Abstract
During muscle contraction, the integrity of the myotendinous junction (MTJ) is important for the transmission of force from muscle to tendon. We evaluated the contractile and structural characteristics of 3-dimensional (3-D) skeletal muscle constructs co-cultured with engineered self-organized tendon constructs (n = 4), or segments of adult (n = 4) or fetal (n = 5) rat-tail tendon. We hypothesized that the co-culture of tendon and muscle would produce constructs with viable muscle–tendon interfaces that remain intact during generation of force. Construct diameter (μm) and maximum isometric force (μN) were measured, and specific force (kPa) was determined. After measure of force, constructs were loaded at a constant strain rate until failure and surface strains were recorded optically across the tendon, the muscle and the interface and used to determine the tangent modulus (passive stiffness) of the construct. Frozen samples were used for Trichrome Masson staining and immunofluorescent analysis of the MTJ-specific protein paxillin. No differences were observed between the groups with respect to diameter, maximum force, or specific force. The MTJ was robust and withstood tensile loading beyond the physiological strain range. The majority of the constructs failed in the muscle region. At the MTJ, there is an increase in the expression and localization of paxillin. In conclusion, using 3 sources of tendon tissue, we successfully engineered 3-D muscle–tendon constructs with functionally viable MTJ, characterized by structural features and protein expression patterns resembling neonatal MTJs in vivo.
INTRODUCTION
The production and use of engineered muscle from myogenic cells harvested from muscle biopsies is potentially a powerful tool for the restoration of muscle function after acute injury, surgery, or disease. Musculoskeletal tissues are highly integrated elements within the context of a living system, none of which is known to achieve adult phenotype without input from other components of the living system. Skeletal muscle has 3 principle tissue interfaces: vascular, neuromotor, and myotendinous. The inclusion of a functional myotendinous junction (MTJ) interface in vitro would greatly expand the potential to control the phenotype of the muscle tissue in culture and thus increase the utility of engineered muscle for virtually all of its possible applications, including surgical implantation. Therefore, the goal of this experiment was to use previously described methods for constructing 3-dimensional (3-D) muscle constructs,1–4 replace the suture anchor system described in these studies with tendon obtained from in vivo tissue or from tendon engineered from fibroblasts in vitro,5 and evaluate the mechanical integrity and contractile and structural characteristics of 3-D skeletal muscle constructs co-cultured with tendon.
Tendons are highly organized connective tissues that transmit forces between muscle and bone (for review see6). They are resilient during the development of tension but flexible enough to conform to their mechanically demanding environment. The mechanical integrity of tendon tissue can be attributed to the parallel fibrils of collagen. In the resting state, the collagen fibrils take on a wavy conformation, defined as the crimp. As a tendon is stretched, the crimped collagen fibrils begin to straighten out, and as a result, the tendon becomes stiffer with increasing application of mechanical strain. The challenge in the co-culture of tendon with embryonic muscle cells to engineer a functional, viable composite construct is to find the optimal compliance characteristics of the tendon to withstand the contractile muscle forces of the newly formed, highly compliant muscle and to aid in guiding the subsequent phenotype development of engineered muscle.
Mechanical transduction of force across the MTJ activates cell signaling pathways that instruct the cells located at the interface to secrete and deposit proteins to form a specialized extracellular matrix (ECM) at the MTJ.7–9 The increased expression of several ECM proteins of muscle and tendon, including focal adhesion kinase, paxillin, integrin-linked kinase, mitogen-activated protein kinase, and talin,10–12 has been shown to occur in response to increased mechanical loading of the MTJ. These proteins provide a conduit by which forces can be transmitted from muscle to tendon. Lack of the expression of these proteins at the MTJ has been shown to lead to structural damage of the interface during contraction.13,14
Our laboratory has successfully engineered self-organized 3-D tendon from cells isolated from rat Achilles tendons.5 The resulting scaffold-free tissue is composed of aligned, small-diameter collagen fibrils, a large number of cells, and an excess of non-collagenous ECM—all characteristics of embryonic tendon. The stress–strain response of the constructs also resembles the non-linear behavior of immature tendons, and the ultimate tensile strength is approximately equal to that of embryonic chick tendon, roughly 2 MPa. These constructs are morphologically and mechanically similar to embryonic tendon and are potentially useful for studying the developmental biology of tendon as well as for clinical use in tendon repair.
This study will evaluate the structural and functional characteristics of 3-D skeletal muscle constructs co-cultured with engineered tendon constructs, or segments of adult (ART) or fetal (FRT) rat-tail tendon. We hypothesized that the co-culture of tendon tissue and muscle would produce constructs with viable muscle–tendon interfaces that remain intact during force production. We used the increased expression and localization of 2 of these proteins at the MTJ, paxillin and talin, as an indication of the mechanical interaction between the tendon and the engineered muscle construct.
MATERIALS AND METHODS
Animal model and animal care
Tissue-engineering studies were conducted using muscle tissue from female Fischer 344 pregnant rats obtained from Charles River Laboratories, Inc. (Wilmington, MA). All animals were acclimated to our colony conditions (light cycle and temperature) for 1 week before any procedure. Rats were housed in pairs in hanging plastic cages (28 × 56 cm) and kept on a 12:12 light:dark cycle at a temperature of 20°C to 22°C. The animals were fed Purina Rodent Chow 5001 laboratory chow and water ad libitum. At day E15 of gestation, surgical procedures were performed to remove the soleus muscles and Achilles and tail tendons from the pregnant rats. E15 fetuses were cesarean delivered, and fetal tail was obtained. Cells isolated from the soleus muscle and Achilles tendon were used to fabricate the engineered constructs, and adult tails and fetal tails were used as anchors for tendon–muscle co-cultures. All surgical procedures were performed in an aseptic environment with animals in a deep plane of anesthesia induced using intraperitoneal injections of sodium pentobarbital (65 mg/kg). Supplemental does of pentobarbital were administered as required to maintain an adequate depth of anesthesia. All animal care and animal surgery were in accordance with The Guide for Care and Use of Laboratory Animals (Public Health Service, 1996, NIH Publication No. 85–23). The University Committee for the Use and Care of Animals approved the experimental protocol.
Preparation of solutions and media
Unless otherwise indicated, all solutions and media were prepared and stored at 4°C before isolation and culture of primary cells and warmed to 37°C in a heated water bath immediately before use. The media, with slight modifications according to Dennis and Kosnik,3,4 were as follows: growth medium (GMA) consisted of 400 mL of HAM F-12 nutrient mixture (Gibco BRL, Carlsbad, CA), 100 mL fetal bovine serum (Gibco BRL), and 5 mL anti-biotic anti-mycotic (ABAM, Sigma, St. Louis, MO). Differentiation medium consisted of 465 mL Dulbecco’s modified Eagle medium (DMEM, Gibco BRL), 35 mL 100% horse serum (Gibco BRL), and 5 mL ABAM. The tissue was dissociated in a dispase and collagenase solution of 20 mL per 2 soleus muscles that consisted of 8 units of dispase (0.4 units/mg, Sigma) and 200 units of Type 4 collagenase (239 units/mg, Gibco BRL) per mL DMEM. Transport medium was prepared in the amount of 5 mL per muscle dissected at the concentration of 2% ABAM in Dulbecco’s phosphate-buffered saline (DPBS, Gibco BRL). Pre-incubation medium was prepared in the amount of 3 mL per plate and consisted of 2.5 mL of 0.05% sodium azide (Sigma, St. Louis, MO) in DPBS solution, 22.5 mL differentiation medium, and 0.25 mL ABAM per muscle dissected.
Preparation of culture dishes and anchors
All tissue constructs were engineered in individual 35-mm plates as described previously.3 Briefly, each 35-mm plate was coated with 1.5 mL of Sylgard (Type 184 silicon elastomer, Dow Chemical Corporation, Midland, MI) and allowed to cure for 3 weeks. One week before use, Sylgard-treated plates were coated with laminin, 1.0 μg/cm2 per plate for muscle constructs and 3.0 μg/cm2 for tendon constructs. Natural mouse laminin (Gibco BRL) was diluted to the appropriate concentration in DPBS pH 7.2, and 3 mL of this solution was used to coat each plate, which was left to dry for 48 h. Salt crystals were dissolved and removed by rinsing the plates with 3 mL DPBS. The plates were then filled with 2 mL GMA and decontaminated using ultraviolet (UV) light (wavelength 253.7 nm) for 90 min and placed in a 37°C, 5% carbon dioxide (CO2) incubator for 1 week before plating muscle cells. For the self-organized tendon constructs, two stainless steel minutien pins (Fine Science Tools, Foster City, CA), 0.20 in diameter, were pinned 15 mm apart in the center of each dish. The plates were filled with 1 mL of growth medium, UV treated for 90 min, and placed in an incubator for 1 week.
Preparation of self-organized tendon constructs
Self-organized tendon (SOT) constructs were engineered as described previously with modifications.14 Briefly, primary rat tendon fibroblasts were obtained from the Achilles tendons of Fischer 344 retired breeder rats (Charles River Laboratories). Achilles tendon cells were isolated by dissociating the tendons in 1 mg/mL type II collagenase (Worthington Biochemical, Lakewood, NJ) in DMEM plus 2 % ABAM. The solution was placed in an incubator at 37°C (Electron Series II Water Jacketed CO2 Incubator; Thermo Forma Scientific, Waltham, MA) overnight to facilitate breakdown of the ECM. After the tissue was dissociated, the cells were pelleted using centrifugation (AccuSpin FR; Beckman Coulter Inc., Fullerton, CA) at 100 g for 5 min, and the supernatant was removed using aspiration. The cells were resuspended with GMA, expanded in 100-mm-diameter tissue culture dishes, and then passaged at approximately 60% confluence. Cells were trypsinized between the first and fourth passages, and 2×105 cells suspended in 2 mL GMA were seeded onto each plate and supplemented with 100 μg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO), a stable derivative of ascorbic acid. Fresh ascorbic acid was added each time the GMA was changed—every 4 days. After approximately 10 to 14 days, differentiation medium consisting of DMEM plus 7% fetal bovine serum, 100 μg/mL L-ascorbic acid 2-phosphate, 2 ng/mL transforming growth factor (TGF)β1 (Peprotech Inc., Rocky Hill, NJ), and 2 μg/mL insulin (Sigma) was substituted for GMA to induce construct formation. The differentiation medium was changed every 3 to 4 days, and fresh ascorbic acid, TGFβ1, and insulin were added each time. Cell layers delaminated after approximately one month, forming a construct 15 mm long and 0.5 mm in diameter constrained by the minutien pins. Constructs were cut in half and pinned onto monolayers of muscle cells.
Preparation of muscle and isolation of satellite cells
Both soleus muscles were surgically removed under aseptic conditions, weighed, sterilized in 70% ethanol, and incubated for 5 min in transport medium. A single-edged razor blade and a pair of no. 5 forceps (Fine Science Tools,) were then used to slice the soleus muscle longitudinally into 3 strips. Next, 35-mm Sylgard-treated plates were sterilized in 70% ethanol, and muscle slices were pinned to length, 2 muscle strips per plate. Then 3 mL pre-incubation medium was added to each plate, and the plates were UV treated for 90 min. The plates were then placed in a 37°C, 5% CO2 incubator for 50 h. After the 50 h incubation period, muscle slices were inspected for contamination, and any infected plates were discarded. The remaining muscle strips were removed from the plates and incubated in dispase and collagenase solution (2 soleus muscles per 20 mL) in a 37°C shaking water bath for 4 h. The dissociation was aided by occasionally shaking each vial slightly by hand. Once the muscle was fully digested (~4 h), the dissociated cells were filtered through a 100-micron filter and centrifuged at 700 g for 10 min at 25°C. Finally, the supernatant was aspirated from the vials, and the pellet was resuspended in GMA to obtain a concentration of 10 mg of dissociated muscle per 2 mL GMA.
Cell culture and myooid formation
After 1 week of incubation, the GMA was aspirated from the previously prepared laminin-coated plates. Two mL of the cell suspension was plated onto each culture dish, which was then placed in a 37°C, 5% CO2 incubator for 5 days. Culture plates were not disturbed for at least 72 h to allow cell adherence to the plates. After 5 days, GMA was changed every 48 h until the cells became confluent (approximately 7 days). Once the cells achieved confluence, they were fed with differentiation medium every 48 h until the myocytes fused to form multinucleated myotubes that began to contract spontaneously. Two weeks after the plating of the muscle stem cells, 2 anchors consisting of engineered SOT constructs or segments of ART or FRT were pinned onto the muscle cell monolayer 15 mm apart (Fig. 1). Approximately 1 week later, monolayers rolled up around the anchors and formed a cylindrical construct. Seven to 10 days post-construct formation, after approximately 14 to 17 days of co-culture, the tendon–muscle constructs were tested for contractile function and tensile strength. A separate set of specimens was prepared for electron microscopy or histology.
FIG. 1.
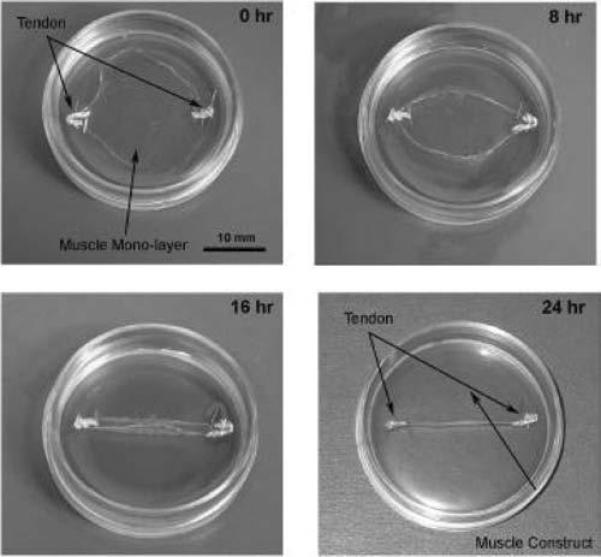
Two weeks after the initial plating of the muscle stem cells, engineered self-organized tendon constructs or segments of adult or fetal rat tail were pinned onto the muscle cell monolayer 15 mm apart. Approximately 1 week later, the monolayer begins to roll up around the tissue anchors and form a cylindrical construct. It takes approximately 24 h from the initiation of delamination for a 3-dimensional construct to form.
Testing of tendon–muscle construct’s contractility
Contractile properties were initially measured 7 to 10 days after the formation of a 3-D construct. The protocol for measuring contractility of engineered muscle constructs was adapted from Dennis et al.1–3 and Irintchev et al.15 Briefly, the pin on one end of the construct was freed from the Sylgard and attached to a force transducer using canning wax. Platinum wire electrodes were positioned on either side of the construct for field stimulation of the entire construct (Fig. 2A). The temperature of the construct was maintained at 37 ± 1°C using a heated aluminum platform. The diameter of the construct was determined and used to calculate cross-sectional area, assuming a circular cross-section. Passive baseline force was measured as the average baseline force preceding the onset of stimulation. Twitches were elicited using a single 1.2-ms pulse at 2.5, 5, 10, and 20 V, whereas maximum tetanic force was determined using a train lasting 1 s and consisting of 1.2-ms pulses at 10 V and 10, 20, 40, 60 and 80 Hz. Data files for each peak twitch force and peak tetanic force trace were recorded at 1,000 samples/s and stored for subsequent analysis using LabVIEW (National Instruments, Austin, TX). Peak tetanic force was normalized using the cross-sectional area to determine maximum specific force.
FIG. 2.
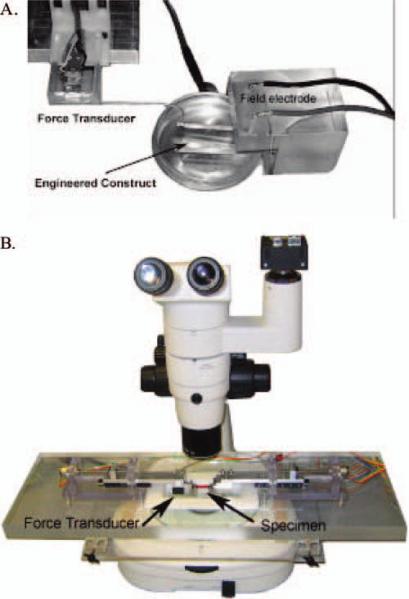
Instrumentation developed for the measurement of the contractile (A) and tensile (B) properties of a 3-dimensional tendon muscle construct. For field stimulation of the entire construct, platinum wire electrodes were positioned on either side of the construct, and a force transducer was attached to one end (A). After the direct field stimulation of the entire construct, the constructs were transferred to a tensile tester to measure stress-strain properties (B). Color images available online at www.liebertpub.com/ten.
Testing of tendon–muscle construct tensile strength
After the testing of contractile properties, the tensile strength of each construct was tested. A tensile tester was custom built around a Nikon SMZ 800 dissecting microscope outfitted with a Basler A102fc digital video camera (Fig. 2B). Dual actuators were driven by stepper motors (Faulhaber, Clearwater, FL) and mounted on crossed roller slides (Del-Tron, Bethel, CT). This enables the specimen to stay in the center of view and allows for the determination of true strain. A custom force transducer, designed to have a resolution of 50 μN, was mounted on one of the cross-heads.16 Grips were machined out of stainless steel and placed at the end of both actuators. First the specimen was gripped on each end with a micro artery clamp (Bear/ARO-Surgical, Newport Beach, CA), which does minimal damage to the tissue but is able to withstand loading (closing pressure of 40 g for vessels 0.2 – 0.9 mm in diameter). Then the artery clamp was placed in the larger grips. The stainless steel grips hung into a trough, submerging the specimen in saline. Uniaxial servomotors and data acquisition were controlled using LabVIEW. The samples were loaded at a constant true strain rate until failure, and synchronized force and image recordings were compiled using LabVIEW.
Histology
Tendons were harvested from adult and 14-day Fisher rat pups for transmission electron microscopy. To determine the structural characteristics of the tendon–muscle interface, the specimens were fixed in a 3% formaldehyde–glutaraldehyde buffer solution in 0.1 M sodium cacodylate, pH 7.4 (Electron Microscopy Sciences, Fort Washington, PA) at 4°C and embedded in Epon (Eponate 12 resin, Ted Pella, Redding, CA). For light microscopy, semi-thin sections (1 μm) were cut using an ultramicrotome (Sorvall, Newtown, CT), mounted on glass microscope slides, and stained with 1% (w/v) toluidine blue solution (Electron Microscopy Sciences). Ultrathin slices (50 nm) were mounted on uncoated copper grids and stained with aqueous uranyl acetate and lead citrate. The ultrastructure of the samples was investigated using a transmission electron microscope (Philips Medical Systems, Bothell, WA) at 60 kV.
For histochemical and immunohistochemical analysis of muscle–tendon structures in vivo and muscle-tendon constructs in vitro, samples were embedded in TBS medium (Triangle Biomedical Sciences, Durham, NC) and then sectioned on a cryostat (10 μm, model HM500, Microm, Waldorf, Germany). For immunostaining, sections were incubated in a blocking buffer (20% calf serum in phosphate-buffered saline for 1 h and then in the solution of primary antibody overnight at 4°C. The following primary antibodies were used: mouse anti-paxillin (Upstate, Lake Placid, NY), mouse anti-talin (Sigma), and rabbit anti-collagen Type I (Chemicon International, Temecula, CA). Depending on the source of primary antibody, 1-h room temperature incubation with anti-mouse or anti-rabbit Cy3- and Cy2-conjugated secondary antibodies (Jackson ImmunoResearch Lab., West Grove, PA) was used for visualization. Nuclei were stained using 5 min incubation with a 4′,6-diamidino-2-phenyindole, dilactate (Sigma) in phosphate-buffered saline with Tris (PBST). The sections were observed and photographed through a Zeiss Axiophot-2 microscope (Carl Zeiss, Jena, Germany). For histochemical analysis, sections were stained using Trichrome Masson staining (Sigma).17
Statistics
Values are presented as mean ± standard error. Statistical analysis was performed using Jump In 5.1 (SAS Institute, Inc., Cary, NC). A one-way analysis of variance was conducted to compare the differences between constructs. Differences were considered significant at p < 0.05.
RESULTS
Morphology of tendon–muscle construct
Distinct invaginations of the collagen fibrils from the tendon into the muscle characterize the MTJ of the adult rat tibialis anterior muscle (Fig. 3). Trichrome Masson staining was used for visualization of the MTJ in adult muscle (Fig. 3A). Blue staining represents collagen in the tendon. The nuclei (dark staining) of the tendon fibroblasts are located in parallel rows flattened between collagen fibers. Red staining represents staining of multinucleated cylindrical skeletal muscle cells—muscle fibers. At the interface, muscle and tendon form a contiguous interaction of cells (Fig. 3 B,C). One week after the co-cultured tendon and muscle cells rolled up into a 3-D construct, approximately 14 to 17 days of co-culture, an interface consisting of collagen fibrils (Fig. 4) and myotubes (Fig. 5) formed and was oriented along the longitudinal axis of the construct. Highly organized collagen fibrils, as seen in adult rats in vivo, characterize the co-culture of the ART with muscle cells (Fig. 4 A,B), with little integration with the muscle construct. Small amounts of collagen in discrete areas of the construct characterize the co-culture of the FRT with the muscle. The presence of cartilage is also indicated, most likely from the developing bones of the FRT. Eell-developed areas of collagen characterize the SOT constructs co-cultured with the muscle. In some constructs, the area is continuous, and in others, areas of myotubes and fat cells break up the areas of collagen.
FIG. 3.

Frozen sections (A) of normal adult myotendinous junction (MTJ) with Mason’s Trichrome staining for tendon collagen (blue), skeletal muscle fibers (red), and cell nuclei (black). Nuclei (dark staining) of fibroblasts in the tendon (tenocytes) are located in parallel rows flattened between collagen fibers. Electron micrographs (B and C) of adult MTJs taken at two different magnifications shows the highly digitated interface between muscle and tendon.
FIG. 4.

Semi-thin sections of muscle constructs engineered with anchors consisting of (A,B) adult rat tail, (C,D) fetal rat tail, and (E,F) self-organized engineered tendon constructs. Color images available online at www.liebertpub.com/ten.
FIG. 5.

Toluidine Blue–stained semi-thin sections of the center of the adult rat tail tendon–muscle construct showing myotubes aligned with the longitudinal axis of the tendon. Color images available online at www.liebertpub.com/ten.
Protein expression at the MTJ
In neonatal tissues, paxillin is expressed in moderate concentrations in the muscle and tendon (Fig. 6A). As the muscle develops, paxillin is preferentially expressed at the neonatal MTJ and is characterized by an increase in the localization between the tendon collagen and muscle (Fig. 6A). In the MTJ of the adult tibialis anterior muscle, paxillin is concentrated at the junction between the collagen fibers and the muscle cells (Fig. 6B). The interface between the ART tendon–muscle constructs looks similar to the neonatal MTJ (Fig. 6C–E). Paxillin is localized to the interface between the tendon and myotubes, although the expression is not as concentrated as observed in adult samples. Talin immunohistochemistry showed similar expression and localization to paxillin (results not shown).
FIG. 6.

Immunostaining of adult and fetal myotendinous junctions (MTJs) and the interfaces of the tendon–muscle construct engineered in vitro using adult rat-tail tendon (ART). Paxillin (red) is clustered at the MTJ in (A) neonatal and (B) adult rat muscle. Immunostaining of an ART tendon–muscle construct for (C) type I collagen and (D) paxillin. (E) 4′,6-diamidino-2-phenylindole was used to stain nuclei.
Contractile properties
The average diameters (μm), maximum isometric force (μN), and specific force (kPa) of the myooid and tendon–muscle constructs were not significantly different (Fig. 7). There were no significant differences between the diameters or isometric or specific force production of the 3 tendon–muscle constructs engineered in this study.
FIG. 7.
(A) Diameter, (B) maximum isometric force, and (C) specific force of muscle-only construct (MO), tendon–muscle constructs (adult rat tail (ART), fetal rat tail (FRT), and engineered self-organized tendon constructs (SOTs). Values are means ± standard errors.
Tensile testing
When the tendon–muscle constructs were subjected to strains of physiological and above-physiological levels, the tendon–muscle interface remained intact and failed at the middle (muscle only) portion of the constructs (for movie see the link “SOT/Muscle Movie” at http://www.personal.umich.edu/~arruda/). The tangent modulus, or passive stiffness, of the muscle constructs was 37.2 kPa ± 10.3 kPa (n = 8).
DISCUSSION
We have constructed 3-D tendon–muscle constructs from co-cultures of myogenic cells from adult soleus muscles with engineered SOT constructs or segments of ART or E-15 FRT. We have extended our muscle-only model to a new model with functional MTJs that withstood tensile loading beyond the physiological strain range. The majority of the constructs failed in the muscle region. In addition, we have shown that, at the MTJ of these engineered constructs, there is an increase in the expression and localization of some of the MTJ-specific proteins, such as paxillin, similar to those found in fetal and neonatal MTJs in vivo.
The specific force of the various tendon constructs was variable, and no statistical difference in specific force between the construct groups was found. Longer culture times or normalization for contractile elements in the cross-section may decrease the variability of data collected.
The MTJ is the structure that transmits force generated by a muscle contraction to the ECM of the muscle and onto the tendon. Folding of the sarcolemma into finger-like projections at the interface between muscle and tendon at sites of myocyte termination characterizes the morphology of the MTJ.18,19 The projections result in an approximately 10-fold increase in the area of muscle–tendon contact over the cross-section of the muscle fiber and ensure that the stresses experienced by the MTJ are shear stresses, thus decreasing the contractile stress applied directly to the sarcolemmal junction.20,21 Therefore, the transmission of force between muscle and tendon is rarely associated with a severance at the interface between muscle and tendon, but rather occurs in the body of the muscle, just proximal to the MTJ.22 Two weeks of co-culture of tendon with the monolayer of myotubes resulted in the formation of MTJs resembling the structure of neonatal MTJs in vivo. The average tangent modulus of the muscle constructs was approximately one quarter of the passive stiffness reported for a young adult rat soleus muscle.23 This may be attributed to the poorer organization of the contractile proteins and the ECM of the myooids than of the adult muscle (Figs. 3, 5). The majority of the constructs failed in the mid-substance. The introduction of the tendon to these cultures induces the formation of an interface that morphologically and mechanically resembles a developing MTJ.
Current literature suggests that the transmission of force between myofibrils and the ECM occurs via 2 parallel systems that link intracellular and extracellular structural proteins: the dystrophin-diphosphoglycerate system and the integrin system (for review see9). The dystrophin-diphosphoglycerate system appears to be the primary system for transmission of force from the lateral surfaces of the muscle fibers to the MTJs.24 The integrins create mechanical links from the ends of the myofibrils by binding actin filaments on the cytoplasmic side and one or more ECM molecules, including vinculin, FAK, paxillin, and talin, on the extracellular side, thus creating a system for longitudinal transmission of force.25–29 It has been suggested that the integrin system is the sensor of tensile strain and elicits electrical or chemical signals that are proportional to the strains they experience. The lack of integrin expression at the MTJ is associated with structural damage during muscle contraction.13 Paxillin has been shown to interact with many cytoskeletal proteins, suggesting that paxillin is a focal adhesion scaffolding protein, which it has been postulated may play a role in integrin-mediated adhesion and focal adhesion dynamics.30,31 Therefore, the expression of paxillin, one of the proteins involved in the integrin system, suggests that our tendon–muscle constructs are experiencing the required tensile stress to invoke expression, localization, and formation of MTJ-like structures.
In cell culture systems, focal adhesions comparable with the MTJ found in vivo integrate the structural components between the myotubes and ECM. Many of the proteins associated with MTJs, including integrin, tensin, paxillin, talin, and vinculin, are also localized at the focal adhesions.27–29,32–34 In association with focal adhesions, paxillin has been shown to play an important role in recruiting structural and signaling molecules and mediating the binding of FAK, vinculin, and other cytoskeletal molecules to focal adhesions,30,31 suggesting that paxillin is a scaffolding protein in the MTJ.30,31 In our study, we compared the expression and localization of paxillin in neonatal, adult, and engineered MTJs. Although paxillin was expressed in adult and neonatal MTJs, its expression was much more diffuse in the neonatal MTJ than the adult, which showed a tight banding of paxillin at the interface of muscle and tendon. The expression and localization of paxillin in the engineered tendon–muscle constructs were similar to that observed in the neonatal tissue, suggesting that the MTJ in our constructs is immature.
Gordon et al. observed that the expression of paxillin is lower in muscles that experience lower load bearing, such as the fast-twitch plantaris and gastrocnemius muscles, than in the greater load-bearing slow-twitch soleus muscle.12 Greater mechanical loading for 1 or 8 days upregulated the expression of paxillin in the soleus and plantaris muscles. These data indicate that, in the hind-limb muscles of rats, the focal adhesion complex proteins such as paxillin can adapt to the mechanical loading state of the muscle.12 Paxillin is expressed in the muscle and tendon in embryonic limb tissues. As mechanical stress is applied to the MTJ, paxillin localizes to the interface between the muscle and tendon. Notice the more-diffuse banding of paxillin in the neonatal sample than in the adult sample in Fig. 6. In the ART sample, paxillin is expressed in the muscle area of the construct, and localization of paxillin occurs at the muscle–tendon interface. Expression of paxillin in the tendon area of the ART construct is not seen. This indicates that the muscle portion of the construct contains undeveloped myofibers and that the construct is experiencing some mechanical stress that is signaling the paxillin to localize at the muscle–tendon interface. Our tendon–muscle constructs were studied 8 days after they obtained their 3-D structure, which was approximately 25 days after initial plating of cells. It could be that the engineered constructs need more time to form the structural elements of the MTJ or that the constructs are not experiencing enough (cyclic) load-bearing strain to achieve an adult-like MTJ phenotype. Future studies will investigate the effects of time in culture and cyclic mechanical loading on the structure and function of the engineered constructs.
In conclusion, using 3 sources of tendon tissue, we have successfully engineered 3-D muscle–tendon constructs with viable MTJs characterized by structural features and protein expression patterns similar to those found in neonatal MTJs developed in vivo. The inclusion of a functional tendon–muscle interface in vitro greatly expands the potential to control the phenotype of the muscle tissue in culture and thus increases the usefulness of engineered muscle for virtually all of its possible applications.
ACKNOWLEDGMENTS
The authors would like to thank Krystyna Pasyk for her technical expertise in electron microscopy. This research was supported by DARPA Biomolecular Motors Program, U.S. Air Force, AFOSR Award Number FA9550–05–1–0015 and by NSF, Civil and Mechanical Systems grant #CMS9988693. Sarah Calve was partially supported by a University of Michigan, GE-Rackham Merit Fellowship.
REFERENCES
- 1.Kosnik PE, Faulkner JA, Dennis RG. Functional development of engineered skeletal muscle from adult and neonatal rats. Tissue Eng. 2001;7:573. doi: 10.1089/107632701753213192. [DOI] [PubMed] [Google Scholar]
- 2.Dennis RG, Kosnik PE, II, Gilbert ME, Faulkner JA. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physio. 2001;280:C288. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 3.Dennis RG, Kosnik PE., II Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In vitro Cell Dev Bio Animal. 2000;36:327. doi: 10.1290/1071-2690(2000)036<0327:EAICPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Baker EL, Dennis RG, Larkin LM. Glucose transporter content and glucose uptake in skeletal muscle constructs engineered in vitro. In vitro Cell Dev Bio Anim. 2003;39:434. doi: 10.1290/1543-706X(2003)039<0434:GTCAGU>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Calve S, Dennis RG, Kosnik PE, II, Baar K, Grosh K, Arruda EM. Engineering of functional tendon. Tissue Eng. 2004;10:755. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- 6.Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 7.Bailey AJ, Shellswell GB, Duance VC. Identification and change of collagen types in differentiating myoblasts and developing chick muscle. Nature. 1979;278:67. doi: 10.1038/278067a0. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez F, Rifkin DB. Cell signaling events: a view from the matrix. Matrix Biol. 2003;22:101. doi: 10.1016/s0945-053x(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 10.Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 11.Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol. 1999;277:C152. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- 12.Gordon SE, Fluck M, Booth FW. Selected contribution: skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90:1174. doi: 10.1152/jappl.2001.90.3.1174. [DOI] [PubMed] [Google Scholar]
- 13.Mayer U, Saher G, Fassler R, Bornemann A, Echter-meyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 14.Lopez MA, Mayer U, Hwang W, Taylor T, Hashmi MA, Jannapureddy SR, Boriek AM. Force transmission, compliance, and viscoelasticity are altered in the alpha7-integrin-null mouse diaphragm. Am J Physiol Cell Physiol. 2005;288:C282. doi: 10.1152/ajpcell.00362.2003. [DOI] [PubMed] [Google Scholar]
- 15.Irintchev A, Rosenblatt JD, Cullen MJ, Zweyer M, Wernig A. Ectopic skeletal muscles derived from myoblasts implanted under the skin. J Cell Sci. 1998;111(Pt 22):3287. doi: 10.1242/jcs.111.22.3287. [DOI] [PubMed] [Google Scholar]
- 16.Dennis RG, Kosnik PE., II . Mesenchymal cell culture instrumentation and methods for evaluating engineered muscle. In: Atala A, Lanza RP, editors. Methods of Tissue Engineering. Academic Press; New York: 2002. p. 307. [Google Scholar]
- 17.Luna L. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. McGraw-Hill; New York: 1968. Masson’s Trichrome method; p. 94. [Google Scholar]
- 18.Tidball JG, Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell Tissue Res. 1989;257:77. doi: 10.1007/BF00221636. [DOI] [PubMed] [Google Scholar]
- 19.Trotter JA. Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel) 1993;146:205. doi: 10.1159/000147459. [DOI] [PubMed] [Google Scholar]
- 20.Tidball JG. Myotendinous junction: morphological changes and mechanical failure associated with muscle cell atrophy. Exp Mol Pathol. 1984;40:1. doi: 10.1016/0014-4800(84)90060-1. [DOI] [PubMed] [Google Scholar]
- 21.Trotter JA, Hsi K, Samora A, Wofsy C. A morphometric analysis of the muscle-tendon junction. Anat Rec. 1985;213:26. doi: 10.1002/ar.1092130105. [DOI] [PubMed] [Google Scholar]
- 22.Tidball JG. Alpha-actinin is absent from the terminal segments of myofibrils and from subsarcolemmal densities in frog skeletal muscle. Exp Cell Res. 1987;170:469. doi: 10.1016/0014-4827(87)90321-1. [DOI] [PubMed] [Google Scholar]
- 23.Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. J Appl Physiol. 1998;85:1011. doi: 10.1152/jappl.1998.85.3.1011. [DOI] [PubMed] [Google Scholar]
- 24.Henry MD, Campbell KP. Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol. 1996;8:625. doi: 10.1016/s0955-0674(96)80103-7. [DOI] [PubMed] [Google Scholar]
- 25.Bockholt SM, Otey CA, Glenney JR, Jr., Burridge K. Localization of a 215-kDa tyrosine-phosphorylated protein that cross-reacts with tensin antibodies. Exp Cell Res. 1992;203:39. doi: 10.1016/0014-4827(92)90037-9. [DOI] [PubMed] [Google Scholar]
- 26.Turner CE, Burridge K. Transmembrane molecular assemblies in cell-extracellular matrix interactions. Curr Opin Cell Biol. 1991;3:849. doi: 10.1016/0955-0674(91)90059-8. [DOI] [PubMed] [Google Scholar]
- 27.Turner CE, Kramarcy N, Sealock R, Burridge K. Localization of paxillin, a focal adhesion protein, to smooth muscle dense plaques, and the myotendinous and neuromuscular junctions of skeletal muscle. Exp Cell Res. 1991;192:651. doi: 10.1016/0014-4827(91)90090-h. [DOI] [PubMed] [Google Scholar]
- 28.Tidball JG, O’Halloran T, Burridge K. Talin at myotendinous junctions. J Cell Biol. 1986;103:1465. doi: 10.1083/jcb.103.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- 30.Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 31.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 32.Bockholt SM, Burridge K. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes Commun. 1995;3:91. doi: 10.3109/15419069509081279. [DOI] [PubMed] [Google Scholar]
- 33.Bozyczko D, Decker C, Muschler J, Horwitz AF. Integrin on developing and adult skeletal muscle. Exp Cell Res. 1989;183:72. doi: 10.1016/0014-4827(89)90419-9. [DOI] [PubMed] [Google Scholar]
- 34.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]



