ad libitum prior to and during treatments.
Nonylphenol and TCPOBOP treatment of wild-type and CAR-null mice
Eight to ten-week old B6129PF1/J male and female mice were randomly split into five treatment groups each (n = 5–6; 3–4 respectively). The mice were fed 0, 50, or 75 mg/kg/day NP mixed in 100 µl honey for 7 days, or injected with 0 or 3 mg/kg TCPOBOP mixed in 100 µl of corn oil one day prior to necropsy. Age matched male and female CAR-null mice were split into similar groups and treated the same as the wild-type mice. All mice were anesthetized by ketamine injection, and euthanized by CO2 asphyxiation. Livers were excised, diced into several pieces; half of the liver was used for microsome preparation, and the other half was placed in TRI-Reagent® (Sigma-Aldrich) for RNA extraction. All samples were stored at −80°C.
Sample preparations
RNA was extracted from approximately half of the liver with TRI-reagent® according to the manufacturer’s instructions followed by DNAse (Promega Corporation, Madison WI) digestion to remove residual genomic DNA. RNA concentrations were determined spectrophotometrically at 260/280 nm (Molecular Devices, Ramsey, MN). Reverse transcription was performed to make cDNA using 200 units Moloney Murine Leukemia Virus–Reverse Transcriptase (MMLV-RT), a 10 mM dNTP mixture, and 0.05 mg random hexamers (Promega Corporation, Madison, WI).
For microsome and cytosol preparation, approximately half of the liver was individually homogenized with a Dounce Homogenizer and protein fractions were prepared as described previously (Van der Hoeven and Coon 1974). Protein concentrations were determined with the Bio-Rad protein assay (Bio-Rad, Hercules, CA) according to manufacturer’s instructions.
Testosterone hydroxylase assays
Testosterone hydroxylase assays were used to measure CYP activity as previously described using thin-layer chromatography to separate the testosterone metabolites (Hernandez et al. 2006). Testosterone metabolites were quantified with a LS5801 liquid scintillation counter (Beckman, Fullerton, CA).
Quantitative Real-time Polymerase Chain Reaction (QPCR)
Quantitative real-time PCR (QPCR) was performed using primers for specific isoforms to Cyp2a, Cyp2b, Cyp2c, and Cyp3a subfamily members, or 18S as the housekeeping gene. All the primers have been previously published (Hernandez et al. 2006), except Cyp2c37. The sequences and annealing temperatures for Cyp2c37 primers are 5’–ATACTCTATATTTGGGCAGG-3 ’ for the forward primer and 5 ’-GTTCCTCCACAAGGCAAC-3’(52.5°C) for the reverse primer. Amplifications of the samples and the standard curve were performed in triplicate using a 96-well MyiQ™ Single Color Reverse Transcription Real-Time PCR detection system (Bio-Rad) with 0.25× SybrGreen (SuperArray Biosciences Co, Frederick, MD) as the fluorescent double strand-intercalating agent to quantify gene expression as described previously (Hernandez et al. 2006; Hernandez et al. 2007) using Muller’s equation to determine relative quantities of each CYP (Muller et al. 2002).
Immunoprecipitation
Liver cytosol from untreated male and female B6129PF1/J mice was used for immunoprecipitation of CAR. Briefly, 10 µl of CAR primary antibody rabbit polyclonal IgG (sc-13065, Santa Cruz Biotechnology, Santa Cruz, CA) was added to 200 µg cytosol from each sample and incubated for 1 hour at 4° C. Resuspended Protein A/G PLUS-Agarose (20 µl)(Santa Cruz Biotechnology) was added and incubated overnight at 4° C on a rocker platform. The next day samples were centrifugated at 1,000×g for 5 minutes at 4° C, and the supernatant was carefully aspirated and discarded. The Pellet was washed one time with 1.0 mL RIPA Buffer (Pierce Biotechnology, Rockford, IL) and three times with 1 mL 1× PBS, each time repeating the centrifugation step mentioned above. Following the final wash, aspiration and discarding of supernatant, the pellet was resuspended in 10 µl 3× electrophoresis sample buffer and 20 µl of water for Western blotting.
Western Blots
Western Blots were performed on 30–50 µg of microsomal protein to measure CYP levels. Proteins were separated by polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% gel, and transferred to 0.45µm nitrocellulose (Bio-Rad Laboratories, Hercules, CA) where the blot was blocked using 5% skim milk/0.3% Tween 20 dissolved in phosphate buffered saline. Pre-stained protein standards (Bio-Rad) were used as molecular weight markers. Primary antibodies were obtained from a variety of sources. Rat anti-mouse Cyp2b10 antibody is a generous gift from Dr. Randy Rose at North Carolina State University, Raleigh, NC, rabbit anti-mouse CAR was obtained from Santa Cruz Biotechnology, rabbit anti-rat CYP3A1 and CYP2C8/9/19 were obtained from Chemicon International (Temicola, CA), mouse anti-human CYP2A6 was obtained from Gentest™ Corporation (San Jose, CA), and rabbit anti-mouse β-actin (Sigma Aldrich, St. Louis, MO) was used as a housekeeper to ensure equal loading of samples. Goat anti-rabbit IgG (Bio-Rad) alkaline-phosphatase coupled secondary antibodies were used for recognizing CYP3A1 and CYP2C8/9/19 primary antibodies. Goat anti-rat (Bio-Rad), rabbit anti-mouse (Gentest™ Corporation), and goat anti-mouse (Bio-Rad) IgG were used to recognize the Cyp2b10, CYP2A6 and β-Actin primary antibodies, respectively. Primary antibodies were diluted 1:1000, and secondary antibodies were diluted 1:500. Bands were either visualized colorimetrically with nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) as substrates (Bio-Rad Laboratories, Hercules, CA), or with a chemilluminescent kit according to the manufacturer’s directions (Bio-rad). Colorimetric blots were scanned on a GS-710 densitometer (Bio-rad, Hercules, CA), and bands were quantified with LabWorks™ image analysis software (UVP laboratory products, Upland, CA). Chemiluminescence was quantified on a Chemi-Doc system with Quantity One software (Bio-Rad).
Zoxazolamine-induced paralysis in NP-treated mice
Wild-type male and female mice were randomly split into treatment groups. Sixteen wildtype and fourteen female CAR-null mice were also split into untreated, 50 mg/kg/day NP, and 3 mg/kg/day TCPOBOP treated groups as described above. Seventeen wildtype and twenty male CAR-null mice were split into untreated, 50 mg/kg/day, 75 mg/kg/day, and 3 mg/kg/day TCPOBPOP and treated concurrently with the female mice. Mice were treated with honey, NP mixed in honey for 7 days, or given one injection of 3 mg/kg TCPOBOP dissolved in corn oil a day prior to the ZOX-challenge. On day 8, mice were injected i.p. with 300 mg/kg Zoxazolamine (ZOX) in corn oil. After injection, initial paralysis was noted, and paralysis time was measured by placing the mice on their backs and measuring the time until they were able to consistently right themselves. Mice that did not right themselves after 11 hours were euthanized.
Statistical Analysis
Statistical tests were performed with StatView software (SAS Institute Inc., Cary, NC). ANOVA was used to compare three or more treatment groups followed by Fisher’s PLSD as the post-hoc test, and Student’s t-tests were used to compare two groups. A p-value of ≤ 0.05 was regarded as significantly different from control values and is shown in figures with an asterisk. Fisher’s exact test was used to compare the survival rates of the Zoxazolamine-treated mice, and ANOVA followed by Fisher’s PLSD was used to compare the paralysis times between groups. A p-value ≤ 0.05 was regarded as significantly different from controls and is shown with an asterisk in the figures.
RESULTS
Testosterone hydroxylase activity
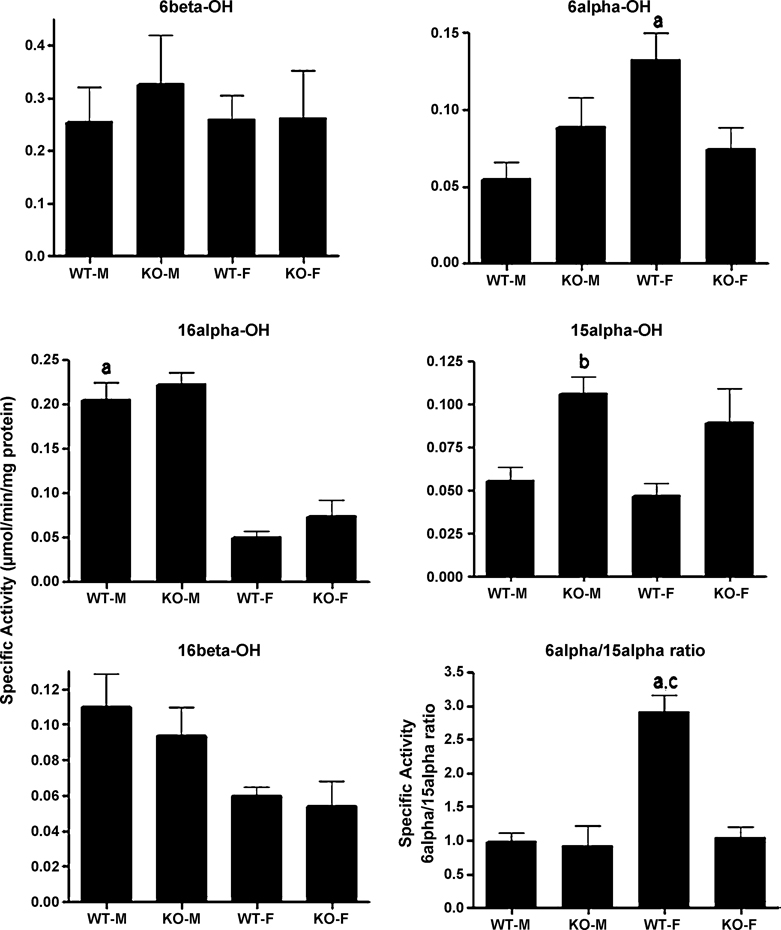
We compared testosterone hydroxylase activity in male and female wild-type and CAR-null mice as testosterone hydroxylation is often used as a biomarker of P450 enzyme activity, such as Cyp2a, 2b, 2c, and 3a subfamily members. Most testosterone hydroxylase activities were unaffected by gender with the exception of the female predominant 6α-OH activity (2.5X-higher in females) and the male predominant testosterone 16α-OH activity (4X-higher in males) (Fig. 1). 16β-OH activity showed a trend towards male predominance, but it was not statistically significant. Hydroxylation of testosterone in the 6α-position is performed primarily by the female predominant Cyp2a4 (Baldwin and LeBlanc 1992). Hydroxylation of testosterone in the 16-position is performed by several enzymes including Cyp2b, 2c, and 2d members (Harada and Negishi 1984; Lee et al. 2006; Ohmori et al. 1993; Yamada et al. 2002). However, Cyp2d9 is the only male predominant enzyme of the three CYPs suggesting Cyp2d9 is the primary 16α-and 16β-OH in males.
Fig. 1. Gender differences in testosterone hydroxylation in wild-type and CAR-null mice.
Testosterone hydroxylation in the 6β-, 16α-, 16β-, and 6α-positions were determined and compared between male and female wild-type and CAR-null mice. The results are shown as mean specific activity (µmol/min/mg protein) ± SD (n = 5–6). An (a) indicates a significant difference between wild-type male (WT-M) and wild-type female (WT-F) mice, a (b) indicates a significant difference between WT-M and CAR-null male (KO-M) mice, and a (c) indicates a significant difference between wild-type female (WT-F) and CAR-null female (KO-F) mice by ANOVA followed by Fisher’s PLSD test as the post-hoc test (p < 0.05).
CAR-null male and female mice demonstrated an increase in testosterone 15α-hydroxylase activity, but this data was only significant in male mice (Fig. 1). The female predominant 6α-hydroxylase activity also decreased in CAR-null females, but was not statistically significant. However, the 6α/15α-OH ratio, which is generally much greater in females, controlled by androgen status, and considered a biomarker of androgen disruption in mice (Wilson et al. 1999), was decreased in CAR-null female mice. Because androgens are CAR inverse agonists, this suggests that CAR is involved in the androgen regulation of the gender predominant CYPs involved in 6α or 15α-OH activity.
Regulation of basal P450 expression by CAR
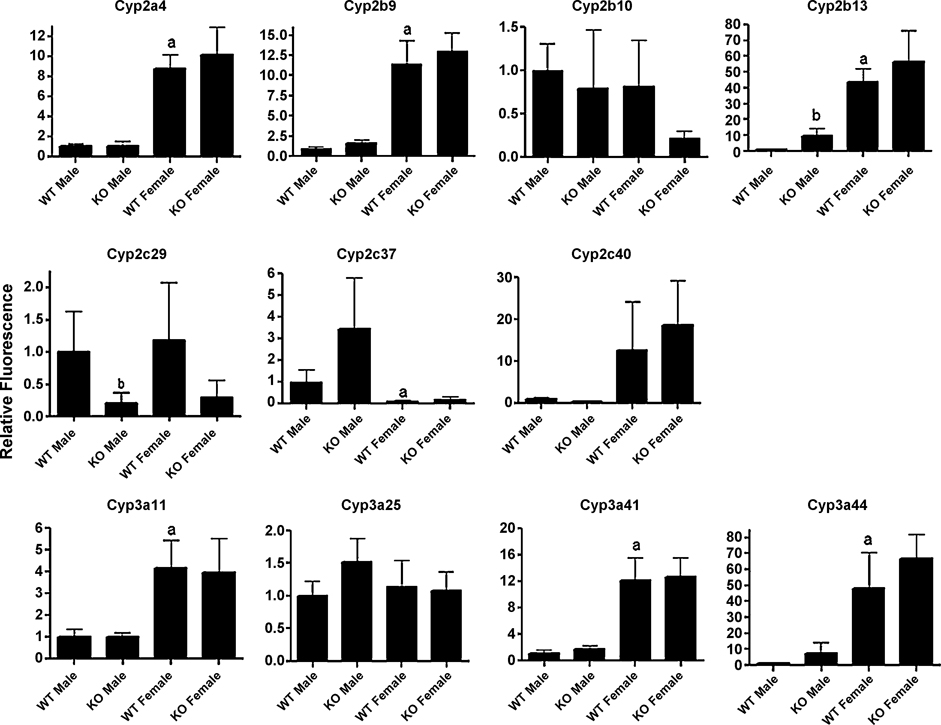
To further characterize the basal regulation of CYPs by CAR in male and female mice, we performed QPCR on several Cyp2 and Cyp3 family members. Seven of the eleven P450s measured by QPCR were female predominant including Cyp2a4, Cyp2b9, Cyp2b13, Cyp3a11, Cyp3a41, and Cyp3a44 (Fig. 2). Of the CYPs we examined by QPCR, only Cyp2c37 was male predominant. Cyp2b13 and Cyp3a44 showed greater than 40-fold higher levels in females, and Cyp2b9, Cyp2c40, and Cyp2a4 showed approximately 9–12.5-fold higher levels in females (Fig. 2). Cyp3a25 was gender neutral as previously published (Hernandez et al. 2006); however, Cyp3a11 was 4.2-fold higher in B6129 female mice than male mice. Our previous studies with FVB/NJ mice showed that Cyp3a11 expression was gender neutral (Hernandez et al. 2006).
Fig. 2. Gender differences in expression of specific CYP isoforms in wild-type and CAR-null mice.
Quantitative real-time PCR (QPCR) was performed as described in the Materials and Methods and used to determine gender and CAR-regulated expression of P450s in untreated mice. The results are shown as relative activity ± SD (n = 5–6). An (a) indicates a significant difference between wild-type male (WT-M) and wild-type female (WT-F) mice, a (b) indicates a significant difference between WT-M and CAR-null male (KO-M) mice, and a (c) indicates a significant difference between wild-type female (WT-F) and CAR-null female (KO-F) mice by ANOVA followed by Fisher’s PLSD as the post-hoc test (p < 0.05).
Untreated CAR-null mice also show perturbed regulation of a couple of P450s relative to their wild-type counterparts. Cyp2c29 is down-regulated greater than 4-fold in both male and female CAR-null mice when compared to wild-type mice; however, only the down-regulation in males was significant. A couple of Cyp2b family members showed trends indicative to gender specific effects of CAR on the basal regulation of CYPs. Cyp2b13 expression in CAR-null male mice was increased nearly 9-fold higher, though its expression in males was still considerably lower than its expression in females. Lastly, Cyp2b10 showed a trend towards down-regulation in CAR-null female mice.
Sexually dimorphic induction of CYPs by NP and TCPOBOP in a CAR-dependent manner
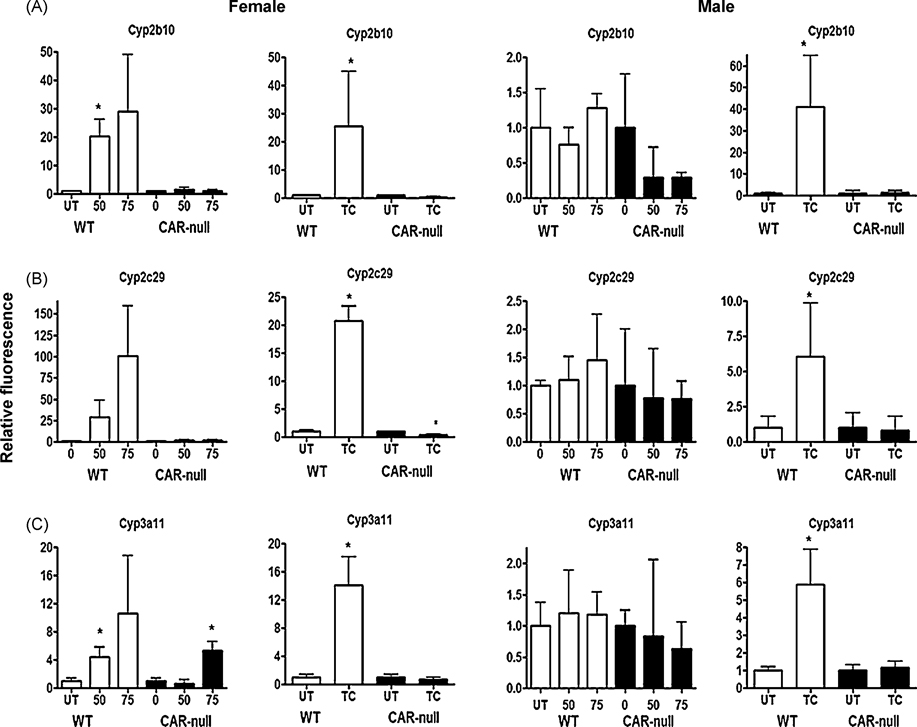
QPCR was performed with male and female wild type and CAR-null mice following treatment with the partial agonist NP at 0, 50, 75 mg/kg, and the full agonist and classical CAR activator TCPOBOP at 3 mg/kg. Of the eleven P450s measured, six P450s consistently responded to NP or TCPOBOP treatment (Fig. 3,4). Three of the eleven P450s measured by Q-CPR (Cyp2b10, Cyp2c29, Cyp3a11) showed a similar trend following treatment with NP or TCPOBOP. The partial agonist NP induced Cyp2b10 (Fig. 3A), Cyp2c29 (Fig. 3B), and Cyp3a11 (Fig. 3C) in a CAR-dependent, female specific manner, but the full agonist TCPOBOP induced these CYPs in a CAR-dependent manner in both males and females (Fig. 3). Interestingly, Cyp3a11 was induced at 75 mg/kg/day NP in CAR-null mice, suggesting activation of PXR by NP (Hernandez et al. 2007; Masuyama et al. 2000). Overall, this data indicates that the induction of these three CYPs by CAR agonists is not gender specific. However, females are more sensitive to the effects of CAR agonists as only females responded to the partial agonist, NP (Fig. 3) (Baldwin and Roling 2008; Hernandez et al. 2007).
Fig. 3. Cyp2b10, Cyp2c29, and Cyp3a11 show similar patterns of gene expression following treatment with NP or TCPOBOP in wild-type and CAR-null male and female mice.
QPCR was performed as described in the Materials and Methods. On the X-axis, 0, 50, 75 refers to treatment with NP and TC refers to treatment with TCPOBOP. Data are expressed as mean ± SD (n = 5–6) for each of the different P450s in males and females. An asterisk indicates a significant difference from the untreated wild-type or CAR-null mice (p < 0.05). Statistical significance was determined by ANOVA followed with Fisher’s PLSD as the post-hoc test in NP-treated mice compared to their corresponding wild-type or CAR-null controls. Statistical significance was determined by Student’s t-tests in TC-treated mice compared to their corresponding wild-type or CAR-null controls.
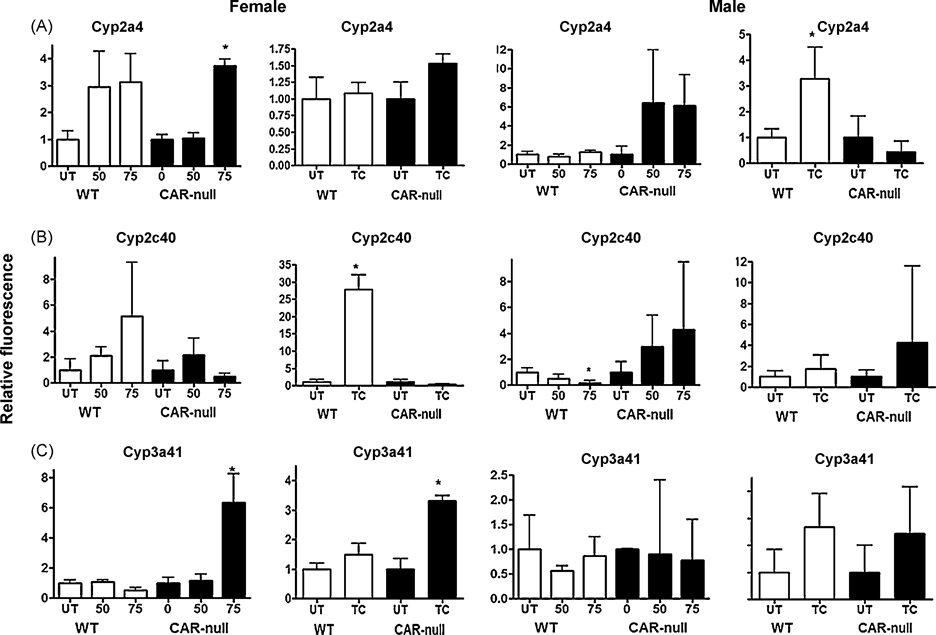
Fig. 4. Cyp2a4, Cyp2c40, and Cyp3a41 show differential patterns of regulation following treatment with NP or TCPOBOP in wild-type or CAR-null male and female mice.
QPCR was performed as described in the Materials and Methods. On the X-axis, 0, 50, 75 refers to treatment with NP and TC refers to treatment with TCPOBOP. Data are expressed as mean ± SD (n = 5–6) for each of the different P450s in males and females. An asterisk indicates a significant difference from the untreated wild-type or CAR-null mice (p < 0.05). Statistical significance was determined by ANOVA followed with Fisher’s PLSD as the post-hoc test in NP-treated mice compared to their corresponding wild-type or CAR-null controls. Statistical significance was determined by Student’s t-tests in TC-treated mice compared to their corresponding wild-type or CAR-null controls.
Three other CYPs did not exhibit diverse changes in their gene expression patterns following treatment with NP or TCPOBOP. Cyp2a4 was significantly induced by NP only in female CAR-null mice, suggesting the activation of PXR (Fig. 4A) similar to that observed for Cyp3a11. Wild-type mice showed trends indicating NP-mediated Cyp2a4 increases in both male in female mice. Cyp2a4 was induced by TCPOBOP in male, but not female mice, suggesting that the induction of the female predominant Cyp2a4 is not very sensitive to CAR activation. Male specific induction of Cyp2a4 by CAR activators has been observed previously, presumably due to low constitutive expression of Cyp2a4 in males (Hernandez et al. 2006; Wei et al. 2002).
Alterations in the gene expression of Cyp2c40 and Cyp3a41 do not show similar patterns or easily explicable patterns following treatment with TCPOBOP or NP (Fig. 4). Both of these CYPs are highly female predominant with Cyp2c40 showing 12.4X; greater expression and Cyp3a41 showing 12.1× greater expression in females. In turn, we observed much greater variability in the gene expression from male mice. For example, in females Cyp2c40 was induced significantly by TCPOBOP and showed an increase in expression following treatment with NP that was not statistically significant (Fig. 4b). Thus, changes in Cyp2c40 expression in female mice followed a similar pattern to Cyp2b10, Cyp2c29, and Cyp3a11. However, in males, TCPOBOP did not induce Cyp2c40, and NP actually reduced the expression of Cyp2c40 in a CAR-dependent manner. Cyp3a41 was induced by NP and TCPOBOP in CAR-null mice but not wild-type mice. In FVB/NJ mice we had previously observed that NP down-regulated Cyp3a41 in females (Hernandez et al. 2006); however, the slight drop in levels observed in this study were not significant. TCPOBOP nor NP altered Cyp3a41 expression in male mice. Overall, gene expression of these female predominant CYPs (Cyp2a4, 3a41) was highly variable in male mice, but typically showed recognizable changes in gene expression in females. Data for several of the CYPs is not shown (i.e. Cyp2b9, 2b13, 3a25, and 3a44) because there is little or no statistically significant changes in expression following either NP or TCPOBOP treatment (Suppl Data, Fig. 1S).
Western blots of hepatic CYPs following TCPOBOP and NP treatment
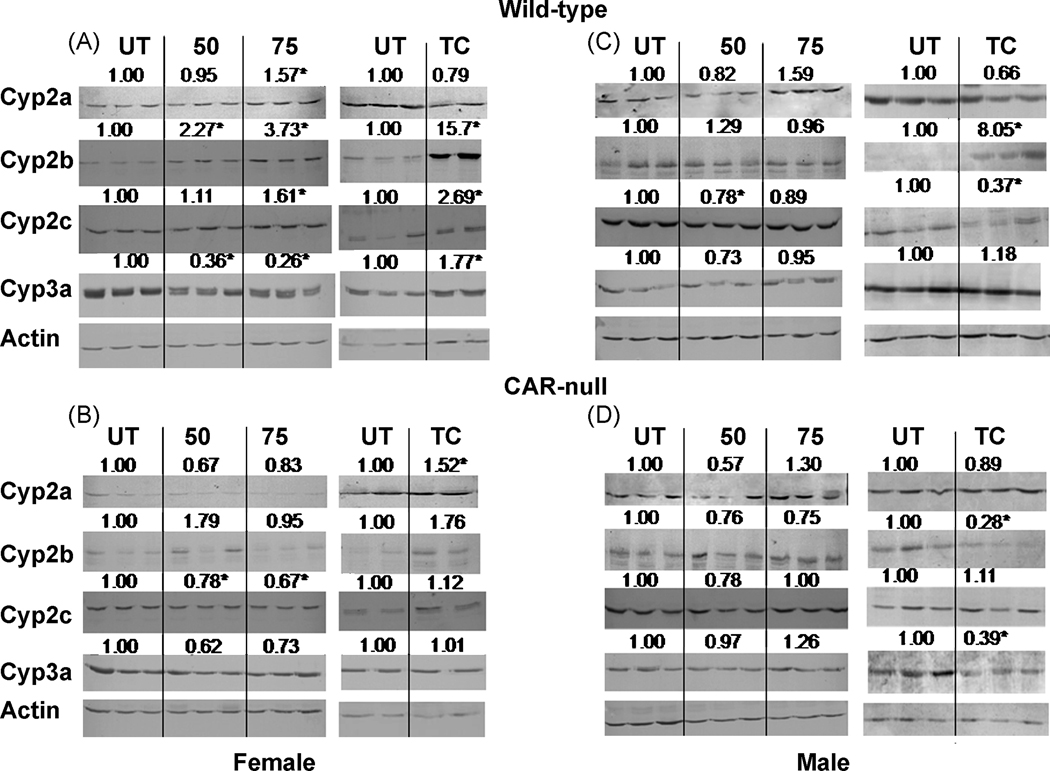
Western blots were performed on male and female, wild-type and CAR-null mice to investigate the effects NP or TCPOBOP on hepatic CYP subfamily levels, determine whether changes in one or more isoform caused significant changes in a CYP subfamily, and to confirm the QPCR data by investigating CYP protein levels. Because more than one isoform in a subfamily may be recognized by an antibody, we refer only to the subfamily. Western blotting confirmed that TCPOBOP and NP induced Cyp2b and Cyp2c subfamily members in wild-type females (Fig. 5A) in a CAR-dependent fashion as treated CAR-null mice failed to demonstrate an increase0 in these CYPs (Fig. 5A,B). Interestingly, Cyp3a subfamily members were up-regulated by TCPOBOP but down-regulated by NP in wild-type female mice (Fig. 5A) and this occurred in a CAR-dependent fashion (Fig. 5B). However, Cyp3a11 mRNA expression was significantly induced by both TCPOBOP and NP in wild-type mice (Fig. 3C). The discrepancy is interesting as previous studies have demonstrated NP-mediated down-regulation of Cyp3a protein (Arukwe et al. 1997; Hernandez et al. 2006; Laurenzana et al. 2002) and mRNA consistent with Cyp3a41 and Cyp3a44 down-regulation (Hernandez et al. 2006). However, our current study did not show down-regulation of either Cyp3a41 or Cyp3a44 by QPCR (Fig. 4C, Suppl Data).
Fig. 5. Western blots of hepatic microsomes from NP and TCPOBOP-treated wild-type and CAR-null male and female mice.
Western blots were performed and visualized as described in the Materials and Methods. Blots were quantified densitometrically and the relative mean differential expression as compared to the controls is reported above the blots. An asterisk indicates a significant difference from the corresponding untreated mice by ANOVA followed by Fisher’s PLSD for NP-treated mice, and Student’s t-test for TC-treated mice (p < 0.05).
In male mice, Western blotting demonstrated that NP caused no significant changes in CYP protein levels in wild-type or CAR-null mice consistent with QPCR. However, QPCR indicated TCPOBOP induced Cyp2a4, Cyp2b10, Cyp2c29, and Cyp3a11, but only the Cyp2b subfamily showed a significant increase in protein levels. In contrast, Cyp2c protein levels were decreased significantly in the TCPOBOP-treated wild-type male mice. Furthermore, several P450s (Cyp2b, 3a) were down-regulated in the TCPOBOP-treated CAR-null male mice, suggesting that CAR-null mice are sensitive to the toxic effects of TCPOBOP at the dose we provided (Fig. 5B).
Immunoprecipitation, Western blots, and Comparison of CAR protein expression between male and female mice
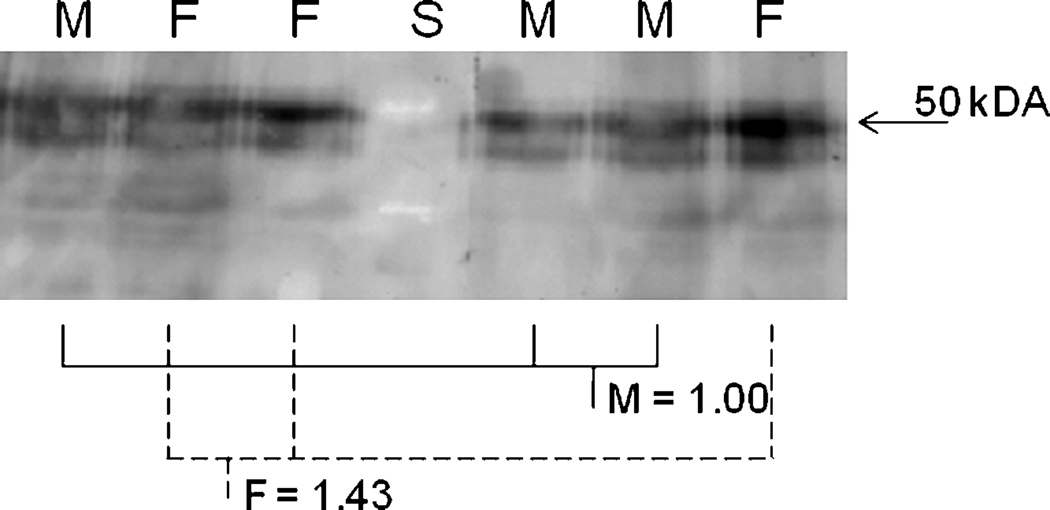
Several studies have indicated that females are more sensitive to CAR agonists than males (Ledda-Columbano et al. 2003). Reasons suggested indicate repression of CAR activity by male androgens, increased activity in females because of the presence of estrogens as CAR agonists (Kawamoto et al. 2000), and greater mRNA expression of CAR in female mice (Petrick and Klaassen 2007). However, protein expression of CAR has not been investigated. Therefore, we examined differences in cytosolic protein expression between male and female B6129 mice. Cytosol was examined because CAR is primarily found in the cytosol compared to the nucleus, and even in phenobarbital-treated animals most CAR remains in the cytosol (Dail et al. 2008). We consider the CAR Western blots semi-quantitative because of the immunoprecipitations, which also eliminates all of the housekeeping genes on the blot. Semi-quantification of Western blots from immunoprecipitated CAR by densitometry found no significant difference between male and female CAR protein expression (Fig. 6). The immunoprecipitations and Western blots were performed a total of three times with different samples and we observed similar results (28, 43, 53% more CAR in females; n = 3–4). In all of the cases, the results were not statistically significant, indicating that differences in CAR protein expression between males and females is not the reason for enhanced CAR activity in female mice.
Fig. 6. Western blots of immunoprecipitated CAR protein from untreated wild-type male and female mice.
Immunoprecipitation and Western blotting was performed as described in the Materials and Methods. Blots were quantified densitometrically and the relative mean expression compared to the male mice is reported. M = male (solid lines); F = female (dashed lines). There were no significant differences as determined by Student’s t-test (p < 0.05).
Pharmacological effects of Zoxazolamine; CAR status, sex, and agonist treatment
Zoxazolamine, a classical CYP substrate, is a muscle relaxant often used to determine the in vivo pharmacological effects of CYP inducers or inhibitors on xenobiotic metabolism. Increased paralysis time following ZOX-treatment indicates inhibition of CYPs and decreased paralysis time indicates induction of CYPs following xenobiotic treatment or genetic alteration (wild-type compared to CAR-null mice). We used ZOX to elucidate the pharmacological effects of sex, CAR status, and agonist treatment (TCPOBOP, NP). Groups of treated and untreated male and female wild-type and CAR-null mice were injected with ZOX and paralysis time measured. This allowed us to compare differences between untreated male and female mice, wild-type and CAR-null mice of each sex, and untreated and treated mice of each sex.
Female B6129 mice were clearly more resistant to the paralyzing effects of ZOX than male B6129 mice (Table 1). None of the female mice died during ZOX-treatment, including CAR-null female mice; several male mice died including some of the wild-type mice and nearly all of the CAR-null male mice. The difference in paralysis time between male and female mice is consistent with the greater number of female predominant CYPs in the Cyp2 and Cyp3 families as these are important families in ZOX metabolism. In addition, comparing untreated wild-type female mice to untreated CAR-null female mice following ZOX-treatment demonstrated that female CAR-null mice are more susceptible to ZOX paralysis than female wild-type mice (Table 1). However, paralysis times were not statistically different when untreated wild-type and CAR-null male mice were compared.
Table 1.
Sex, CAR-status, and xenobiotic exposure influence Zoxazolamine-induced paralysis time.
| Females | ||
|---|---|---|
| Treatment | Survival | Mean Paralysis Time |
| WT-UT | 8/8 | 55 + 15 |
| WT-NP (50) | 6/6 | 1 + 2a |
| WT-TC | 2/2 | 0 + 0a |
| CAR-null UT | 6/6 | 105 + 38c |
| CAR-null NP | 6/6 | 64 + 15b,c |
| CAR-null TC | 2/2 | 80 + 10c |
| Males | ||
| Treatment | Survival Mean Paralysis Timee | |
| WT-UT | 2/5 | 203 + 80 |
| WT-NP (50) | 5/5 | 246 + 107 |
| WT-NP (75) | 4/5 | 127 + 89 |
| WT-TC | 2/2 | 305 + 175 |
| CAR-null UT | 1/6 | 600 |
| CAR-null NP (50) | 0/5d | n.a. |
| CAR-null NP (75) | 0/5d | n.a. |
| CAR-null TC | 1/4 | 163 |
different by ANOVA followed by Fisher’s PLSD as compared to WT control.
different by ANOVA followed by Fisher’s PLSD as compared to CAR-null control.
different by ANOVA followed by Fisher’s PLSD as compared to similarly treated WT mouse.
different by Fisher’s exact test compared to the similarly treated WT mouse.
mean paralysis time does not include mice that did not survive Zoxazolamine challenge
n.a. = not applicable
Treatment of mice with NP or TCPOBOP significantly altered ZOX paralysis of wild-type mice. NP and TCPOBOP markedly decreased ZOX paralysis time in a similar fashion in wild-type female mice despite TCPOBOP’s greater potency towards CAR. ZOX paralysis was unaffected by TCPOBOP-treatment in CAR-null mice demonstrating that the TCPOBOP-mediated decrease in ZOX paralysis is CAR-dependent. Similarly, the NP-mediated decrease in ZOX paralysis is in part CAR-dependent in female mice. However, NP-treated CAR-null mice show a small but significant decrease in paralysis time, suggesting activation by PXR in addition to CAR (Table 1)(Hernandez et al. 2007; Masuyama et al. 2000).
Wild-type male mice treated with NP showed a significantly greater survival rate than CAR-null male mice treated with NP because none of the CAR-null mice survived. This suggests that NP activates CAR and induces CYPs, and/or CAR-mediates basal regulation of CYPs. There were no significant differences between untreated and NP-treated male wild-type mice; however, there was a slight trend suggesting greater survival in wild-type NP-treated mice compared to untreated mice. Untreated and NP-treated mice that survived the ZOX-challenge showed no difference in paralysis time (Table 1) reflecting the poor CYP induction in male wild-type mice by NP. All of the TCPOBOP treated wild-type male mice survived but many of the untreated wild-type, CAR-null, and TCPOBOOP-treated CAR-null mice did not. Overall, TCPOBOP showed similar data to the NP treated male mice as significant changes in paralysis time and survival were not observed potentially due to small sample size (Table 1). Overall, the difference between males and females following treatment is indicative of the increased sensitivity of female mice to CAR agonists (Fig. 3–5).
Discussion
CAR is a xenobiotic responsive transcription factor critical in the regulation of CYPs and other detoxification genes (Pascussi et al. 2008). CAR is also constitutively active (Baes et al. 1994), and CAR expression is associated with CYP expression in human liver (Wortham et al. 2007). For example, Therefore, we hypothesized CAR may basally regulate some CYP enzymes. The basal regulation of CYPs by CAR is highly apparent pharmacologically in ZOX-treated mice. Untreated CAR-null mice were significantly more sensitive to ZOX-induced paralysis than untreated wild-type mice, especially in females (Table 1). The increased sensitivity in female CAR-null mice to ZOX is associated with slightly reduced Cyp2b10 and Cyp2c29 levels, and a shift to the male pattern testosterone 6α/15α-OH ratio (Fig. 1,2).
Untreated male CAR-null mice did not show a significant increase in sensitivity to ZOX based on survival and paralysis time (Table 1). However, CAR-null male mice showed significantly lower basal expression of Cyp2c29 coupled with 9-fold higher expression of Cyp2b13, and greater 15α-OH activity (Fig. 1,2), a biomarker of Cyp2a activity (Burkhart et al. 1985). Overall, ZOX and QPCR data suggest that CAR is moderately involved in the basal regulation of some CYPs in males and females including CYPs in the Cyp2b and Cyp2c subfamilies.
Corroborating evidence for basal regulation of CYPs by CAR exists in humans as recent data based on associations between CYP expression levels and nuclear receptors indicates that several nuclear receptors (HNF4α, CAR, PXR) are involved in the basal regulation of CYPs (Slatter et al. 2006; Vyhlidal et al. 2006; Wortham et al. 2007). Recent data investigating the expression and activity of a broad scope of xenobiotic detoxification genes indicates that the basal regulation of several CYPs are controlled by HNF4α > CAR > PXR (Wortham et al. 2007). The dominance of HNF4α is not surprising because it directly controls the expression of CAR (Ding et al. 2006) and the glucocorticoid receptor (GR), which controls the expression of PXR (Pascussi et al. 2000). Colinearity among expression levels indicates that HNF4α directly controls CYP2C9, while HNF4α’s regulation of CAR controls CYP2A6, CYP2B6, CYP2C8, and CYP2C19 (Wortham et al. 2007). In summary, CAR expression is associated with the expression of CYP2A6, 2B6, 2C8, and 2C19 suggesting CAR basally regulates these genes in humans. Our data indicates that Cyp2b13, Cyp2c29, and potentially Cyp2b10 are controlled in part by CAR in mice.
Furthermore, HNF4α is important in the sexually dimorphic expression of hepatic CYPs (Wiwi et al. 2004). Several CYP members, including Cyp2a4, Cyp2b9, Cyp2b13, Cyp3a11, Cyp3a41, and Cyp3a44, are female predominant, which in part explains the significantly diminished paralysis time in female mice compared to male mice. Thus, lower basal levels of several drug metabolizing CYPs in male B6129 mice appears to have a pharmacological consequences (Table 1). HNF4α negatively controls Cyp2a4 and Cyp2b9 in male mice (Wiwi et al. 2004). We did not observe a significant change in either of these enzymes; although Cyp2b9 was increased slightly (2.1X) in CAR-null mice relative to wild-type male mice (Fig. 2). HNF4α positively regulates Cyp2b10, Cyp2b13, Cyp3a41, and Cyp3a44 (Wiwi et al. 2004), but the involvement of CAR had not been tested. We observed a significant increase in Cyp2b13 in CAR-null male mice relative to wild-type mice. Interestingly, microarray data from HFN4α-null mice also show a significant increase in Cyp2b13 and Cyp2b9 expression exclusively in males (Holloway et al., 2008). However, only the Cyp2b13 data is significant in CAR-null males (Fig. 2) indicating that HNF4α is the primary factor repressing the regulation of Cyp2b9 in males, while CAR is the primary factor repressing the regulation Cyp2b13 in males.
We also observed a nearly 4-fold drop in Cyp2b10 in CAR-null female mice relative to wild-type mice, and a 3.5-fold increase in Cyp3a44 in the CAR-null male mice compared to the wild-type mice. However, the expression changes in both of these CYPs was not statistically significant, which is consistent with the reduced basal regulatory control of CYPs by CAR compared to HNF4α (Wortham et al. 2007). The basal regulation of Cyp2c subfamily members in mice by HNF4α has not been investigated (Wiwi et al. 2004). Interestingly, CAR-null mice recently backcrossed to the B6 background show significant repression of Cyp2b10 in both sexes (Cho et al. 2008). This suggests that similar to rats (Yoshinari et al. 2001), mice may demonstrate strain specific sexually dimorphic effects related to CAR.
Other sex and/or strain differences may be observed as further work is performed with B6 and B6-CAR-null mice instead of B6129 and B6129-CAR-null mice. However, current data suggests that strain will not have much effect on CAR activity because other than the sex difference in basal Cyp2b10 expression in CAR-null males; most differences between genders are related to the overall repressed sensitivity of CAR activity especially after treatment with NP, a weaker, partial agonist with a shorter half-life and an unknown mechanism of activation (indirect or direct activation of CAR). Other groups have also observed overall repressed sensitivity of CAR activity in male mice (Petrick and Klaassen 2007; Ledda-Columbano et al. 2003) and human males (Lamba et al. 2003). Furthermore, similar sex differences in CYP2a, 2b, 2c, and 3a basal subfamily expression have been observed in other strains (Wiwi et al., 2004; Hernandez et al., 2006; Holloway et al., 2008) as well as repressed induction in males compared to females following NP treatment (Hernandez et al., 2006). Taken together, this suggests that future research investigating CAR activity with pure inbred strains will reveal similar results as with B6129 mice.
Testosterone hydroxylase assays were also performed to assess P450 activity in various mouse models to determine basal regulation of CYP activity by CAR, and determine differences between males and females. CAR represses 15α-OH activity, especially in male mice. 15α-OH activity is associated with Cyp2a4 activity in female mice (Burkhart et al. 1985), but this activity has not been fully characterized in male mice. Cyp2a4 is expressed at much greater levels in females than males, and while 6α-OH activity declines in males relative to females, 15α-OH activity does not. Thus, the CYP that makes up the difference in 15α-OH activity in males needs further characterization, but may be another Cyp2a subfamily member such as Cyp2a5.
Because both 6α- and 15α-OH activities were perturbed by either sex or CAR-status, we examined the 6α/15α-OH ratio. The 6α/15α-OH ratio was decreased greater than 2.5-fold in female CAR-null mice compared to their wild-type counterparts (Fig. 1). Therefore, CAR-null female mice had a similar 6α/15α-OH ratio to male mice. The 6α/15α-OH ratio, which is typically much greater in females, is in part controlled by androgens and considered a biomarker of androgen disruption or androgen status (Wilson et al. 1999). This suggests that CAR is a key player in the recognition of androgen status in the liver, consistent with its repression by androstanes and other androgens (Forman et al. 1998). It is interesting to speculate that perturbations in steroid metabolism and testosterone 6α/15α-OH ratio associated with exogenous estradiol or endocrine disrupting xenobiotics such as indole-3-carbinol, DDT and vinclozolin (Sierra-Santoyo et al. 2005; Wilson et al. 1999) may be in part be mediated by the activation of CAR (Kawamoto et al. 2000). This is consistent with our theory that CAR is a steroid sensor and protector of the endocrine system (Kretschmer and Baldwin 2005).
In addition, we investigated differential responses of male and female wild-type and CAR-null, mice to the moderately potent CAR agonist NP and the highly potent CAR agonist TCPOBOP. A robust and significant decrease in paralysis time was observed in wild-type female mice treated with NP or TCPOBOP, as paralysis was nearly lost in both treatments. This is consistent with an increase in several CYPs as measured by QPCR (Fig. 3,4) and Western blotting (Fig. 5) following NP or TCPOBOP treatment. In turn, CAR-null NP- and TCPOBOP-treated mice responded poorly to ZOX-treatment and lacked demonstrative CYP induction demonstrating that NP and TCPOBOP are mediating their effects through CAR. Interestingly, CAR-null females treated with NP but not TC showed a significant albeit diminished decrease in paralysis time compared to untreated CAR-null females, suggesting activation of PXR (Baldwin et al. 2005; Hernandez et al. 2007; Masuyama et al. 2000; Mikamo et al. 2003) and induction of Cyp3a subfamily members in a CAR-independent fashion as observed by QPCR (Fig. Fig. 3c, 4a,c).
NP or TCPOBOP treated wild-type male mice did not respond as well as the NP or TCPOBOP treated wild-type female mice to ZOX. However, survival was increased in NP- and TCPOBOP-treated wild-type males compared to the untreated wild-type males and treated and untreated CAR-null males, suggesting that CYP levels were increased slightly in a CAR-mediated fashion. This is consistent with the CAR-dependent increase in CYP levels measured by QPCR (Fig. 3,4) and Western blotting (Fig. 5) in TCPOBOP-treated mice. Previously induction of Cyp2b subfamily members by TCPOBOP (Wei et al. 2000) and NP in FVB/NJ mice (Hernandez et al. 2006) was associated with ZOX clearance and indicates the importance of Cyp2b in ZOX metabolism (Hernandez et al. 2006). However, we observed no significant increases in CYP RNA (Fig. 3,4) or protein (Fig. 5) levels in NP-treated B6129 male mice, but did observe significantly greater survival compared to untreated mice. The lack of CYP induction by NP in B6129 male mice compared to FVB/NJ male mice may be related to differences in the mouse strain. FVB/NJ male mice show lower expression levels of several CYPs (Hernandez et al. 2006) and thus induction of these CYPs may be more sensitive markers than in the B6129 mice. This suggests that there were increases in CYPs that we did not measure by QPCR, or slight induction of several CYPs in a non-statistically significant manner made a biological impact. It should be noted that paralysis time was not changed between surviving untreated and NP-treated male mice, and this indicates weak or no induction of CYPs in the male mice (Fig. 3–5, Table 1).
In general, significantly greater responses to NP and TCPOBOP were observed in female mice than male mice in a CAR-dependent fashion. For example, reductions in ZOX paralysis time were greater in treated female mice than treated male mice. Furthermore, QPCR and Western blots of NP-treated mice showed induction of CYPs almost exclusively in females (Fig. 3,4,5)(summarized in Table 2). Previously, we had observed significantly greater induction of CYPs in NP-treated females than males (Hernandez et al. 2006) in a study with FVB/NJ mice that led us to investigate the role of CAR in NP-mediated sexually dimorphic induction. However we did not observe the near abrogation of CYP induction by NP in males we observed in this study. This large sexually dimorphic difference was especially apparent in the female specific CAR-mediated induction of Cyp2b10, Cyp2c29, and Cyp3a11 by NP measured by QPCR. Western blots also showed significant dose-dependent changes in Cyp2a, Cyp2b, Cyp2c, and Cyp3a levels only in wild-type females, but not wild-type males treated with NP. However, TCPOBOP did not typically show sexually dimorphic induction. TCPOBOP-mediated CYP induction occurred in both sexes as measured by QPCR (Fig. 3,4), but showed a slight (about 0.5×) attenuated response as measured by Western blotting (Fig. 5, summarized in Table 2).
Table 2.
Summary of the effects of TCPOBOP and NP on CYP expression in male and female, wild-type and CAR-null mice.
| Female | Male | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q-PCR | Western | Q-PCR | Western | ||||||||
| CYP | Genotype | 50 | 75 | TC | NP | TC | 50 | 75 | TC | NP | TC |
| Cyp2a4 | WT | + | + | N.C. | +* | N.C | N.C. | N.C. | +* | N.C. | N.C. |
| KO | N.C. | +* | N.C. | N.C. | +* | + | + | N.C. | N.C. | N.C. | |
| Cyp2b10 | WT | +* | +* | +* | +* | +* | N.C. | N.C. | + | N.C. | + |
| KO | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. | --* | |
| Cyp2c29 | WT | + | + | +* | +* | +* | N.C. | N.C. | +* | --* | --* |
| KO | N.C. | N.C. | --* | --* | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. | |
| Cyp2c40 | WT | +* | +* | +* | +* | +* | --* | --* | N.C. | --* | --* |
| KO | N.C. | N.C. | N.C. | --* | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. | |
| Cyp3a11 | WT | +* | + | +* | --* | +* | N.C. | N.C. | +* | N.C. | N.C. |
| KO | N.C. | +* | N.C. | N.C | N.C. | N.C. | N.C. | N.C. | N.C. | --* | |
| Cyp3a41 | WT | N.C. | -- | N.C. | --* | +* | N.C. | N.C. | N.C. | N.C. | N.C. |
| KO | N.C. | +* | +* | N.C | N.C. | N.C. | N.C. | N.C. | N.C. | --* | |
N.C.= no change
+ = trend indicating increasing levels of isoform or subfamily
-- = trend indicating decreasing levels of isoform or subfamily
Asterisk indicates statistically significant trend.
Several other studies have demonstrated sexually dimorphic differences in response to CAR ligands. For example, only male WKY rats, but not female WKY rats, respond to phenobarbital-treatment because of low CAR levels in the WKY female rats (Yoshinari et al. 2001). Cyp2a4 is only induced by TCPOBOP in male mice potentially due to the low Cyp2a4 expression in females (Fig. 4)(Wei et al. 2002). In contrast, female mice are more sensitive to the hepatic proliferative effects of TCPOBOP than male mice (Ledda-Columbano et al. 2003), and we have previously observed greater induction of several P450s in females compared to males following NP-treatment (Hernandez et al. 2006). There is precedence for a NR1I nuclear receptor regulating basal CYP expression in a gender specific fashion. PXR-null male mice demonstrate greater Cyp3a activity than there wild-type counterparts, but PXR-null female mice demonstrate reduced Cyp3a activity compared to their wild-type counterparts (Anakk et al. 2004). There are several putative explanations for the greater response in females to CAR activation, including higher expression of CAR in female liver than male liver (Petrick and Klaassen 2007). However, we did not measure increased CAR protein expression when comparing wild-type male and female mice (Fig. 6). There are other potential explanations for increased CAR activity in females. For example, estradiol is a CAR agonist in mice (Kawamoto et al. 2000), and additional xenobiotic activators may act in an additive fashion within the mixture of chemicals found in the liver. Lastly, androgens and their metabolites repress CAR activity, which may decrease the efficaciousness of CAR in males (Forman et al. 1998).
Taken together, one or all three of these mechanisms in combination may explain the increased CAR activity and induction of CYPs in adult NP-treated female B6129 mice relative to male mice. The ability of TCPOBOP to overcome reduced CAR activity in male mice and show similar increases in CYPs in both sexes may be related to its high potency and saturation of CAR. However, CAR activity is considerably greater in treated females than males, especially when mice are treated with the weaker partial activator (and potential indirect activator), NP, instead of a potent direct acting agonist such as TCPOBOP. Because environmentally we are primarily exposed to a large number of weak agonists, sexually dimorphic differences in CAR activity may cause significant sex-dependent differences in our detoxification rates, especially CYP2B6. It is interesting to hypothesize that sex differences in CAR activity may in part explain the female predominance of human CYP2B6 (Lamba et al. 2003b). Further, PCBs which activate CAR, have greater effects on thyroid hormones and thyroid hyperplasia in females than males (Hagmar et al. 2001). This is presumably due to the greater expression of CAR in human females (Lamba et al. 2003a) and therefore faster CAR activated thyroid hormone clearance (Qatanani et al. 2005). Lastly, most drugs are cleared faster in human females than males (Meibohm et al. 2002; Wolbold et al. 2003). Exposure to an increasing number of pharmaceuticals and environmental chemicals such as NP may help explain increased clearance of drugs in females and lead to potential disparities in clearance rates, or increased drug-drug/drug-toxicant interactions.
Supplementary Material
Supplementary data 1 & 2: Cyps showing little or no change following treatment with NP or TCPOBOP in wild-type or CAR-null male and female mice. QPCR was performed as described in the Materials and Methods. On the X-axis, 0, 50, 75 refers to treatment with NP and TC refers to treatment with TCPOBOP. Data are expressed as mean ± SD (n = 5–6) for each of the different P450s in males and females. An asterisk indicates a significant difference from the untreated wild-type or CAR-null mice (p < 0.05). Statistical significance was determined by ANOVA followed with Fisher’s PLSD as the post-hoc test in NP-treated mice compared to their corresponding wild-type or CAR-null controls. Statistical significance was determined by Student’s t-tests in TC-treated mice compared to their corresponding wild-type or CAR-null contr
Acknowledgements
Research support for this study was provided by start-up funds from Clemson University and NIH grants GM008012 (wsb), CA127415 (wh), and DK46546 (ddm). Juan P. Hernandez was supported in part by a NSF-AGEP award and an NIH Kirchstein fellowship, 1F31ES014113-01A1.
Footnotes
Note: The authors have no conflicts of interest to declare
References
- Acevedo R, Villanueva H, Parnell PG, Chapman LM, Gimenez T, Gray SL, Baldwin WS. The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated MMTVneu mice and its potential effects on breast cancer incidence and latency. J. Appl. Toxicol. 2005;25:339–353. doi: 10.1002/jat.1078. [DOI] [PubMed] [Google Scholar]
- Anakk S, Kalsotra A, Kikuta Y, Huang W, Zhang J, Staudinger JL, Moore DD, Strobel HW. CAR/PXR provide directives for Cyp3a41 gene regulation differently from Cyp3a11. Pharmacogenomics J. 2004;4:91–101. doi: 10.1038/sj.tpj.6500222. [DOI] [PubMed] [Google Scholar]
- Arukwe A, Knudsen FR, Goksoyr A. Fish zona radiata (eggshell) protein: A sensitive biomarker for environmental estrogens. Environ. Health Perspect. 1997;105:418–422. doi: 10.1289/ehp.97105418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi H-S, Martinoli MG, Simha D, Moore DD. A New Orphan Member of the Nuclear Hormone Receptor Superfamily That Interacts with a Subset of Retinoic Acid Response Elements. Mol. Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin WS, LeBlanc GA. The anti-carcinogenic plant compound indole-3-carbinol differentially modulates P450-mediated steroid hydroxylase activities in mice. Chem-Biol. Interac. 1992;83:155–169. doi: 10.1016/0009-2797(92)90043-k. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA. A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures. Toxicol. Sci. 2008 doi: 10.1093/toxsci/kfn206. in press: 10.1093/toxsci/kfn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA, Peterson S, Chapman LM. Effects of nonylphenol on hepatic testosterone metabolism and the expression of acute phase proteins in winter flounder (Pleuronectes americanus): Comparison to the effects of Saint John's Wort. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2005;140:87–96. doi: 10.1016/j.cca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Burkhart BA, Harada N, Negishi M. Sexual dimorphism of testosterone 15 alpha-hydroxylase mRNA levels in mouse liver. cDNA cloning and regulation. J. Biol. Chem. 1985;260:15357–15361. [PubMed] [Google Scholar]
- Cai Y, Dai T, Ao Y, Konishi T, Chuang KH, Lue Y, Chang C, Wan YJ. Cytochrome P450 genes are differentially expressed in female and male hepatocyte retinoid X receptor alpha-deficient mice. Endocrinology. 2003;144:2311–2318. doi: 10.1210/en.2002-0129. [DOI] [PubMed] [Google Scholar]
- Cho SW, Lee EK, Lee YJ, Cho BJ, Lee Y, Evans RM, Moore DD, Park YJ. Keystone Symposium: Nuclear Receptors. British Columbia, CA: Orphan Brothers, Whistler; 2008. Opposing regulation of Cyp3A expression by CAR and PXR in proplthiouracil-induced hypothyroid mice; p. #302. [Google Scholar]
- Dail MB, Shack LA, Chambers JE, Burgess SC. Global liver proteomics of rats exposed for five days to phenobarbital identifies changes associated with cancer and with cyp metabolism. Toxicol. Sci. 2008 doi: 10.1093/toxsci/kfn198. Epub ahead of print: 10.1093/toxsci/kfn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Lichti K, Kim I, Gonzalez FJ, Staudinger JL. Regulation of constitutive androstane receptor and its target genes by fasting, cyclic AMP, HNF4a and the coactivator PGC-1a. J. Biol. Chem. 2006;281:26540–26551. doi: 10.1074/jbc.M600931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, LeCluyse EL, Negishi M, Goldstein JA. Regulation of human CYP2C9 by the constitutive androstane receptor: discovery of a new distal binding site. Mol. Pharmacol. 2002;62:737–746. doi: 10.1124/mol.62.3.737. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, D'Costa DJ, Robertson GR, Liddle C. Transcriptional regulation of the human CYP3A4 gene by constitutive androstane receptor. Mol. Pharmacol. 2002;62:359–365. doi: 10.1124/mol.62.2.359. [DOI] [PubMed] [Google Scholar]
- Hagmar L, Rylander L, Dyremark E, Klasson-Wehler E, Erfurth EM. Plasma concentrations of persistent organochlorines in relation to thyrotropin and TH levels in women. Int Arch Occup Environ Health. 2001;74:184–188. doi: 10.1007/s004200000213. [DOI] [PubMed] [Google Scholar]
- Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol. Rev. 2003;55:649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- Harada N, Negishi M. Mouse liver testosterone 16 alpha-hydroxylase (cytochrome P-450(16) alpha). Purification, regioselectivity, stereo specificity, and immunochemical characterization. J. Biol. Chem. 1984;259:12285–12290. [PubMed] [Google Scholar]
- Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS. Gender Specific Induction of Cytochrome P450s in Nonylphenol-Treated FVB/NJ Mice. Toxicol. Appl. Pharmacol. 2006;216:186–196. doi: 10.1016/j.taap.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Huang W, Chapman LM, Chua S, Moore DD, Baldwin WS. The environmental estrogen, nonylphenol, activates the constitutive androstane receptor (CAR) Toxicol. Sci. 2007;98:416–426. doi: 10.1093/toxsci/kfm107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpour F, Moore R, Negishi M, Sueyoshi T. Serine 202 regulates the nuclear translocation of constitutive active/androstane receptor. Mol. Pharmacol. 2006;69:1095–1102. doi: 10.1124/mol.105.019505. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, Moore DD. Induction of bilirubin clearance by the constitutive androstane receptor (CAR) Proc. Natl. Acad. Sci. USA. 2003;100:4156–4161. doi: 10.1073/pnas.0630614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JP, Ferguson SS, Negishi M, Goldstein JA. Phenytoin induction of the cyp2c37 gene is mediated by the constitutive androstane receptor. Drug Metab. Dispos. 2006;34:2003–2010. doi: 10.1124/dmd.106.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Kakizaki S, Yoshinari K, Negishi M. Estrogen activation of the nuclear orphan receptor CAR (constitutive active receptor) in induction of the mouse Cyp2b10 gene. Mol. Endocrinol. 2000;14:1897–1905. doi: 10.1210/mend.14.11.0547. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, Negishi M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 2003;64:1069–1075. doi: 10.1124/mol.64.5.1069. [DOI] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: Xenosensors of Endocrine Disrupters? Chem-Biol. Interac. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, Fackenthal JD, Rogan PK, Ring B, Wrighton SA, Schuetz EG. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J. Pharmacol. Exp. Ther. 2003;307:906–922. doi: 10.1124/jpet.103.054866. [DOI] [PubMed] [Google Scholar]
- Laurenzana EM, Weis CC, Bryant CW, Newbold RR, Delclos KB. Effect of dietary administration of genistein, nonylphenol or ethinyl estradiol on hepatic testosterone metabolism, cytochrome P-450 enzymes, and estrogen receptor alpha expression. Food Chem. Toxicol. 2002;40:53–63. doi: 10.1016/s0278-6915(01)00095-3. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Pibiri M, Concas D, Molotzu F, Simbula G, Cossu C, Columbano A. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059–1065. doi: 10.1093/carcin/bgg063. [DOI] [PubMed] [Google Scholar]
- Lee C, Hutson JR, Tzau VK, Riddick DS. Regulation of constitutive mouse hepatic cytochromes P450 and growth hormone signaling components by 3-methylcholanthrene. Drug Metab. Dispos. 2006;34:1530–1538. doi: 10.1124/dmd.106.009936. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y, M K, Takafumi K, MacDonald PN. Endocrine disrupting chemicals, phthalic acid and nonylphenol, activate Pregnane X Receptor-mediated transcription. Mol. Endocrinol. 2000;14:421–428. doi: 10.1210/mend.14.3.0424. [DOI] [PubMed] [Google Scholar]
- Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41:329–342. doi: 10.2165/00003088-200241050-00002. [DOI] [PubMed] [Google Scholar]
- Mikamo E, Harada S, Nishikawa J, Nishihara T. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol. Appl. Pharmacol. 2003;193:66–72. doi: 10.1016/j.taap.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Noshiro M, Negishi M. Pretranslational regulation of sex-dependent testosterone hydroxylases by growth hormone in mouse liver. J. Biol. Chem. 1986;261:15923–15927. [PubMed] [Google Scholar]
- Ohmori S, Taniguchi T, Rikihisa T, Kanakubo Y, Kitada M. Species differences of testosterone 16-hydroxylases in liver microsomes of guinea pig, rat and dog. Xenobiotica. 1993;23:419–426. doi: 10.3109/00498259309057030. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem MJ. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 2003;1619:243–253. doi: 10.1016/s0304-4165(02)00483-x. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: Crosstalk and consequences. Annu. Rev. Pharmacol. Toxicol. 2008;48:1.1–1.31. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol. Pharmacol. 2000;58:1441–1450. doi: 10.1124/mol.58.6.1441. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD. Importance of hepatic induction of constitutive androstane receptor (CAR) and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab. Dispos. 2007;35:1806–1815. doi: 10.1124/dmd.107.015974. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Endo Y, Mashino M, Kuroiwa M, Ohara A, Jarukamjorn K, Nemoto N. Regulation of the expression of two female predominant CYP3A mRNAs (CYP3A41 and CYP3A44) in mouse liver by sex and growth hormones. Arch. Biochem. Biophys. 2002;404:234–242. doi: 10.1016/s0003-9861(02)00329-6. [DOI] [PubMed] [Google Scholar]
- Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem. J. 2007;401:735–741. doi: 10.1042/BJ20061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Santoyo A, Hernandez M, Albores A, Cebrian ME. DDT increases hepatic testosterone metabolism in rats. Arch. Toxicol. 2005;79:7–12. doi: 10.1007/s00204-004-0603-y. [DOI] [PubMed] [Google Scholar]
- Slatter JG, Templeton IE, Castle JC, Kulkami A, Rushmore TH, Richards K, He Y, DAi X, Cheng OJ, Caguyong M, Ulrich RG. Compendium of gene expression profiles comprising a baseline model of the human liver drug metabolism transcriptome. Xenobiotica. 2006;36:938–962. doi: 10.1080/00498250600861728. [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonylphenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ. Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoshi P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J. Biol. Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, Sueyoshi T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase IAI gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- Suino K, Peng L, Reynolds R, Li Y, Cha JY, Repa JJ, Kliewer SA, Xu HE. The nuclear xenobioitic receptor CAR: structural determinants of constitutive activation and heterodimerization. Mol. Cell. 2004;16:893–905. doi: 10.1016/j.molcel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Van der Hoeven TA, Coon MJ. Preparation and properties of partially purified cytochrome P450 and NADPH-cytochrome P450 reductase from rabbit liver microsomes. J. Biol. Chem. 1974;249:6302–6310. [PubMed] [Google Scholar]
- Vyhlidal CA, Gaedigk R, Leeder JS. Nuclear receptor expression in fetal and pediatric liver: correlation with CYP3A expression. Drug Metab. Dispos. 2006;34:131–138. doi: 10.1124/dmd.105.005967. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J. Biol. Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc. Natl. Acad. Sci. USA. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacog. J. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Wilson VS, McLachlan JB, Falls JG, LeBlanc GA. Alteration in sexually dimorphic testosterone biotransformation profiles as a biomarker of chemically induced androgen disruption in mice. Environ Health Perspect. 1999;107:377–384. doi: 10.1289/ehp.99107377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, Waxman DJ. Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4a-deficient mice. Mol Endocrinol. 2004;18:1975–1987. doi: 10.1210/me.2004-0129. [DOI] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nussler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003;38:978–988. doi: 10.1053/jhep.2003.50393. [DOI] [PubMed] [Google Scholar]
- Wortham M, Czerwinski M, He L, Parkinson A, Wan YJ. Expression of constitutive androstane receptor, hepatic nuclear factor 4a, and P450 oxidoreductase genes determines interindividual variability in basal expression and activity of a broad scope of xenobiotic metabolism genes in the human liver. Drug Metab Dispos. 2007;35:1700–1710. doi: 10.1124/dmd.107.016436. [DOI] [PubMed] [Google Scholar]
- Xiong H, Yoshinari K, Brouwer KL, Negishi M. Role of constitutive androstane receptor in the in vivo induction of Mrp3 and CYP2B1/2 by phenobarbital Drug Metab. Dispos. 2002;30:918–923. doi: 10.1124/dmd.30.8.918. [DOI] [PubMed] [Google Scholar]
- Yamada H, Gohyama N, Honda S, Hara T, Harada N, Oguri K. Estrogen-dependent regulation of the expression of hepatic Cyp2b and 3a isoforms: assessment using aromatase-deficient mice. Toxicol. Appl. Pharmacol. 2002;180:1–10. doi: 10.1006/taap.2002.9366. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Sueyoshi T, Moore R, Negishi M. Nuclear receptor CAR as a regulatory factor for the sexually dimorphic induction of CYP2B1 gene by phenobarbital in rat livers. Mol Pharmacol. 2001;59:278–284. doi: 10.1124/mol.59.2.278. [DOI] [PubMed] [Google Scholar]
- Yoshinori K, Kobayashi K, Moore R, Kawamoto T, Negishi M. Identification of the nuclear receptor CAR: HSP90 complex in mouse liver and recruitment of protein phosphotase 2A in response to phenobarbital. FEBS Lett. 2003;548:17–20. doi: 10.1016/s0014-5793(03)00720-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1 & 2: Cyps showing little or no change following treatment with NP or TCPOBOP in wild-type or CAR-null male and female mice. QPCR was performed as described in the Materials and Methods. On the X-axis, 0, 50, 75 refers to treatment with NP and TC refers to treatment with TCPOBOP. Data are expressed as mean ± SD (n = 5–6) for each of the different P450s in males and females. An asterisk indicates a significant difference from the untreated wild-type or CAR-null mice (p < 0.05). Statistical significance was determined by ANOVA followed with Fisher’s PLSD as the post-hoc test in NP-treated mice compared to their corresponding wild-type or CAR-null controls. Statistical significance was determined by Student’s t-tests in TC-treated mice compared to their corresponding wild-type or CAR-null contr