Abstract
Sensory systems adapt to changing environmental influences by coordinated alterations in structure and function. These alterations are referred to as plastic changes. The gustatory system displays numerous plastic changes even in receptor cells. This review focuses on the plasticity of gustatory structures through the first synaptic relay in the brain. Unlike other sensory systems, there is a remarkable amount of environmentally induced changes in these peripheral-most neural structures. The most consistent and largest changes occur to stimuli that also impact on homeostatic systems, especially when the environmental manipulation is instituted during early development.
Introduction
The taste system is inherently plastic. This is due in no small part to the turnover of receptor cells every 10 days in mammals.1 Therefore, even at the location where stimuli first contact this sensory system, there is ample opportunity for experience-induced functional changes. Under the influence of varying environmental factors, different proportions of receptor membrane components can be incorporated as new taste receptor cells are born. Such factors may relate to the hormonal, dietary, and developmental status of the animal. Beyond the receptors, the peripheral and central gustatory systems may have their own capacity for plasticity, reflecting not only receptor cell alterations but also interactions of afferent gustatory signals with other neural programs.
While the primary goal of Edmond Rolls’ review (see p. XXX) is not to address plasticity directly, he provides numerous examples of how experience influences cortical taste responses. The reader of his paper will appreciate the exquisite organization of higher gustatory neural levels and the ability of higher-order gustatory structures to adapt to environmental influences. In contrast, this review will focus on gustatory plasticity in more peripheral structures. In particular, I will emphasize the role of diet in alterations in gustatory function and structure in the peripheral gustatory system and at the first central relay. Readers are encouraged to read other important work that summarizes findings related to topics about gustatory organization, development, and higher-order plasticity.2
Given the inherent plastic nature of the gustatory system, one might expect the literature to be filled with examples of how increased or decreased exposure to specific stimuli through dietary means results in corresponding changes in taste receptor cell function. It is my opinion that this simply is not so. In fact, one of the main points that I wish to emphasize in this review is how most of the manipulations that are effective in altering the neurobiology of gustation in mammals have (and may require) a clear hormonal and/or systemic antecedent. Thus, other physiological systems involved in homeostasis have important influences on plasticity of gustatory function and structure.
Plasticity of Receptor Cell Function in Insects
Some of the best examples of clear changes in taste receptor cell function following selective stimulation derive from work done with insects. For example, Glendinning et al.3,4 demonstrated that selective exposure to caffeine in the caterpillar of Manduca sexta results in a selective attenuated response to that stimulus only in cells that are highly sensitive to “bitter” tasting solutions. No other receptor cell is affected, nor are responses to other bitter stimuli affected. Other examples in which alterations in the environment of insects have direct effects on taste receptor function are also available; the common element is that changes are correlated with survival.5
Plasticity of Receptor Cell Function in Adult Mammals
Early work in which diet was manipulated in order to alter taste receptor cell function often provided conflicting results.6–9 Indeed, a conclusion from these initial studies was that the gustatory system is remarkably resistant to environmental manipulations.9 The most consistent manipulation that was successful in altering peripheral taste responses, however, was dietary sodium depletion. Feeding adult rats a sodium-deficient diet for 10 days selectively decreased NaCl responses in the chorda tympani nerve10 (Figure 1). The parallel between the insect work by Glendinning3,4 and the rat work by Contreras10 is that a single stimulus was altered with a corresponding, specific alteration in taste function. In the case of dietary sodium restriction, there are likely changes in hormonal levels that regulate sodium balance. Specifically, aldosterone should be elevated after 10 days of sodium restriction. Since aldosterone is a potent regulator of epithelial sodium channel (ENaC) function in other sodium-transporting epithelia, it may also have similar effects on altering ENaC function in taste receptor cells. Indeed, ENaCs have an important role in sodium taste transduction in adult rodents.11 However, there is an apparent paradox in taste tissue. In all other tissue with ENaCs, aldosterone increases sodium-driven currents.12 In contrast, an apparent increase in aldosterone decreases sodium-elicited responses in taste receptor cells,10 even though aldosterone appears to increase the amount of the taste response blockable by the pharmacological agent amiloride.13
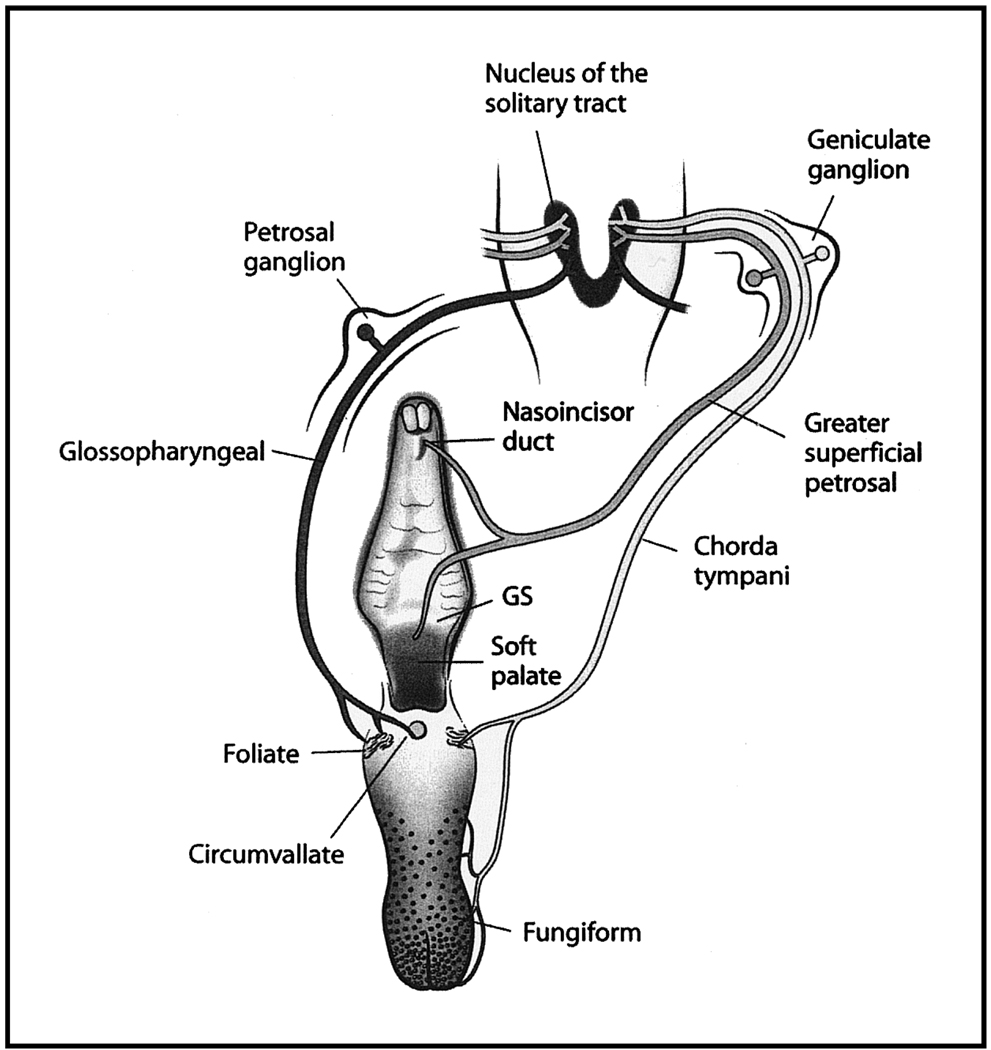
Figure 1.
Organization of the peripheral gustatory system and of the first synaptic relay in the brain, the nucleus of the solitary tract. The tongue and palate are shown with their receptive fields. The chorda tympani nerve innervates taste buds located in fungiform papillae and in the anterior foliate papillae. The glossopharyngeal nerve innervates taste buds in posterior foliate papillae and in the circumvallate papilla. The greater superficial petrosal nerve innervates taste buds in the nasoincisor duct, in the geschmacksstreifen (GS), and on the soft palate. Each nerve has cell soma in the respective ganglia (chorda tympani and greater superficial petrosal nerve in geniculate ganglia and glossopharyngeal nerve in the petrosal ganglia). All three nerves project unilaterally to the nucleus of the solitary tract.
Issues related to low-sodium diets producing attenuated salt taste responses are complicated further by a recent study in which immunohistochemistry for ENaC subunits and whole-cell patch clamp recordings were done in foliate, circumvallate, and fungiform taste buds (Figure 1). Following procedures that elevate aldosterone, there was an increase in immunopositive staining for beta and gamma ENaC subunits in all types of taste buds compared with those of normal rats.14 Interestingly, large changes in staining occurred in circumvallate taste buds, which are usually poorly sensitive to sodium salt stimuli and to the amiloride block14 (Figure 1). Furthermore, there were significant increases in sodium-driven currents recorded by whole-cell patch clamp procedures in aldosterone-treated rats in all populations of taste buds.14 Thus, even though there is a decrease in primary afferent function (at least for the anterior tongue) following dietary sodium depletion, it appears that taste tissue is sensitive to aldosterone. To date, the mechanisms that reconcile these data are not established; however, it may be that an abundance of ENaC is made in response to elevated aldosterone but they are not inserted into the receptor membrane or that they are not functional until phosphorylated.
Plasticity of Brainstem Gustatory Neurons in Adult Mammals
A considerable number of studies have examined the effects of diet and/or metabolic changes on taste responses in the rodent nucleus of the solitary tract (NTS), the first central relay of the gustatory system (see Figure 1). Studies have shown that dietary sodium restriction has significant effects on gustatory coding processes for salt taste in the NTS in the same direction as noted above for chorda tympani nerve alterations.15,16 Therefore, sodium restriction produces attenuated sodium salt responses through the NTS. Other studies have shown that increased blood glucose and insulin have corresponding effects in NTS neurons to selectively decrease responses to sugars.17,18 Additionally, satiety factors serve to modify NTS responses in a specific way.18,19–21 Therefore, it is clear that alterations in digestive physiology have a significant effect on taste responses in neurons located at the first synaptic relay along the taste system. This, in turn, may have significant effects on other physiological systems such as cardiovascular function due to the anatomical proximity of associated neural structures and their circuits with the NTS.22
The association between gut function and taste function in the brain stem can best be illustrated through the use of a conditioned taste aversion paradigm. Chang and Scott23 paired gastrointestinal illness with the taste of saccharin. Following conditioning, the sweet taste elicited larger responses in NTS neurons than in controls, the effect was specific to the stimulus and only affected one group of neurons, and the neurons responded best to “sweet” stimuli. Thus, a previously neutral stimulus became much more effective in eliciting responses in a subset of neurons once the stimulus took on characteristics that were associated with illness and toxicity.
In summary, the most prevalent and clearest examples of where functional plasticity occurs in peripheral or medullary neurons in adults seems to require an association with gut function or regulation. It may be, therefore, that at least lower-level gustatory neurons are plastic not necessarily to limited or enhanced experience, but are plastic primarily when there is an adaptive physiological advantage.
Developmental Manipulations and Their Effects on Gustatory Organization
While there are clear examples of how the adult gustatory system is susceptible to environmental influences, it is during development that the largest and most wide-spread plastic changes occur. Environmental influences have their greatest effect when superimposed on a dynamic, developing gustatory system. The following section examines structural and functional changes following experimental manipulations introduced during development, again with a focus on diet-related alterations.
Developmental Dietary Sodium Restriction and Its Effect on Taste Receptor Cell Function
The largest change in chorda tympani function during normal rat development occurs to sodium- and lithium-salt responses.24–26 In fact, responses to some concentrations increase three-fold from the first through the third week postnatally.24–26 A corresponding increase in ENaC function is the underlying mechanism responsible for such a large increase.27 Therefore, there is an increase in a single transduction pathway accounting for the largest age-related increase in taste function. Given the magnitude of response change with age, we tested the hypothesis that alteration of sodium salt taste stimulation would have significant effects on gustatory development by altering the sodium content of the diet during pre- and postnatal development.
Reducing the sodium content in food to 0.03% beginning before embryonic day 8 (E8) and maintained through at least postnatal day 28 (P28) decreased NaCl responses in the chorda tympani nerve by as much as 60% compared with controls.28–30 Responses to other stimuli were unaffected.28–30 The selective decrease in sodium salt-elicited responses reflects the absence of functional ENaCs.29,31
The period during which the sodium-restricted diet must be initiated to achieve the suppressed responses is before taste buds first appear on the anterior tongue (before E8).30 Therefore, our original hypothesis relating altered gustatory stimulation with altered function (i.e., stimulus-induced plasticity) is not appropriate in this experimental paradigm. Instead, consistent with the examples noted earlier, the severe sodium restriction may be interfering with the development of other physiological systems that impact on the development of ENaC function in gustatory system much later in development. For example, the low-sodium diet may affect the production of aldosterone by the adrenals. Electrolyte levels in the mother’s milk and in the offspring’s blood are not altered as a result of dietary sodium restriction.29,32 It is also unlikely that plasma sodium levels are altered prenatally, 33 further indicating that hormonal and/or growth factors34 play an important role in regulating sodium transport through ENaCs. Moreover, it appears that most of the machinery is in place for sodium taste transduction well before taste responses occur in rats fed a sodium-replete or a sodium-restricted diet.
Taste receptor cells of both sodium-restricted and normal rats are immunopositive for antibodies directed at ENaCs by P1.35 These cells also appear to have normal numbers of transcript available for each of the three subunits that form ENaCs.36 Finally, early postnatal rats fed a sodium-replete diet (not restricted) have sodium-elicited currents in whole-cell patch recordings,37 suggesting that channels are functional in certain portions of taste receptor cells even though sodium responses in primary afferents are nearly absent. These findings collectively indicate that most of the molecular processing occurs in sodium-restricted rats. What appears to be missing are post-translational processes related to insertion into the apical domain (where they contact stimuli) and/or phosphorylation. If most of the molecular processing has occurred with the exception of final steps, it could be predicted that ENaC function in taste cells are especially labile.
Indeed, the dietary effects on the peripheral gustatory system described above are reversible. When a sodium-replete diet is restored in restricted rats at any time postnatally, chorda tympani responses recover to control levels in about 15 days, with a corresponding increase in functional ENaCs.29 A single, 30-mL ingestive bout of physiological saline is also sufficient to recover sodium taste responses,38,39 and this recovery is not dependent on direct stimulation of taste receptor cells with sodium. If sodium-restricted rats are allowed to drink physiological saline sufficient to produce recovery, but are not allowed to absorb sodium due to a pre-injection of the diuretic drug furosemide, they do not recover normal chorda tympani function.38 Therefore, as emphasized earlier for normal development, the sodium transducer is not under control of stimulation of the receptor with sodium, but is probably under control of regulatory factors released following absorption. As with recovery via ingestion of food, there is a period of 10 to 12 days needed for recovery following sodium ingestion. 39 This suggests that post-ingestive effects are targeted toward newly proliferating cells, because functional recovery is seen following a complete cycle of taste receptor cell turnover (i.e., about 10 days).39 It is the newly forming taste receptor cells that have the functional ENaCs and not ones present when “recovery” begins. There is, however, an age-dependent process operational. Recovery is dependent on processes beginning at or beyond the age of weaning, because some of the putative factors present in normal milk are not used by early-restricted rats for recovery of the response.40 Therefore, taste receptor cells have to be developmentally competent to recover sodium responses.
An important qualification regarding taste receptor cell susceptibility is that not all populations of taste receptors are susceptible to early dietary sodium restriction. Taste receptor cells on the palate (Figure 1) are not affected by early sodium restriction.41 Greater superficial petrosal nerve recordings reveal that early sodium-restricted rats respond normally to palatal stimulation with sodium and non-sodium salts.41 Thus, only a restricted population of taste receptors is vulnerable to early NaCl restriction. The bases of the differential susceptibilities are not currently known.
Plasticity of Neuron/Target Matching
Since receptor cell turnover is a normal, ongoing process occurring throughout the lifespan of the animal, it presents a formidable challenge for primary afferent neurons: single primary afferents have continually changing receptive fields. Therefore, these neurons must exhibit a degree of plasticity by either maintaining contact with individual receptor cells throughout the lifespan of the cell and then re-establishing functional contacts with new receptors, or by remodeling their synaptic contacts with existing receptor cells. This is especially profound in the developing animal, in which the receptor cell turnover system is superimposed on the development of gustatory structures. For example, there are age-related processes that determine the correct number of available neurons (e.g., through programmed cell death) as well as factors that determine proper numbers and location of taste buds that may be independent of each other.42 There must then be a match between individual taste buds and the final numbers of innervating neurons.
Developmental studies in rat show that the innervation pattern of single taste buds is relatively static from P10 to P40.43 This is during a developmental period when taste buds grow to match the number of innervating neurons.44 The match is a simple function in which the size of the taste bud is directly related to the number of innervating neurons—the larger the taste bud the more neurons that innervate it.44 The function can be disrupted by dietary sodium restriction begun at E3 and continued throughout the animal’s pre- and postnatal development. 45 The factor primarily responsible for the mismatch is decreased taste bud size and not a change in numbers of innervating neurons.44,45 The decreased taste bud size, in turn, relates to fewer numbers of taste bud cells and changes in taste bud cell kinetics compared with normally fed adults (e.g., lifespan).46 Once sodium-restricted rats are returned to a sodium-replete diet, the match is established,45 indicating that rules governing taste bud size, perhaps as influenced by the number of innervating neurons, are maintained even after a long-term disruption in the taste bud size due to a number of innervating neuron functions. The approach taken in these studies was to examine the match between single taste buds and multiple neurons referred to as innervation patterns. Complementary questions regarding how many taste buds are innervated by single neurons, referred to as receptive fields, have revealed important principles about age-related plasticity in the peripheral gustatory system.
Functionally, the receptive fields of single neurons are modified normally during development. This brings up an interesting hypothesis that taste function shapes how many taste buds become connected to single taste afferents. In developing sheep, there is a selective pruning of receptive fields for neurons that become responsive to sodium stimuli, while the receptive fields of other neuron types remain relatively large.47 These data are important not only from a developmental framework (because they reveal structure/function relationships between taste receptors and afferent neurons during gustatory development), but also because they strongly suggest that such relationships may be modifiable through environmental factors. Such studies await examination.
Plasticity of Central Gustatory Structures Following Developmental Manipulations
Elimination of Taste Receptor Cells During Development
Removal of taste receptors during a period in which connections are being made in the NTS has been used successfully to examine the plasticity of the anatomical organization of primary afferents as they project into the brain. Lasiter et al.48 destroyed taste receptors on the anterior tongue and then examined the terminal field organization of gustatory nerves in the NTS. They found that destruction of taste buds between P2 and P7 permanently reduced the chorda tympani terminal field in the NTS by 30%. This reduction persisted even though taste buds regenerated and even though normal numbers of geniculate ganglion neurons (Figure 1) were present.48 The investigators concluded that the relatively large reduction probably reflects changes in neuronal activity during a sensitive period.48 However, neither peripheral nor central functional responses were recorded in these rats. Nonetheless, it brings up a potential interesting contrast between peripheral function that appears related more to physiological changes than to activity-dependent processes, which may be a mechanism involved in central organization.
Selective Exposure to Taste Stimuli
To directly assess the role of taste-elicited activity on development of terminal fields, Lasiter et al.49,50 adapted the artificial rearing procedures developed by Hall.51 Rat pups were isolated from their mothers by P4, and then fed intragastrically.49,50 Thereafter, they could be selectively exposed to taste stimuli by way of an oral cannula. They found that limited gustatory stimulation was sufficient in producing normal terminal field development in the NTS.49,50 In contrast, water stimulation failed to produce normal NTS terminal fields.50 Therefore, similar to when taste receptors were destroyed,48 rats exposed only to water early in development failed to develop the normal organization of taste inputs into the brain stem. Interestingly, at least 3 days of exposure to 150 mM NaCl begun at P4 resulted in normal terminal fields.50 Therefore, the collective work from Lasiter points to an important role of taste-elicited activity during a time when taste receptors become functional. A drastic alteration in gustatory experience leads to profound changes in NTS development.
Effect of Early Sodium Restriction on NTS Development
In contrast to the morphological and functional effects of early dietary sodium restriction on peripheral gustatory development (see “Plasticity of Receptor Cell Function in Adult Mammals”), there appear to be even more profound, yet selective, effects on central development. In contrast to Lasiter’s work showing that decreased amounts of gustatory stimulation led to decreased terminal field size, dietary sodium restriction during pre- and postnatal development, which also produces attenuated sodium salt elicited activity (see previous section), resulted in both enlarged and irregularly-shaped chorda tympani terminal fields.52 In fact, a 9-day period of sodium restriction limited to E3 to E12 produced a permanent alteration in the chorda tympani terminal field.53 In all manipulations of early dietary sodium, the largest effect was localized to the dorsal zone of the field.52,53 Thus, a highly plastic change at the first central gustatory relay is caused by dietary restriction conducted before taste receptors appear (i.e., around birth). Although severe sodium restriction must occur during early embryonic development, expression of the altered effects is delayed until the terminal field normally expands (i.e., during the first 3 postnatal weeks).54 The delayed effect on central development parallels the delayed effect of ENaC function described earlier,27 suggesting that the enlarged field may be partially related to activity-dependent effects.
It is critical to note that the central anatomical effects are not generalizable to all gustatory nerves; the size and topography of the projections from the greater superficial petrosal nerve (Figure 1) are unaffected by dietary manipulations.41 This is somewhat surprising because the chorda tympani and greater superficial petrosal nerves both have their cell soma in the geniculate ganglion, both are branches of the facial nerve, and both are responsive to sodium stimulation. However, it is consistent with an activity-dependent mechanism, because the greater superficial petrosal nerve is unaffected by early dietary sodium restriction (see “Developmental Dietary Sodium Restriction and Its Effect on Taste Receptor Cell Function”).41 Therefore, there is a clear dichotomy between the chorda tympani and greater superficial petrosal nerves in the physiological and anatomical susceptibility to early sodium restriction. Such a dichotomy may relate to the developmental time course of the two nerves and the associated molecular and cellular events that occur within each system.
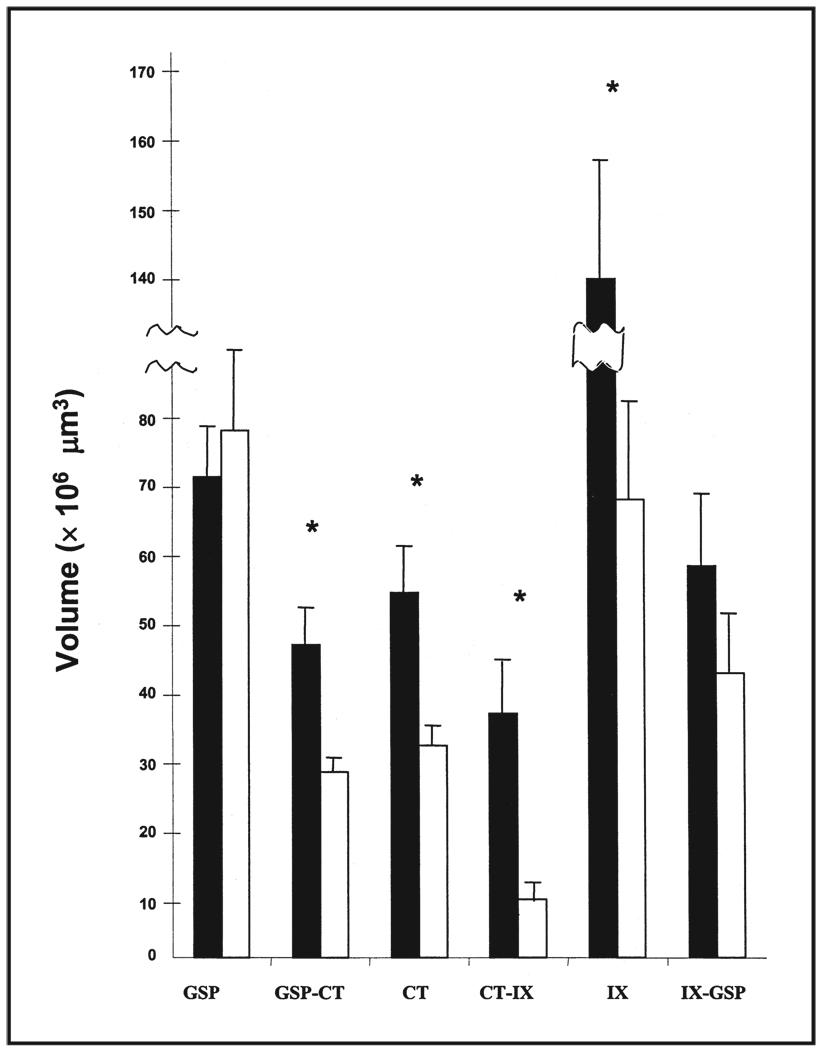
One of the limitations of labeling primary afferent fields in the past has been that only one label has been examined at a time, especially when labeling was accomplished in live animals. This was due in part because the anterograde tracer, horseradish peroxidase, was the universally preferred tracer that yielded the most sensitive identification of terminals. However the reaction from horseradish peroxidase only allowed visualization of a single terminal field in each animal. While this technique was very useful in providing much of the work provided above, questions related to relative positions of a single terminal field with others could not be answered. To remedy this technical problem, we recently developed fluorescent labeling procedures so that the chorda tympani, the greater superficial petrosal, and the glossopharyngeal nerves could each be labeled in the same rat.55 With the use of a confocal laser microscope, we could then visualize the volumes of each field, their degree of overlap, and the position of the three fields relative to the others. Additionally, we could more easily compare the effects of early dietary sodium restriction on gustatory afferent field development with that of controls. Figure 2 shows that the total terminal field size of the greater superficial petrosal nerve is similar between control and restricted rats, as found earlier by Sollars and Hill.41 Also consistent with previous work,52 the terminal field of the chorda tympani nerve was significantly larger in sodium-restricted rats compared with controls. However, unlike a previous report,52 we found that the glossopharyngeal nerve was almost twice as large in sodium-restricted rats compared with controls. The fidelity of the labels in our fluorescently labeled tissue is much better than those achieved using horseradish peroxidase as the tracer, and has revealed much more of the terminal field organization of the glossopharyngeal nerve.
Figure 2.
Mean total terminal field volumes of the greater superficial petrosal (GSP), chorda tympani (CT), and glossopharyngeal (IX) nerves in the nucleus of the solitary tract in restricted (solid bars) and control (open bars) rats. The fields of overlap are denoted by the dual labels (e.g., GSP-CT). SEM are shown for each mean and asterisks denote p < 0.05.
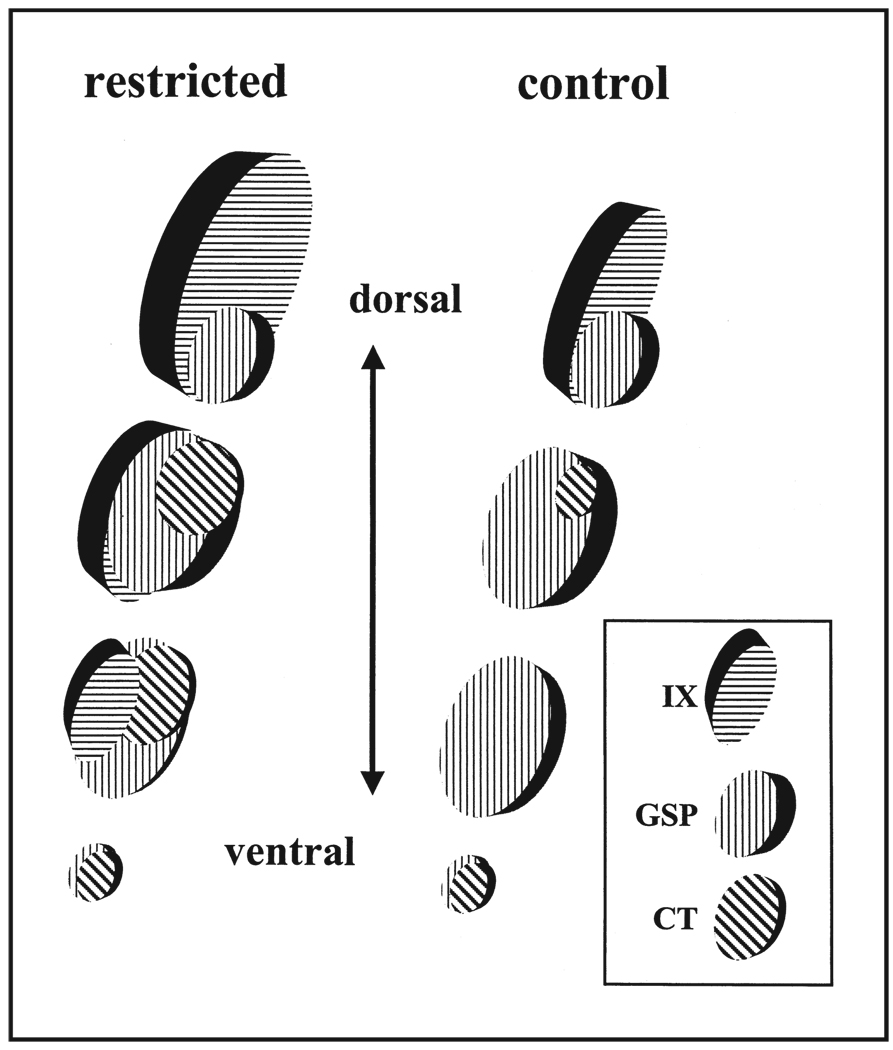
As noted above, this labeling technique also allows a view of the relative positions of each terminal field compared with others, thereby helping to identify the area most affected in the NTS by sodium restriction. By dividing the NTS into three dorsal to ventral zones, we found that the largest changes occurred in the dorsal zone of the chorda tympani nerve terminal field (Figure 3). Specifically, the glossopharyngeal nerve, which is the dorsal-most field of the three, extends ventrally into the chorda tympani field in sodium-restricted rats. This does not occur in controls. Furthermore, as seen in a previous study,52 the dorsal region of the chorda tympani field in restricted rats was much larger than in controls (Figure 3). Pittman and Contreras56 provided an interesting contrast to our dietary-induced effects. By feeding rats a high-sodium diet throughout pre- and postnatal development, the chorda tympani terminal field was abnormally small compared with controls, and the zone most affected was also the dorsal zone. Thus, low- and high-sodium diets produce opposite effects in terminal field development. These results further illustrate the remarkable amount of plasticity in the lower neural levels of the gustatory system and the specificity of such changes, not only in the nerves affected but also in the zone to which they project. Further molecular studies that focus on early events of terminal field organization should provide clues about this specificity.
Figure 3.
Model of terminal field organization in the nucleus of the solitary tract from dorsal to ventral zones in restricted and control rats. The fields of overlap for the respective fields are shown at four dorsal-ventral levels. See text for details of overlap among the three terminal fields and the diet-related differences. IX, glossopharyngeal nerves; GSP, greater superficial petrosal nerves; CT, chorda tympani nerves.
There are also striking postsynaptic morphological alterations that occur as a result of early developmental manipulations. Specific cell types in the rostral pole of the adult rat NTS restricted of dietary sodium from E3 show pronounced and permanent increases in dendritic length and number, whereas other cell types are spared.57 Neurons affected by dietary restriction are putative relay neurons.58 These data strongly suggest that the morphological effects of early dietary manipulations are specific to certain cell types and may relate to their function.57 It is unclear, however, whether changes in terminal field organization affect the dendritic organization of the NTS or vise versa. Specificially, do the postsynaptic changes in dendrites govern the abnormally enlarged terminal fields or do the enlarged terminal fields govern the abnormally large dendrites on putative relay neurons? Alternatively, are other factors operating to modify both independently? Regardless of the mechanism, both terminal field and dendritic alterations as a result of the early dietary manipulation are permanent. Restoration of a sodium-replete diet exaggerates instead of restores normal morphology.52,57 This has important implications for brain stem development of non-gustatory structures as well as permanently affecting taste-elicited behaviors.
The consequences of early environmental manipulations are also expressed functionally in NTS neurons that are similar to, but significantly different from, what is predicted from the peripheral gustatory system effects. NTS neurons in sodium-restricted rats respond with lower response frequencies to sodium salts, while activity to non-sodium salts and to non-salt stimuli are normal. 59 However, unlike in the periphery, sodium-restricted rats fed a sodium-replete diet for at least 5 weeks at adulthood have NTS neurons that are more responsive to sodium salts than in controls.59 Accordingly, these neurons have an apparent shift in the stimulus to which they respond best. The central functional changes, like the morphological changes, are permanent and specific.
Finally, the terminal fields of NTS neurons in the next central gustatory relay, the parabrachial nucleus, are similar among control, sodium-restricted, and “recovered” rats.60 Thus, the parabrachial nucleus is resistant to the dietary-induced changes seen at the lower neural levels. This resistance may reflect different developmental processes and convergence of visceral and multiple gustatory inputs in the parabrachial nucleus (chorda tympani, greater superficial petrosal, and glossopharyngeal nerves) present in normal rats.61,62
Plasticity in Adults Induced by Nerve Section
Coupled with the ongoing turnover of taste receptor cells, taste receptor cell structure and function is dependent upon innervation (i.e., they are trophically dependent). 63,64 Loss of innervation results in a loss of the normal morphological appearance and responses of taste buds, while restoration of innervation results in a restoration of normal taste bud morphology and function.64,65 Therefore, these bidirectional interactions between taste buds and the neurons that innervate them further illustrate how plastic the gustatory system is, even at adulthood. In essence, this is a system in which environmental effects can have an impact on new taste cells as they regenerate instead of through normal turnover. Thus, the effects on newly forming cells can be assessed without the many developmental processes that operate on the entire animal, as would be the case for the developmental studies presented above. Our strategy has been to induce a wholesale degeneration and then regeneration of taste receptors on one side of the tongue by doing a unilateral chorda tympani nerve section and leaving the other side intact. Then, as receptors regenerate, we can examine the effects of environmental effects such as dietary sodium restriction on taste function.
Through this experimental paradigm, we showed that the adult regenerating gustatory system is especially susceptible to environmental manipulations.66 Unilateral chorda tympani nerve section in rats fed a sodium-restricted diet at adulthood results in a regenerated nerve that has attenuated responses specific to sodium salts and, as expected, the underlying mechanism relates to a decrease in ENaC function.66 Therefore, environmental factors exert their effects on the function of ENaCs in receptor cells that newly form after an extensive loss of previous taste receptor cell generations. There are other, novel effects.
In the same rat, the contralateral, uncut nerve is supersensitive to sodium salts.66 This supersensitivity does not occur immediately after sectioning the contralateral chorda tympani nerve, but responses increase systematically following an initial subnormal response within 2 days post-section (i.e., approximately 25% of controls). As found in the regenerated nerve, the neurophysiological alterations are selective and relate to functional changes in ENaCs. Moreover, the alterations in the intact nerve occur in the absence of reinnervation of the originally sectioned chorda tympani nerve.66
These large effects occur only under certain conditions. Chorda tympani sectioning must be accompanied by a sodium-restricted diet to produce response alterations; the presence of only one of these conditions fails to produce functional changes. This points to important interactions between events that occur in response to nerve section and the physiological effects of maintenance on a low-sodium diet. While the mechanism has not been identified, findings indicate that the immune system is involved in these processes.67 In fact, recent preliminary studies suggest that immune-derived cells are normally activated in unilaterally sectioned rats within days after denervation, and that these immune cells may maintain normal ENaC function.68 Much fewer activated immune cells are present following nerve section if the rat is placed on a sodium-restricted diet, and therefore normal function is compromised. While this is an attractive candidate compensatory mechanism that may occur in normal rats, there is no current explanation for the long-term effects in which the contralateral, intact side becomes supersensitive to sodium salt stimulation with time following nerve section.
In concert with the hypothesis that the immune system is involved in this phenomenon, it can be predicted that similar results would occur if other procedures also activated the immune system in the tongue. This has been shown in separate groups of sodium-restricted rats in which the glossopharyngeal nerve, the greater superficial petrosal nerve (Figure 1), or a non-gustatory nerve that innervates gustatory papillae (the trigeminal nerve) was sectioned.69 Another group received thermal injury to the antero-ventral tongue. Recordings from the chorda tympani nerve were then obtained between 4 and 10 days post-sectioning. We found that substantial and selective suppression of sodium salt responses in the chorda tympani occurred in a graded fashion generally related to the distance from the target field of the injury (e.g., anterior vs. posterior tongue) to anterior tongue taste buds. The order of effectiveness was: chorda tympani section > trigeminal section > thermal injury = glossopharyngeal section > greater superficial petrosal section.69 There was no effect of greater superficial petrosal nerve section on chorda tympani nerve responses. These results support the hypothesis that local, diffusible factors liberated from immune-derived cells as a result of neural and/or epithelial damage are involved in regulating the transduction pathway responsible for sodium salt sensation, and that these factors may become evident through dietary sodium restriction. They also point to the interesting idea that the age of taste receptor cells may determine their susceptibility to environmental influences.
Conclusion
This review has explored plasticity in the peripheral gustatory system and in the first central relay, with a focus on dietary influences. Experience-related changes in function are found in insect and mammalian taste receptor cells. In both instances, the clearest examples that alter transduction pathways relate to adapting to an aversive or toxic environment (in insects) or to significant alterations in homeostatic systems in mammals (e.g., sodium regulation). In mammals, functional alterations also occur in the first central relay of the gustatory system, illustrating that plastic changes are present as the message initially enters the brain.
While plasticity can occur in mature gustatory systems, the largest and most widespread alterations occur when instituted during development. Peripheral gustatory function can be significantly and selectively affected by institution of severe dietary manipulations before and during establishment of peripheral gustatory structures. Again, this appears to be more related to hormonal or growth factor influences than to activity-dependent factors. In contrast, central morphological and functional changes appear to relate to both activity-dependent and activity-independent mechanisms. Furthermore, unlike peripheral effects, the central changes are permanent. These findings collectively point to the importance of diet, especially dietary components during early development, in shaping the lifelong structure and function of the taste system. The implication for all plastic changes in the neurobiology of the gustatory system is that the nervous system adapts to the environment and may be expressed by behavioral and/or regulatory changes.
Acknowledgments
This work was supported by NIH grants DC00407 and DC03576.
References
- 1.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doty RL. Handbook of Olfaction and Gustation. Vol. 2. Basel: Marcel Dekker; 2003. [Google Scholar]
- 3.Glendinning JI, Ensslen S, Eisenberg Me, Weiskopf P. Diet-induced plasticity in the taste system of an insect: localization to a single transduction pathway in an identified taste cell. J Exper Biol. 1999;202:2091–2102. doi: 10.1242/jeb.202.15.2091. [DOI] [PubMed] [Google Scholar]
- 4.Glendinning JI, Brown H, Cappor M, Davis A, Gbedemah A, Long E. A peripheral mechanism for behavioral adaptation to specific “bitter” taste stimuli in an insect. J Neurosci. 2001;21:3688–3696. doi: 10.1523/JNEUROSCI.21-10-03688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renwick JA. Variable diets and changing taste in plant-insect relationships. J Chem Ecol. 2001;27:1063–1076. doi: 10.1023/a:1010381509601. [DOI] [PubMed] [Google Scholar]
- 6.Warren RP, Pfaffmann C. Early experience and taste aversion. J Comp Physiol Psychol. 1959;52:263–271. doi: 10.1037/h0047655. [DOI] [PubMed] [Google Scholar]
- 7.Capretta PJ, Rawls LH., 3rd Establishment of a flavor preference in rats: importance of nursing and weaning experience. J Comp Physiol Psychol. 1974;86:670–673. doi: 10.1037/h0036158. [DOI] [PubMed] [Google Scholar]
- 8.Rozin P, Gruss L, Berk G. Reversal of innate aversions: attempts to induce a preference for chili peppers in rats. J Comp Physiol Psychol. 1979;93:1001–1014. doi: 10.1037/h0077632. [DOI] [PubMed] [Google Scholar]
- 9.Wurtman JJ, Wurtman RJ. Sucrose consumption early in life fails to modify the appetite of adult rats for sweet foods. Science. 1979;205:321–322. doi: 10.1126/science.451607. [DOI] [PubMed] [Google Scholar]
- 10.Contreras RJ, Frank ME. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979;73:569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 12.Garty H, Benos DJ. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988;68:309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- 13.Herness MS. Aldosterone increases the amiloride-sensitivity of the rat gustatory neural response to NaCl. Comp Biochem Physiol Comp Physiol. 1992;103:269–273. doi: 10.1016/0300-9629(92)90578-e. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol. 1999;405:406–420. doi: 10.1002/(sici)1096-9861(19990315)405:3<406::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs KM, Mark GP, Scott TR. Taste responses in the nucleus tractus solitarius of sodium-deprived rats. J Physiol. 1988;406:393–410. doi: 10.1113/jphysiol.1988.sp017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura R, Norgren R. Repeated sodium depletion affects gustatory neural responses in the nucleus of the solitary tract of rats. Am J Physiol. 1997;273(4 part 2):R1381–R1391. doi: 10.1152/ajpregu.1997.273.4.R1381. [DOI] [PubMed] [Google Scholar]
- 17.Giza BK, Scott TR. Intravenous insulin infusions in rats decrease gustatory-evoked responses to sugars. Am J Physiol. 1987;252(5 part 2):R994–R1002. doi: 10.1152/ajpregu.1987.252.5.R994. [DOI] [PubMed] [Google Scholar]
- 18.Giza BK, Deems RO, Vanderweele DA, Scott TR. Pancreatic glucagons suppresses gustatory responsiveness to glucose. Am J Physiol. 1993;265(6 part 2):R1231–R1237. doi: 10.1152/ajpregu.1993.265.6.R1231. [DOI] [PubMed] [Google Scholar]
- 19.Gleen JF, Erickson RP. Gastric modulation of gustatory afferent activity. Physiol Behav. 1976;16:561–568. doi: 10.1016/0031-9384(76)90216-x. [DOI] [PubMed] [Google Scholar]
- 20.Giza BK, Scott TR. Blood glucose selectively affects taste-evoked activity in the rat nucleus tractus solitarius. Physiol Behav. 1983;31:643–650. [PubMed] [Google Scholar]
- 21.Giza BK, Scott TR, Antonucci RF. Effect of cholecystokinin on taste responsiveness in rats. Am J Physiol. 1990;258:R1371–R1379. doi: 10.1152/ajpregu.1990.258.6.R1371. [DOI] [PubMed] [Google Scholar]
- 22.Norgren R. Handbook of Physiology. The Nervous System, Sensory Processes. vol 3. Bethesda: American Physiological Society; 1984. Central neural mechanisms of taste; pp. 1087–1128. [Google Scholar]
- 23.Chang FC, Scott TR. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci. 1984;4:1850–1862. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DL, Almli CR. Ontogeny of chorda tympani nerve responses to gustatory stimuli in the rat. Brain Res. 1980;197:27–38. doi: 10.1016/0006-8993(80)90432-1. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T. Chorda tympani responses to gustatory stimuli in developing rats. Jpn J Physiol. 1980;30:631–643. doi: 10.2170/jjphysiol.30.631. [DOI] [PubMed] [Google Scholar]
- 26.Ferrell MF, Mistretta CM, Bradley RM. Development of chorda tympani taste responses in rat. J Comp Neurol. 1981;198:37–44. doi: 10.1002/cne.901980105. [DOI] [PubMed] [Google Scholar]
- 27.Hill DL, Bour TC. Addition of functional amiloride-sensitive components to the receptor membrane: a possible mechanism for altered taste responses during development. Brain Res. 1985;352:310–313. doi: 10.1016/0165-3806(85)90121-x. [DOI] [PubMed] [Google Scholar]
- 28.Hill DL, Mistretta CM, Bradley RM. Effects of dietary NaCl deprivation during early development on behavioral and neurophysiological taste responses. Behav Neurosci. 1986;100:390–398. doi: 10.1037//0735-7044.100.3.390. [DOI] [PubMed] [Google Scholar]
- 29.Hill DL. Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol. 1987;393:413–424. doi: 10.1113/jphysiol.1987.sp016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill DL, Przekop PR., Jr Influences of dietary sodium on functional taste receptor development: a sensitive period. Science. 1988;241:1826–1828. doi: 10.1126/science.3175625. [DOI] [PubMed] [Google Scholar]
- 31.Ye Q, Stewart RE, Heck GL, Hill DL, DeSimone JA. Dietary Na+-restriction prevents development of functional Na+ channels in taste cell apical membranes: proof by in vivo membrane voltage perturbation. J Neurophysiol. 1993;70:1713–1716. doi: 10.1152/jn.1993.70.4.1713. [DOI] [PubMed] [Google Scholar]
- 32.Stewart RE, Tong H, McCarty R, Hill DL. Altered gustatory development in Na+-restricted rats is not explained by low Na+ levels in mothers’ milk. Physiol Behav. 1993;53:823–826. doi: 10.1016/0031-9384(93)90194-k. [DOI] [PubMed] [Google Scholar]
- 33.Kirksey A, Pike RL, Callahan JA. Some effects of high and low sodium intakes during pregnancy in a rat. II. Electrolyte concentrations of maternal plasma, muscle, bone and brain and of placenta, amniotic fluid, fetal plasma and total fetus in normal pregnancy. J Nutr. 1962;77:43–51. doi: 10.1093/jn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 34.Stewart RE, DeSimone JA, Hill DL. New perspectives in gustatory physiology: transduction, development, and plasticity. Am J Physiol. 1997;272(1 part 1):C1–C26. doi: 10.1152/ajpcell.1997.272.1.C1. [DOI] [PubMed] [Google Scholar]
- 35.Stewart RE, Lasiter PS, Benos DJ, Hill DL. Immunohistochemical correlates of peripheral gustatory sensitivity to sodium and amiloride. Acta Anat (Basel) 1995;153:310–319. doi: 10.1159/000147740. [DOI] [PubMed] [Google Scholar]
- 36.Binder DR, Posner ED, Hill DL. Amiloride-sensitive epithelial sodium channel (ENaC) transcription in the dietary sodium-restricted rat. Program No. 632.8. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. [Accessed Sept. 30, 2004]. Available online at www.sfn.org. [Google Scholar]
- 37.Kossel AH, McPheeters M, Lin W, Kinnamon SC. Development of membrane properties in taste cells of fungiform papillae: functional evidence for early presence of amiloride-sensitive sodium channels. J Neurosci. 1997;17:9634–9641. doi: 10.1523/JNEUROSCI.17-24-09634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przekop P, Jr, Mook DG, Hill DL. Functional recovery of the gustatory system after sodium deprivation during development: how much sodium and where. Am J Physiol. 1990;259(4 part 2):R786–R791. doi: 10.1152/ajpregu.1990.259.4.R786. [DOI] [PubMed] [Google Scholar]
- 39.Stewart RE, Hill DL. Time course of saline-induced recovery of the gustatory system in sodium-restricted rats. Am J Physiol. 1996;270(4 part 2):R704–R712. doi: 10.1152/ajpregu.1996.270.4.R704. [DOI] [PubMed] [Google Scholar]
- 40.Phillips LM, Stewart RE, Hill DL. Cross fostering between normal and sodium-restricted rats: effects on peripheral gustatory function. Am J Physiol. 1995;269(3 part 2):R603–R607. doi: 10.1152/ajpregu.1995.269.3.R603. [DOI] [PubMed] [Google Scholar]
- 41.Sollars SI, Hill DL. Lack of functional and morphological susceptibility of the greater superficial petrosal nerve to developmental dietary sodium restriction. Chem Senses. 2000;25:719–727. doi: 10.1093/chemse/25.6.719. [DOI] [PubMed] [Google Scholar]
- 42.Mistretta CM, Hill DL. Development of the taste system. In: Doty RL, editor. Handbook of Olfaction and Gustation. Vol. 2. Basel: Marcel Dekker; 2003. pp. 759–782. [Google Scholar]
- 43.Krimm RF, Hill DL. Neuron/target matching between chorda tympani neurons and taste buds during postnatal rat development. J Neurobiol. 2000;43:98–106. [PubMed] [Google Scholar]
- 44.Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- 45.Krimm RF, Hill DL. Early dietary sodium restriction disrupts the peripheral anatomical development of the gustatory system. J Neurobiol. 1999;39:218–226. doi: 10.1002/(sici)1097-4695(199905)39:2<218::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Hendricks S, Brunjes PC, Hill DL. Developmental taste receptor cell kinetics. Chem Senses. 2002;27:A58. [Google Scholar]
- 47.Nagai T, Mistretta CM, Bradley RM. Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity. J Neurosci. 1988;8:64–72. doi: 10.1523/JNEUROSCI.08-01-00064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lasiter PS, Kachele DL. Effects of early postnatal receptor damage on development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Dev Brain Res. 1990;55:57–71. doi: 10.1016/0165-3806(90)90106-9. [DOI] [PubMed] [Google Scholar]
- 49.Lasiter PS, Diaz J. Artificial rearing alters development of the nucleus of the solitary tract. Brain Res Bull. 1992;29:407–410. doi: 10.1016/0361-9230(92)90076-a. [DOI] [PubMed] [Google Scholar]
- 50.Lasiter PS. Effects of orochemical stimulation on postnatal development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Bull. 1995;38:1–9. doi: 10.1016/0361-9230(95)00063-k. [DOI] [PubMed] [Google Scholar]
- 51.Hall WG. The ontogeny of feeding in rats: I. Ingestive and behavioral responses to oral infusions. J Comp Physiol Psych. 1979;93:977–1000. doi: 10.1037/h0077771. [DOI] [PubMed] [Google Scholar]
- 52.King CT, Hill DL. Dietary sodium chloride deprivation throughout development selectively influences the terminal field organization of gustatory afferent fibers projecting to the rat nucleus of the solitary tract. J Comp Neurol. 1991;303:159–169. doi: 10.1002/cne.903030114. [DOI] [PubMed] [Google Scholar]
- 53.Krimm RF, Hill DL. Early prenatal critical period for chorda tympani nerve terminal field development. J Comp Neurol. 1997;378:254–264. [PubMed] [Google Scholar]
- 54.Walker BR, Hill DL. Emergence of afferent fiber terminal field alterations in sodium restricted rats. Society for Neuroscience Abstracts. 1995;21:1657. [Google Scholar]
- 55.May OL, Hill DL. Organization and plasticity of gustatory nerve terminal fields revealed by triple fluorescent labeling. Chem Senses. 2003 in press. [Google Scholar]
- 56.Pittman DW, Contreras RJ. Dietary NaCl influences the organization of chorda tympani neurons projecting to the nucleus of the solitary tract in rats. Chem Senses. 2002;27:333–341. doi: 10.1093/chemse/27.4.333. [DOI] [PubMed] [Google Scholar]
- 57.King CT, Hill DL. Neuroanatomical alterations in the rat nucleus of the solitary tract following early maternal NaCl deprivation and subsequent NaCl repletion. J Comp Neurol. 1993;333:531–542. doi: 10.1002/cne.903330406. [DOI] [PubMed] [Google Scholar]
- 58.Lasiter PS, Wong DM, Kachele DL. Postnatal development of the rostral solitary nucleus in rat: dendritic morphology and mitochondrial enzyme activity. Brain Res Bull. 1989;22:313–321. doi: 10.1016/0361-9230(89)90059-2. [DOI] [PubMed] [Google Scholar]
- 59.Vogt MB, Hill DL. Enduring alterations in neurophysiological taste responses after early dietary sodium deprivation. J Neurophysiol. 1993;69:832–841. doi: 10.1152/jn.1993.69.3.832. [DOI] [PubMed] [Google Scholar]
- 60.Walker BR, Hill DL. Developmental sodium restriction and gustatory afferent terminal field organization in the parabrachial nucleus. Physiol Behav. 1998;64:173–178. doi: 10.1016/s0031-9384(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 61.Ogawa H, Hayama T, Ito S. Convergence of input from tongue and palate to the parabrachial nucleus neurons of rats. Neurosci Lett. 1982;28:9–14. doi: 10.1016/0304-3940(82)90200-2. [DOI] [PubMed] [Google Scholar]
- 62.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 63.Zalewski AA. Trophic functions of the neuron. VI. Other trophic systems. Neuronal and tissue specification involved in taste bud formation. Annal N Y Acad Sci. 1974;228:344–349. doi: 10.1111/j.1749-6632.1974.tb20523.x. [DOI] [PubMed] [Google Scholar]
- 64.Cheal M, Dickey WP, Jones LB, Oakley B. Taste fiber responses during reinnervation of fungiform papillae. J Comp Neurol. 1977;172:627–646. doi: 10.1002/cne.901720406. [DOI] [PubMed] [Google Scholar]
- 65.Cheal M, Oakley B. Regeneration of fungiform taste buds: temporal and spatial characteristics. J Comp Neurol. 1977;172:609–626. doi: 10.1002/cne.901720405. [DOI] [PubMed] [Google Scholar]
- 66.Hill DL, Phillips LM. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci. 1994;14(5 part 1):2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips LM, Hill DL. Novel regulation of peripheral gustatory function by the immune system. Am J Physiol. 1996;271(4 part 2):R857–R862. doi: 10.1152/ajpregu.1996.271.4.R857. [DOI] [PubMed] [Google Scholar]
- 68.McCluskey LP. Unilateral chorda tympani nerve section induces a bilateral increase in lingual macrophages in control-fed but not Na+-restricted rats. Chem Sens. 2003 in press. [Google Scholar]
- 69.Hendricks SJ, Sollars SI, Hill DL. Injury-induced functional plasticity in the peripheral gustatory system. J Neurosci. 2002;22:8607–8613. doi: 10.1523/JNEUROSCI.22-19-08607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]