Abstract
Adult meadow voles (Microtus pennsylvanicus) are solitary in the spring–summer reproductive season, but during winter months, females and males are socially tolerant and aggregate in groups. This behavioral difference is triggered by day length: female meadow voles housed in short, winter-like day lengths form same-sex partner preferences, whereas those housed in long, summer-like day lengths are less social. The present study demonstrates that same-sex social attachments in short day lengths are not exclusive; females formed concurrent attachments with more than one individual, and with non-kin as well as siblings. Partner preferences between females were established within one day of cohousing and did not intensify with greater durations of cohabitation. Males also formed same-sex social attachments, but unlike female affiliative behavior, male partner preferences were not significantly affected by day length. These data are discussed in the context of field behavior and the physiological mechanisms supporting social behavior in voles.
Keywords: social behavior, partner preference, prosocial, sex differences, day length, affiliation, group-living, rodent
1. INTRODUCTION
The social and reproductive systems of arvicoline (formerly microtine) rodents are diverse, including social monogamy, polygamy, and territoriality among females, males, or both sexes [1]. Neurobiological analysis of social behavior of the genus Microtus has focused primarily on prairie voles, a socially monogamous species that forms long-lasting attachments with an opposite sex partner [2,3]. In contrast, meadow voles (Microtus pennsylvanicus), in common with the vast majority of rodents, are polygamous and display multiple paternity within litters [4–6]. During winter months in the field, or when housed in short day lengths (SDs) in the laboratory, meadow voles become non-reproductive and shift their behavior to a social phenotype. Non-sexual social behavior forms the basis for group living in many species [7] and is an important component of complex societies. Investigations of meadow vole social behavior may yield insights into the mechanisms that support sociality outside of reproductive contexts.
Among field populations of meadow voles in several habitats, the reproductive season is characterized by female maintenance of exclusive territories that rarely overlap those of other females [4, 8]. Male home ranges in spring and summer are larger than those of females, and substantially overlap those of other males, as well as multiple females [4, 8, 9]. Summer contact between males and females is limited to reproductive activity, as suggested by the absence of captures of opposite sexed adult meadow voles in a single trap [8], an event that occurs regularly in socially monogamous prairie voles [2]. In winter, meadow vole home ranges contract and those of multiple individuals overlap substantially. Social groups formed at the end of the breeding season typically are initially comprised of a female and her most recent offspring, with immigrant males joining the group throughout the fall [10]. Females also migrate during this period [11], and by late December to early January, social constellations no longer represent family lineages [10]. These mixed-sex groups consist of 3–10 voles that sleep in clusters of 2–3 [12].
Seasonal changes in meadow vole behavior are concomitant with seasonal changes in reproductive physiology in both sexes, and social behavior appears to be at least partly dependent on the reduced secretion of gonadal steroids. In winter months, male meadow voles caught in the field are typically nonscrotal [13]. Intermale aggression towards unfamiliar individuals increases in spring months as the gonads undergo recrudescence [14, 15], and castration of field-caught males reduces intermale aggression [13]. Females housed in winter day lengths have smaller uteri than females housed in summer-like long days (LDs) [16]. These SD females can form selective partner preferences for either a male or female cage mate [16–18], whereas LD females exhibit markedly reduced same-sex social behavior in laboratory partner preference tests [16] and in the field [4, 19]. Exposure to endogenous or exogenous estradiol reduces same-sex huddling behavior in females [16]. Ovariectomy does not increase social behavior in LD female meadow voles, however, suggesting that seasonal differences beyond altered ovarian hormone secretion affect variation in behavior. One contributing factor may be significant differences in oxytocin receptor distributions in the brains of females in LDs versus SDs [17]; oxytocin mediates opposite sex partner preference formation in prairie voles [20] and may affect nonsexual social behavior [21].
Little additional information is available about long-term same-sex attachments in meadow voles. The present experiments characterize several aspects of same-sex social bond formation, including the role of gender and day length, the capacity to form concurrent attachments to multiple partners, the influence of kinship between partners, and the effects of duration of cohabitation.
Specific male-male social preferences have rarely been documented in rodents [22]. Because male meadow voles aggregate with other males in winter [12], and SD-housed males display equal preferences for the odors of SD males and females [23], we speculated that SD males might form specific social attachments with other males. Male meadow voles are considered less territorial than females [1, 5] but do engage in agonistic encounters with neighbors in summer months [14, 19]; seasonal agonism is particularly directed towards unfamiliar individuals [15], but is less pronounced in males than females [19, 24]. As there was no basis for an expectation that LD males would be as antisocial as LD females, we tested the hypothesis that males more readily form partner preferences with each other than do females during summer-like LDs.
The typical winter social group of up to 10 meadow voles [12] provides an opportunity for the formation of multiple social attachments. However, Parker & Lee [18] reported that the female meadow vole does not form a new social bond after her original female partner is removed from their communal cage. In their study, exposure to the first and second partner occurred consecutively in adulthood. This leaves open the possibility that multiple attachments in meadow voles can occur under other circumstances, such as when the potential partners are available concurrently. Same-sex social preferences have previously been demonstrated only between meadow vole littermate pairs. We determined whether more than one bond can form between littermates, as well as whether multiple bonds could form between non-kin.
Finally, we examined the effects of different cohousing intervals on partner preference formation, in order to determine minimum exposure intervals that preferentially promote the formation of same-sex bonds and whether duration of exposure affects the strength of the partner preference.
2. METHODS
2.1. Animals
Our breeding stock was originated from meadow voles generously supplied by Michael Ferkin of the University of Memphis and Zuoxin Wang of Florida State University. Breeding pairs were continuously cohoused in LDs (14:10 light:dark cycle). Offspring were weaned as singletons, pairs, or trios, as described below, and transferred to SDs (10:14 light:dark cycle) or maintained in LDs. Dark onset was 16:00 PST in both photoperiods. Voles were housed in clear plastic cages (48 × 25 × 15 cm) furnished with pine bedding, paper nest chambers (Shepherd Shacks, Shepherd Specialty Papers, Kalamazoo, MI), cotton nesting material (Nestlets, Ancare, Bellmore, NY), and opaque plastic refuge tubes. Breeders were housed in opaque cages containing a plastic nest box. Food (mouse chow no. 5015, Purina Mills, St. Louis MO) and tap water were available ad libitum. Ambient temperature was 21 ± 1°C. Animal care and experimental procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley.
2.2. Behavioral Testing
Tests were conducted as described in Beery et al. [16]. Briefly, the apparatus consisted of three plastic cages: a rear chamber connected by separate tubes to two front chambers. One member of each test-pair was designated the focal (untethered) vole. On the day of the behavioral test, the other member of the co-housed test-pair (the partner) and an unfamiliar vole from the same treatment condition (the stranger) were tethered in separate front chambers. Tethers were affixed to the chamber lids and permitted movement of the tethered vole throughout half the chamber. The positions of the partner and stranger (left versus right chamber) were alternated between tests of each type. Tethered voles were acclimated to the chamber for 5 min before the focal animal was placed in the rear chamber and allowed to move freely for the duration of the 3 hour test. Apparatuses were washed thoroughly after each contact with voles.
Continuous digital footage of social tests was recorded in MPEG format using a video camera (Sony DCR-SR42). Experimenters were absent from the test room during recording of social behavior. Video files were scored at 4x speed by an experimenter using a custom program to record counts and durations of presence in each chamber and of huddling, defined as side-by-side contact of the focal and tethered voles. Descriptions of interactions were recorded as annotations at the end of each file. Scoring was conducted without knowledge of treatment groups.
2.3. Experimental Design
Experiment 1 assessed the degree of same-sex partner preference formation in male meadow voles housed in LDs and SDs. Forty LD-born males were weaned at 19–20 days of age as sibling pairs and transferred to SDs or maintained in LDs (n = 10 pairs/day length). Partner preference tests were conducted between 80–100 days on a focal vole provided access to its cage-mate (the partner) and an unfamiliar male (the stranger) of similar age that also had been cohoused in a same-sex pair.
Experiment 2 tested whether females can form social bonds with more than one individual concurrently. Fifty-four female meadow voles were weaned into groups of three individuals (18 trios). Nine trios were comprised of littermates, as in previous tests of same-sex partner preference [16, 18], and 9 trios were assembled by cohousing females from three different litters weaned on the same date (final n = 8 trios; data from one behavioral test were discarded after a vole became un-tethered). Focal females were tested for partner preference between 80–100 days of age with a randomly selected member of the trio and a stranger. A subset of focal females (n = 7) was given a second partner preference test one week later, in which the focal female was retested with her other cage-mate and a second novel stranger.
Experiment 3 assessed the time course of same-sex social bond formation. 52 female meadow voles were weaned into solo housing in SDs and paired at various intervals (n = 9 pairs/group, except 7 pairs in the 6 h group). Pairings between non-sibling females of the same age were in effect for 1.5 months, 2 weeks, 1 week, 1 day, and 6 hours prior to behavioral testing at 72±2 days of age. A control group consisted of unpaired females of the same age tested with two strangers.
2.4. Data analysis
Total time spent huddling with the partner versus stranger was compared within each treatment group using t-tests assuming unequal variances; groups that huddled significantly more with the partner than the stranger were considered to exhibit a partner preference. Partner preference in individual trials was inferred when the focal vole spent at least twice as much time in side-by-side contact with the familiar as with the unfamiliar vole (as in prior studies [16, 18]). Effects of day length (LD or SD) were also analyzed using t-tests assuming unequal variance.
Differences between more than two treatment groups were analyzed by ANOVA. Significant ANOVAs were followed by pairwise comparisons using Tukey’s HSD. Statistical analyses were performed using JMP 7.0 (SAS Institute Inc., Cary, NC). Means ± SEM are reported throughout.
Data from a previous study [16] involving LD and SD female pairs, cohoused as littermates from weaning and tested in the same apparatus as in the present study, are shown for comparison in multiple figures. The period of data collection overlapped for these studies.
3. RESULTS
3.1. Experiment 1: social preference formation in males
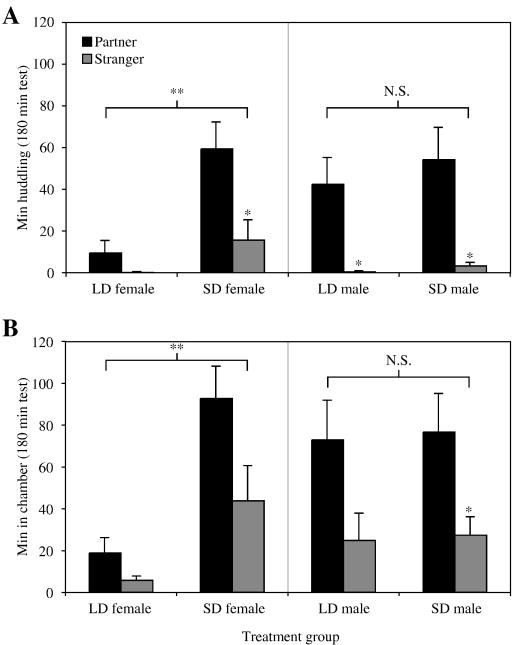
Both LD and SD males huddled extensively with their cage-mates, and displayed a significant preference for huddling with their partner over an unfamiliar male (each p < 0.05; Fig. 1A, right panel). Unlike females (Fig. 1A,B left panels), males showed no significant differences as a function of day length (LD vs. SD) in total or partner-specific huddling time, or time spent in vole-occupied chambers (Fig. 1A, B right panels). In both day lengths, males spent little time huddling with strangers. Stranger-directed huddling behavior was not of significantly longer duration in SD males than LD males (p = 0.17) but occurred somewhat more frequently; 5/10 focal males in SDs huddled with the stranger compared to 1/10 males in LDs (p = 0.07, Fisher’s exact test).
Fig 1.
Male (right panels) and female (left panels) same-sex behavior huddling. Data for females (shown for comparison) have been previously published [16]. (A) Mean (± SEM) time focal voles spent huddling with a same-sex partner (■) or stranger ( ) during a 3 h test. (B) Mean time focal voles spent in the same chamber as the partner or the stranger during a 3 h test. Asterisks above the grey bars denote significant within-group differences in time spent with partners versus strangers. Asterisks above the brackets denote significant differences in total huddling or chamber time between bracketed groups. Males in both long and short day lengths exhibited significant partner preferences, and male behavior did not differ between day lengths although SD males were somewhat more likely than LD males to huddle with strangers (p < 0.06). n = 10/group, *: p < 0.05, **: p < 0.01, N.S.: not significant.
) during a 3 h test. (B) Mean time focal voles spent in the same chamber as the partner or the stranger during a 3 h test. Asterisks above the grey bars denote significant within-group differences in time spent with partners versus strangers. Asterisks above the brackets denote significant differences in total huddling or chamber time between bracketed groups. Males in both long and short day lengths exhibited significant partner preferences, and male behavior did not differ between day lengths although SD males were somewhat more likely than LD males to huddle with strangers (p < 0.06). n = 10/group, *: p < 0.05, **: p < 0.01, N.S.: not significant.
3.2. Experiment 2: concurrent preference formation with multiple partners
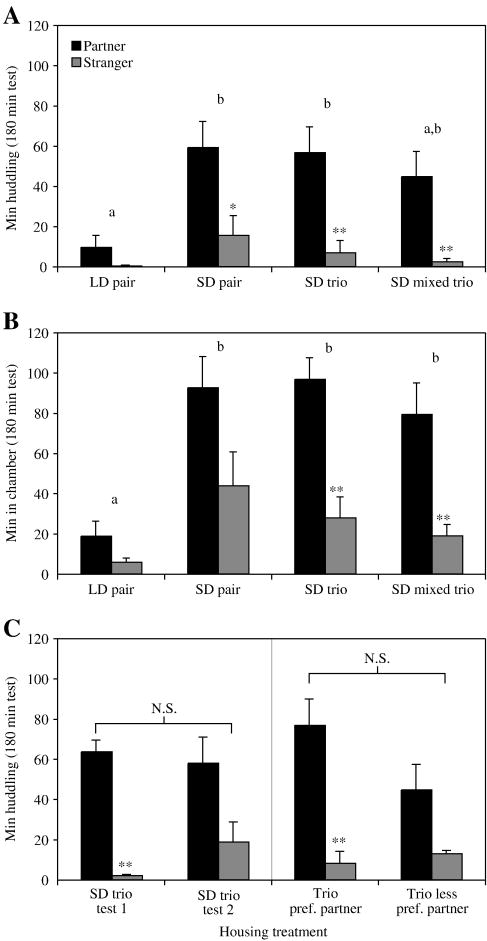
Female meadow voles housed in SDs in same-sex trios of littermates exhibited selective partner preferences for cage-mates over strangers when one of the cage-mates was randomly selected as the partner in behavioral tests (p < 0.01, t-test, “SD trio” in Fig. 2A). Total huddling time of trio-housed SD females was indistinguishable from that of SD females housed in same-sex pairs from weaning (i.e., with only one female cage-mate, “SD pair” in Fig.2A).
Fig 2.
Comparison of behavior of trio-housed voles from either a single litter (SD trio) or three distinct litters (SD mixed trio) to pair-housed voles. (A) Mean (± SEM) time focal females spent huddling with a same-sex partner (■) or stranger ( ) during a 3 h test. Focal voles from both same-litter and mixed-litter trios displayed significant partner preferences for a randomly selected cage-mate. (B) Mean time focal females spent in the same chamber as the partner or the stranger during a 3 h test. Time spent in occupied chambers by voles of both trio types was indistinguishable from the behavior of SD pairs, and significantly different from LD pairs. (C) Focal voles from 7 trios were tested one week after the initial test (test 1) but with the second cage mate (test 2). The left panel displays huddling data from these voles in tests 1 and 2. Total huddling and partner specific huddling did not differ between tests 1 and 2, although voles displayed a non-significant tendency to huddle with the stranger more during the second test (p = 0.19). The right panel displays the same 14 tests divided into groups based on whether the test was the one in which a given focal vole huddled with its partner more (Trio pref. partner) or less (Trio less pref. partner) than in the other test for that focal vole. Even after sorting of paired tests, no significant difference in preference for the two partners was detectable. Asterisks denote significant differences within groups (between partners and strangers). Letters denote differences between treatment groups — groups with the same letter are not statistically different (ANOVA followed by Tukey’s HSD). The letters N.S above a bracket indicate no significant differences in total huddling times between groups. *: p < 0.05, **: p < 0.01.
) during a 3 h test. Focal voles from both same-litter and mixed-litter trios displayed significant partner preferences for a randomly selected cage-mate. (B) Mean time focal females spent in the same chamber as the partner or the stranger during a 3 h test. Time spent in occupied chambers by voles of both trio types was indistinguishable from the behavior of SD pairs, and significantly different from LD pairs. (C) Focal voles from 7 trios were tested one week after the initial test (test 1) but with the second cage mate (test 2). The left panel displays huddling data from these voles in tests 1 and 2. Total huddling and partner specific huddling did not differ between tests 1 and 2, although voles displayed a non-significant tendency to huddle with the stranger more during the second test (p = 0.19). The right panel displays the same 14 tests divided into groups based on whether the test was the one in which a given focal vole huddled with its partner more (Trio pref. partner) or less (Trio less pref. partner) than in the other test for that focal vole. Even after sorting of paired tests, no significant difference in preference for the two partners was detectable. Asterisks denote significant differences within groups (between partners and strangers). Letters denote differences between treatment groups — groups with the same letter are not statistically different (ANOVA followed by Tukey’s HSD). The letters N.S above a bracket indicate no significant differences in total huddling times between groups. *: p < 0.05, **: p < 0.01.
Trios formed at weaning with unrelated individuals from 3 different litters also formed significant partner preferences (p < 0.01, t-test, “SD mixed trio” in Fig. 2A), and their preferences were indistinguishable from those of same-litter SD pairs and trios. The total time both SD trio types spent in vole-occupied chambers was significantly greater than that of LD pairs (ANOVA followed by Tukey’s HSD; Fig. 2B). 7/8 focal females in the same-litter trio condition and 7/9 females in the mixed-litter trio condition spent at least twice as much time huddling with the partner than the stranger, indicative of a preference for that cage-mate.
Seven trios were re-tested one week after the initial partner preference test. In the repeat test the original focal vole was offered a choice between its other cage-mate and a novel stranger. Neither total time huddling nor huddling time with the partner differed between tests 1 and 2 (Fig. 2C, left panel); voles huddled extensively in tests with either cage-mate, with a non-significant increase in huddling with the stranger in the second test (p = 0.19).
The possibility remains that each focal vole formed a preference for only a single cage-mate, and the first and second tests did not differ because an equal proportion of the group preferred the first partner as preferred the second partner. This can be examined by considering the trials for each vole by ranked preference (sorting the more preferred and less preferred partner for each focal vole into two groups) rather than by the order of testing (test 1 vs. test 2). This view (Fig. 2C, right panel) reveals no significant difference in total huddling time (p = 0.26) or huddling time with partners (p = 0.13) even after opportunity for such a difference to appear is maximized.
3.3. Experiment 3: time required for partner preference formation
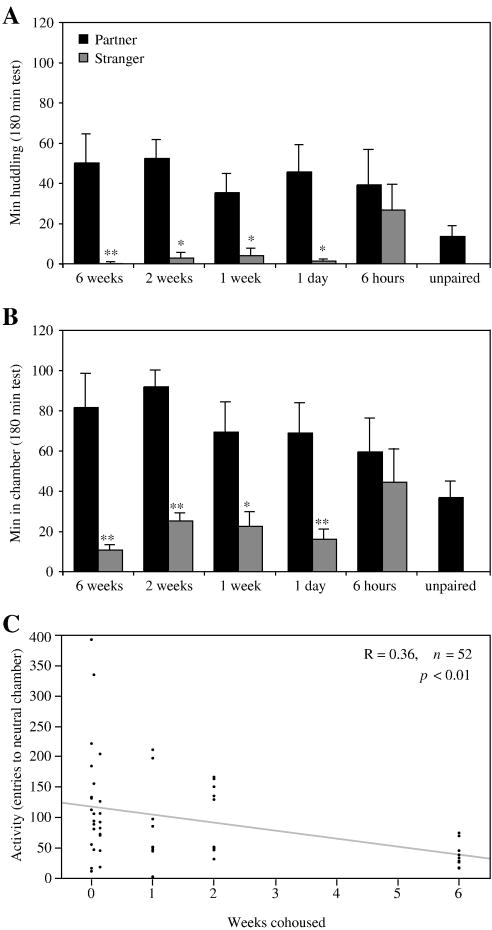
Pairs of unrelated females were tested for partner preference formation after 6 weeks, 2 weeks, 1 week, 1 day, or 6 hours of cohabitation with a same-aged partner (Fig. 3). An unpaired control group was offered a choice of two unfamiliar females from different litters. Cohousing durations of 1 day or longer resulted in significantly greater huddling with the partner than the stranger (Fig. 3A), and more time spent in the partner’s than the stranger’s chamber (Fig. 3B). Total huddling times and partner huddling times did not differ between cohousing durations treated as separate groups (ANOVA, p = 0.53), nor by duration of cohousing (linear regression, p = 0.23)
Fig 3.
Social behavior following different cohousing durations. Females voles were cohoused for 6 weeks, 2 weeks, 1 week, 1 day, 6 hours, or were not cohoused (unpaired) prior to the preference test. (A) Mean (± SEM) time focal voles spent huddling with a same-sex partner (■) or stranger ( ) during a 3 h test. Unpaired voles were presented with two strangers and the mean huddling time is displayed. (B) Mean time focal voles spent in the same chamber as the partner or the stranger during a 3 h test. Asterisks above the grey bars denote significant differences within groups (between partners and strangers). All cohousing durations of 1 day or longer resulted in significant partner preference formation. Voles cohoused for 6 hours spent significantly more total time huddling than unpaired voles (p < 0.05, t-test). (C) Activity was quantified by the number of times the focal vole entered the central neutral empty chamber from either side chamber that contained a tethered vole. Focal voles were most active in tests after shorter cohousing durations (p < 0.01, linear regression). Activity continued to decrease with longer cohousing periods, despite the lack of change in overall huddling times. *: p < 0.05, **: p < 0.01.
) during a 3 h test. Unpaired voles were presented with two strangers and the mean huddling time is displayed. (B) Mean time focal voles spent in the same chamber as the partner or the stranger during a 3 h test. Asterisks above the grey bars denote significant differences within groups (between partners and strangers). All cohousing durations of 1 day or longer resulted in significant partner preference formation. Voles cohoused for 6 hours spent significantly more total time huddling than unpaired voles (p < 0.05, t-test). (C) Activity was quantified by the number of times the focal vole entered the central neutral empty chamber from either side chamber that contained a tethered vole. Focal voles were most active in tests after shorter cohousing durations (p < 0.01, linear regression). Activity continued to decrease with longer cohousing periods, despite the lack of change in overall huddling times. *: p < 0.05, **: p < 0.01.
After 6 hours of cohousing, females spent significantly more time huddling than did controls (p < 0.05, t-test) but 4/7 meadow voles huddled more with the stranger than the partner. Even among voles that huddled more with their partners, only two huddled at least twice as much with the partner as with the stranger. Likewise, unpaired control females who huddled during tests with two strangers did so with both females, in some cases distributing their time evenly between the two voles and in others huddling predominantly with one stranger. Social attachments may begin to form during the first hours of cohousing, but they do not manifest as partner preferences during this time.
Although cohousing durations of 1 day to 1.5 months all induced partner preferences and equivalent amounts of total huddling, exploratory behavior varied with the duration of cohousing. Meadow voles that cohabited for 0 days or 6 hours explored most extensively, as measured by number of entries into the central chamber, whereas those cohoused for 6 weeks showed the least exploratory activity (Fig. 3C, p < 0.01).
4. DISCUSSION
4.1 Male behavior
Male meadow voles exhibited strong partner preferences for cage-mates over unfamiliar males, demonstrating specific same-sex affiliation. Specific social relationships between males are a common feature of many primate species [26], as well as a variety of other groups including alliances and coalitions in large carnivores [27–30] and cetaceans [31, 32], as well as bachelor groups in ungulate species [33, 34]. Male prairie voles occasionally cohabitate in the field and can form same-sex partner preferences in the laboratory [22]; explicitly tests of social preference formation have not often been attempted, but social bonds might be expected between males of other social species where groups contain multiple males. Male-male bonds likely facilitate group cohesion in winter months, and male pairs are overrepresented in nesting constellations [10]. In long day lengths, male bonds might be a byproduct of the capacity to form social bonds in short day lengths, with females [17], or with their own offspring [35–37].
Somewhat surprisingly, partner preferences between males did not vary significantly with day length. In field tests of social behavior, male meadow voles behaved more aggressively towards unfamiliar same-sex individuals as summer approached and gonadal development progressed, although aggression towards nestmates did not increase [15]. In a separate experiment, agonism in LD–housed and summer-caught males was greater towards males with a familiar odor than towards unfamiliar males [24], suggesting that the “dear enemy” effect [38] is not operative in male meadow voles. Degree of familiarity may affect the extent of social behavior. Male cage-mates were cohoused in pairs from weaning in this study; this amount of cohabitation is unlikely to occur during summer day lengths in nature, as males disperse from their natal nest and would not remain in contact with a littermate unless they coincidentally dispersed to overlapping territories. Consequently, greater sociability of LD males relative to LD females cohoused under the same circumstances may be more indicative of the LD female’s extreme territoriality than of tolerance among LD males. Males housed in LDs were somewhat less likely than SD males to huddle with a stranger, as expected, but again this difference was less pronounced than in females.
4.2 Concurrent attachments in females and attachments to non-kin
Female partner preferences formed in SDs were previously demonstrated to be highly selective [16, 18], but their exclusivity was unknown. Experiment 2 demonstrated that SD females can form more than one concurrent social attachment. If the focal meadow vole had only formed an attachment to one of her cage-mates, in half the tests the randomly selected partner would be the other cage-mate, and the focal vole would not be expected to huddle with this partner. In that scenario, the average partner huddling time should be close to half that of SD pairs. Instead, SD sibling trios showed huddling equivalent to that of SD sibling pairs, and on re-test with the second cage-mate and a new stranger they displayed the same level of huddling with the second partner. Finally, there were no significant differences between the less and more preferred partners in the trio, confirming formation of multiple equivalent social bonds.
Because all prior demonstrations of same-sex social bonding in female meadow voles were with littermates [16, 18] we considered the possibility that partner preferences do not reflect novel attachment, per se, but rather an olfactory preference for the odor of kin over non-kin. If that were the case, sibling trios would prefer each of their partners over a stranger, but non-sibling trios might display a preference for only one partner or for neither. This possibility can be discounted on the basis of the behavior of focal voles in mixed-litter trios, who formed specific social attachments for both non-sibling cage-mates. In prior studies of meadow voles, recognition of kin has appeared to result from familiarity rather than phenotype matching on an olfactory cue [39–41] although this has not been studied exhaustively [42]. Evidently the familiarity established with a non-sibling partner post-weaning is sufficient for social bond formation.
The discovery that meadow voles can form multiple social bonds is compatible with field data on group size, but would not be inferred from prior laboratory studies. Female meadow voles did not display partner preferences for a novel female after 10 days of cohousing instituted three weeks after separation from their former cage and littermate [18]. The failure to bond with the second cage-mate in that circumstance could reflect an insufficient duration of cohousing with the second partner (unlikely in light of the data from Experiment 3); or perhaps social preferences do not form easily between adult females, as they most often cohabit with female offspring or siblings and are joined by non-kin males (also unlikely in light of data from Experiment 3). Alternatively, temporal factors may account for this observation. In the field, social groups close to new membership some time after they are formed [15, 43], which might be mediated by the onset of refractoriness to SDs and concomitant increase in estradiol secretion [16]. A further explanation may be that a first set of bonds precludes future bonds. This appears to be the case in prairie voles, as aggression toward unfamiliar conspecifics of both sexes increases after mating and pair-bond formation [44]. It remains unknown whether prairie voles in the laboratory can form attachments with multiple opposite-sex partners present concurrently.
4.3 Timecourse
Not only do same-sex preferences develop between multiple loosely related meadow voles, but they can form quite rapidly. Experiment 3 demonstrated that the extent of partner preference did not differ among pairs of females cohoused for 1 day, 1 week, 2 weeks, or 1.5 months. Significant partner preferences were not formed during a 6 hour cohousing interval, and the majority of SD control females huddled with one or both strangers. Thus, the time course of same-sex social bond formation in meadow voles is similar to same- and opposite-sex social bond formation in prairie voles in the absence of mating [22, 45, 46]. The demonstration of rapid formation of social bonds between SD females will enable future studies that manipulate oxytocin over a period of a few days to assess the effects of this peptide on non-sexual affiliative behavior.
Although the formation of specific partner preferences in adult voles was first identified in the context of monogamy in prairie voles, it is not surprising that non-sexual social bonds develop between adult peers in species that cohabit in groups. In meadow voles, these bonds form with both kin and non-kin, between same-sex individuals of both sexes, and concurrently with multiple individuals. Significant preferences for familiar individuals form in less than a day, and the conditions under which they develop echo field data — females and males cohabit in groups in the winter, and in summer females become highly territorial. The establishment of these parameters of social preferences in meadow voles informs our understanding of the requirements for social bond formation, and lays the groundwork for studies of the underlying biological mechanisms of this type of affiliation.
Acknowledgments
We are grateful to Michael Ferkin and to Zuoxin Wang for providing the meadow voles used to develop our colony, and to Christiana Tuthill for laboratory management. Elanor Schoomer and Erica Maulhardt assisted with the care and maintenance of breeding voles, and Joseph Driscoll assisted with behavioral scoring. This research was supported by NIH grant MH-61171 and a NDSEG fellowship to A. Beery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boonstra R, Krebs CJ, Gaines MS, Johnson ML, Craine ITM. Natal philopatry and breeding systems in voles (Microtus spp) J Anim Ecol. 1987;56:655–673. [Google Scholar]
- 2.Getz LL. Social structure and aggressive behavior in a population of Microtus pennsylvanicus. J Mammal. 1972;53:310–317. [Google Scholar]
- 3.Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim Behav. 2008;75:1143–1154. [Google Scholar]
- 4.Madison DM. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol. 1980;7:65–71. [Google Scholar]
- 5.Boonstra R, Xia X, Pavone L. Mating system of the meadow vole, Microtus pennsylvanicus. Behav Ecol. 1993;4:83–89. [Google Scholar]
- 6.Berteaux D, Bety J, Rengifo E, Bergeron JM. Multiple paternity in meadow voles (Microtus pennsylvanicus): investigating the role of the female. Behav Ecol Sociobiol. 1999;45:283–291. [Google Scholar]
- 7.Lacey EA, Sherman PW. The ecology of sociality in rodents. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological & evolutionary perspective. Chicago: University of Chicago Press; 2007. pp. 243–54. [Google Scholar]
- 8.Webster AB, Brooks RJ. Social behavior of Microtus pennsylvanicus in relation to seasonal changes in demography. J Mammal. 1981;62:738–751. [Google Scholar]
- 9.Ostfeld RS, Pugh SR, Seamon JO, Tamarin RH. Space use and reproductive success in a population of meadow voles. J Anim Ecol. 1988;57:385–394. [Google Scholar]
- 10.Madison DM, Fitzgerald RW, McShea WJ. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav Ecol Sociobiol. 1984;15:9–17. [Google Scholar]
- 11.Baird DD, Birney EC. Characteristics of dispersing meadow voles Microtus pennsylvanicus. Am Midl Nat. 1982;107:262–283. [Google Scholar]
- 12.Madison DM, McShea WJ. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles - a microtine model. Am Zool. 1987;27:899–908. [Google Scholar]
- 13.Turner BN, Iverson SL, Severson KL. Effects of castration on open-field behavior and aggression in male meadow voles (Microtus pennsylvanicus) Can J Zool. 1980;58:1927–1932. [Google Scholar]
- 14.Turner BN, Iverson SL. The annual cycle of aggression in male Microtus pennsylvanicus, and its relation to population parameters. Ecology. 1973;54:967–981. [Google Scholar]
- 15.McShea WJ. Social tolerance and proximate mechanisms of dispersal among winter groups of meadow voles, Microtus pennsylvanicus. Anim Behav. 1990;39:346–351. [Google Scholar]
- 16.Beery AK, Loo TJ, Zucker I. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm Behav. 2008;54:153–159. doi: 10.1016/j.yhbeh.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker KJ, Phillips KM, Kinney LF, Lee TM. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115:1349–1356. [PubMed] [Google Scholar]
- 18.Parker KJ, Lee TM. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J Comp Psychol. 2003;117:283–289. doi: 10.1037/0735-7036.117.3.283. [DOI] [PubMed] [Google Scholar]
- 19.Ferkin MH, Seamon O. Odor preference and social behavior in meadow voles, Microtus pennsylvanicus: seasonal differences. Can J Zool. 1987;65:2931–2937. [Google Scholar]
- 20.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 21.Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- 22.DeVries AC, Johnson CL, Carter CS. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster) Can J Zool. 1997;75:295–301. [Google Scholar]
- 23.Ferkin MH, Gorman MR. Photoperiod and gonadal hormones influence odor preferences of the male meadow vole, Microtus pennsylvanicus. Physiol Behav. 1992;51:1087–1091. doi: 10.1016/0031-9384(92)90098-m. [DOI] [PubMed] [Google Scholar]
- 24.Ferkin MH. Seasonal differences in social behavior among adult and juvenile meadow voles, Microtus pennsylvanicus. Ethology. 1988;79:116–125. [Google Scholar]
- 25.Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 26.Van Hooff JAREM, Van Schaik CP. Male bonds - affiliative relationships among nonhuman primate males. Behaviour. 1994;130:309–337. [Google Scholar]
- 27.Packer C, Pusey AE. Cooperation and competition within coalitions of male lions: kin selection or game theory? Nature. 1982;296:740–742. [Google Scholar]
- 28.Caro TM, Collins DA. Male cheetah social organization and territoriality. Ethology. 1987;74:52–64. [Google Scholar]
- 29.Zabel CJ, Glickman SE, Frank LG, Woodmansee KB, Keppel G. Coalition formation in a colony of prepubertal spotted hyenas. In: Harcourt HA, de Waal FBM, editors. Coalitions and alliances in humans and other animals. New York: Oxford University Press; 1992. pp. 112–35. [Google Scholar]
- 30.de Villiers MS, Richardson PRK, van Jaarsveld AS. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus) J Zool. 2003;260:377–389. [Google Scholar]
- 31.Connor RC, Smolker RA, Richards AF. 2 levels of alliance formation among male bottle-nosed dolphins (Tursiops sp.) Proc Nat Acad Sci USA. 1992;89:987–990. doi: 10.1073/pnas.89.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor RC, Heithaus MR, Barre LM. Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance’. Proc Biol Sci. 2001;268:263–267. doi: 10.1098/rspb.2000.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruckstuhl KE, Neuhaus P. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol Rev. 2002;77:77–96. doi: 10.1017/s1464793101005814. [DOI] [PubMed] [Google Scholar]
- 34.Bowyer RT. Sexual segregation in ruminants: Definitions, hypotheses, and implications for conservation and management. J Mammal. 2004;85:1039–1052. [Google Scholar]
- 35.Parker KJ, Kinney LF, Phillips KM, Lee TM. Paternal behavior is associated with central neurohormone receptor binding patterns in meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115:1341–1348. doi: 10.1037//0735-7044.115.6.1341. [DOI] [PubMed] [Google Scholar]
- 36.Parker KJ, Lee TM. Interaction of photoperiod and testes development is associated with paternal care in Microtus pennsylvanicus (meadow voles) Physiol Behav. 2002;75:91–95. doi: 10.1016/s0031-9384(01)00636-9. [DOI] [PubMed] [Google Scholar]
- 37.Ferkin MH. Adult-weanling recognition among captive meadow voles (Microtus pennsylvanicus) Behaviour. 1989;108:114–124. [Google Scholar]
- 38.Fisher J. Evolution and bird sociality. In: Huxley J, Hardy AC, Ford EB, editors. Evolution as a process. London: Allen & Unwin; 1954. pp. 71–83. [Google Scholar]
- 39.Gavish L, Hofmann JE, Getz LL. Sibling recognition in the prairie vole, Microtus ochrogaster. Anim Behav. 1984;32:362–366. [Google Scholar]
- 40.Ferkin MH, Rutka TF. Mechanisms of sibling recognition in meadow voles. Can J Zool. 1990;68:609–613. [Google Scholar]
- 41.Paz-y-Miño CG, Tang-martínez Z. Effects of isolation on sibling recognition in prairie voles, Microtus ochrogaster. Anim Behav. 1999;57:1091–1098. doi: 10.1006/anbe.1999.1082. [DOI] [PubMed] [Google Scholar]
- 42.Holmes WG, Mateo JM. Kin recognition in rodents - critical issues, empirical evidence and model systems. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological & evolutionary perspective. Chicago: University of Chicago Press; 2007. pp. 216–28. [Google Scholar]
- 43.McShea WJ, Madison DM. Communal nesting between reproductively active females in a spring population of Microtus pennsylvanicus. Can J Zool. 1984;62:344–346. [Google Scholar]
- 44.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 45.Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 46.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]