Abstract
N-arachidonoyl ethanolamide or anandamide is an endocannabinoid found in most tissues where it acts as an important signaling mediator in a number of physiological and pathophysiological processes. Consequently, intense effort has been focused on understanding all its biosynthetic and metabolic pathways. Herein we report human alcohol dehydrogenase-catalyzed sequential oxidation of anandamide to N-arachidonoyl glycine, a prototypical member of the class of long chain fatty acyl glycines, a new group of lipid mediators with a wide array of physiological effects. We also present a straightforward synthesis for a series of N-acyl glycinals including N-arachidonoyl glycinal, an intermediate in the alcohol dehydrogenase-catalyzed oxidation of anandamide.
Keywords: N-arachidonoyl ethanolamide, anandamide, cannabinoids, cannabinoid receptors, N-arachidonoyl glycine, N-arachidonoyl glycinal, LC-MS
The endocannabinoid system, consisting of cannabinoid receptors, the endogenous ligands that bind these receptors (endocannabinoids) and the enzymes that are involved in their biosynthesis and metabolism, is known to play an important role in several physiological and pathophysiological processes including pain, inflammation, drug addiction, obesity, cancer and Alzheimer’s disease.1 Included in the list of endocannabinoids, is the widely studied N-arachidonoyl ethanolamide2, (anandamide or AEA) which belongs to a growing class of endogenous N-acyl ethanolamides (N-linoleoyl ethanolamide3 (LEA), N-oleoyl ethanolamide4 (OEA) and N-palmitoyl ethanolamide5 (PEA) have also been found endogenously).
Due to the diverse array of biological effects of anandamide, intensive research has focused on its biosynthetic6 and metabolic pathways.7-10 Studies have been carried out on the metabolism of anandamide by hydrolysis of the amide bond via the enzyme fatty acid amide hydrolase7 (FAAH), acknowledged as the chief enzyme responsible for termination of anandamide signaling in vivo, as well as oxidative metabolism with cyclooxygenases8 (COX), lipoxygenases9 (LOX) and cytochrome P450s10 (CYP 450) on the acyl chain of anandamide. However relatively little attention has been paid to the metabolism of the ethanolamine portion of the molecule. Anandamide has a primary alcohol group which makes it a potential substrate for several enzymes. Oxidation of anandamide can result in the formation of N-arachidonoyl glycine which belongs to another class of endogenous bioactive lipids, the long chain fatty acyl glycines or N-acyl glycines. Of these, N-arachidonoyl glycine exerts antinociceptive and anti-inflammatory effects,11 N-oleoyl glycine,12 produces hypothermia when administered to rodents, and N-palmitoyl glycine is a potent inhibitor of heat-evoked firing of nociceptive neurons in the rat dorsal horn, and also causes transient calcium influx in rat dorsal root ganglion cells and F11 cells.13 N-arachidonoyl glycine has been recently identified as an endogenous ligand for the orphan G-protein coupled receptors GPR18 and GPR92, adding to the emerging evidence that this class of lipids can function as intercellular messengers via cell surface receptors.14-15
Despite considerable interest in the biology and pharmacology of N-acyl glycines, not much is known about their biosynthetic pathways. Previously we reported doubly labeled N-arachidonoyl glycine formation following incubation of d8-arachidonic acid and d5-glycine in the presence of rat brain membranes.11 Additionally, N-arachidonoyl glycine formation was also observed after incubation of anandamide with RAW 264.7 cells.16 Recently Mueller et al reported cytochrome c-catalyzed biosynthesis of N-oleoyl glycine17 and N-arachidonoyl glycine18 by incubation of oleoyl CoA and arachidonoyl CoA with glycine and hydrogen peroxide. Here we report the human alcohol dehydrogenase- (ADH) catalyzed sequential oxidative metabolism of anandamide to N-arachidonoyl glycine which proceeds with the formation of the intermediate N-arachidonoyl glycinal.
All human alcohol dehydrogenase isoenzymes are NAD+-dependent, zinc metalloenzymes that are known to catalyze reversible oxidation of alcohols, including beverage ethanol and biologically important long chain alcohols like retinol; ω-hydroxy fatty acids; and 3β-hydroxysteroids.19-21 Each ADH isoenzyme is a dimer, comprised of two subunits and each subunit is comprised of two domains, a catalytic domain and the coenzyme NAD+ binding domain. Although seven ADH genes (ADH1-ADH7) have been identified in humans, the protein product of ADH6 gene has not been identified in vivo.19,22 Based on their amino acid sequences as well as their enzymatic and electrophoretic properties, ADH isoenzymes have been assigned to five distinct classes.23 All human ADH isoenzymes are primarily expressed in liver except for ADH7 which is mainly localized in epithelial tissue of the gastrointestinal tract like stomach mucosa.24,25 While the present findings show anandamide as a new substrate for human ADH7, its oxidation to N-arachidonoyl glycine may implicate ADH as one of the possible pathways in the generation of fatty acyl glycines from fatty acyl ethanolamides in vivo. In addition, a high yielding and straightforward synthesis of a series of N-acyl glycinals, intermediates in the alcohol dehydrogenase-catalyzed metabolism of fatty acyl ethanolamides has also been developed.
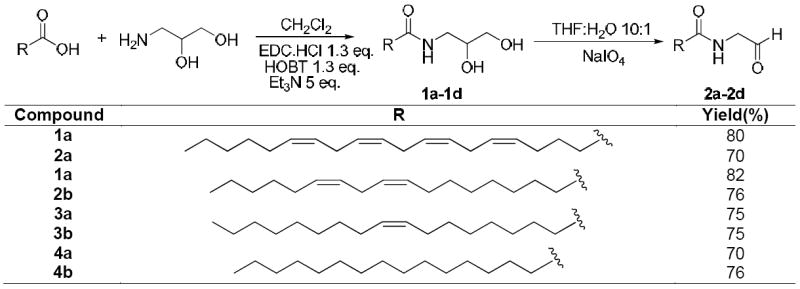
In an effort to explore ADH-catalyzed metabolism of anandamide, we first attempted chemical synthesis of N-arachidonoyl glycinal, the potential intermediate in the oxidation of anandamide to N-arachidonoyl glycine. Our initial efforts focused upon the direct oxidation of anandamide. A literature report, on the pyridiniumchlorochromate26 (PCC) oxidation of arachidonoyl alcohol to arachidonal was confirmed, however attempted oxidation of anandamide with this reagent led to complex mixtures that did not contain the product. Similarly, other conventional oxidants, DMSO27 based and tetrapropylammonium perruthenate28 (TPAP) among others failed to give the aldehyde. A more indirect two step route was adopted utilizing sodium periodate oxidation of the diol 1a according to Scheme 1. The diol 1a was synthesized by conjugation of arachidonic acid with 1,2-dihydroxy-3-aminopropane using combination of 1-ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride (EDC.HCl), N-hydroxybenzotriazole (HOBT) and triethylamine (Et3N) in dichloromethane at room temperature. Addition of arachidonic acid and amine in one portion gave a mixture of N-acylated (~30%) and O-acylated products, however a good yield of required N-acylated product 1a was obtained by slow addition of arachidonic acid to a mixture of 1,2-dihydroxy-3-aminopropane, EDC.HCl, HOBT and Et3N in dichloromethane over a period of 8 h under high dilution.29 The diol 1a was cleanly oxidized to aldehyde 2a in ~ 70% yield using sodium periodate in THF/H2O mixture.29 The analogous compounds 2b-2d were synthesized from their respective diols 1b-1d using the above methodology in 70 to 75% yields. The structures of all the compounds were confirmed by 1H and 13C NMR analysis.29
Scheme 1. Synthesis of N-acyl-(1,2-dihydroxypropyl)amides (1a-1d) and N-Acyl glycinals (2a-2d).
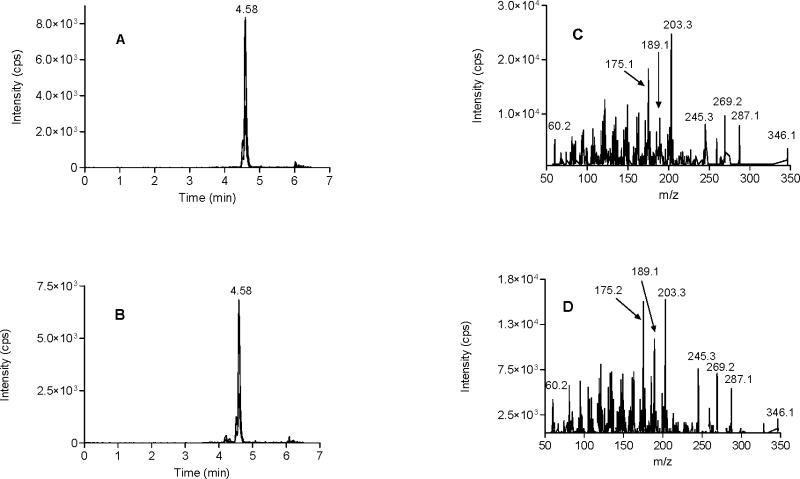
Purified recombinant human ADH7 isoenzyme was used to determine if anandamide was a substrate for human alcohol dehydrogenases.29 Initial attempts to monitor this reaction by following the production of NADH on a spectrophotometer at 340 nm were complicated by the apparent sequential oxidation of the alcohol to the acid and the apparent dismutation of the aldehyde to the corresponding alcohol and acid.30 Consequently, we chose to monitor the progress of the reactions by the multiple reaction monitoring method (MRM) using liquid chromatography-tandem mass spectrometry (LC/MS/MS). N-arachidonoyl glycinal is the potential product in the ADH-catalyzed oxidation of anandamide. Fig. 1A shows the MRM LC/MS/MS chromatogram of N-arachidonoyl glycinal standard in the positive ion mode where the mass of the parent ion (m/z 346) was paired with the mass of glycinal (m/z 60) and arachidonoyl (m/z 287) ions. Analysis of the assays containing anandamide, NAD+ and ADH7 using the above MRM method gave chromatograms with identical retention times as the synthetic sample (Fig. 1B). This peak was not observed in the absence of enzyme indicating that the product was formed by ADH7-catalyzed reaction. The identity of the product from the enzymatic assays was further confirmed by complete mass spectral fragmentation analysis using ESI MS/MS. Fig. 1C presents the product ion spectrum for N-arachidonoyl glycinal standard in the positive ion mode. The signature ions in the spectrum include the parent ion (m/z 346), the arachidonoyl (m/z 287) and the glycinal (m/z 60) fragments. Fig. 1D shows the product ion spectrum for the ADH7-synthesized N-arachidonoyl glycinal and this spectrum shows all the major fragments and is qualitatively identical to that of synthetic standard confirming ADH7 catalyzed synthesis of N-arachidonoyl glycinal from anandamide.
Figure 1.
Chromatographic and mass spectral fragmentation analysis of standard and ADH7-synthesized N-arachidonoyl glycinal. LC/MS/MS MRM chromatograms of standard and ADH7-synthesized N-arachidonoyl glycinal (A and B). MS/MS spectra of standard and ADH7-synthesized N-arachidonoyl glycinal (C and D).
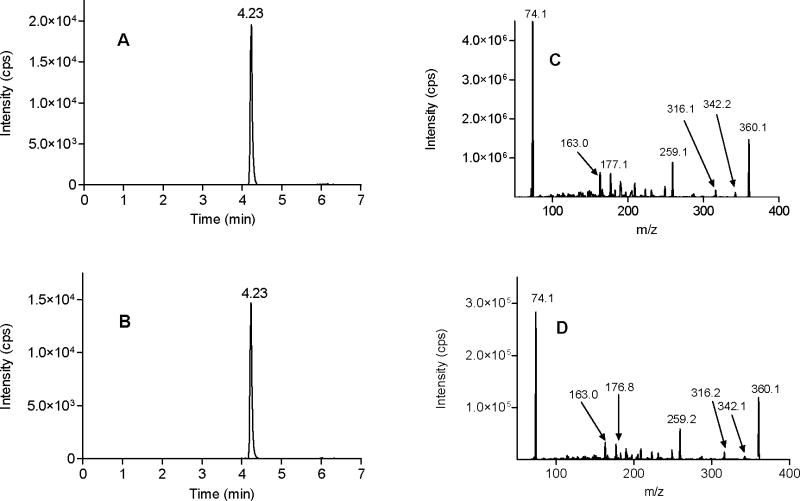
To explore if ADH7 also oxidized N-arachidonoyl glycinal to N-arachidonoyl glycine, we analyzed enzymatic assays for the presence of N-arachidonoyl glycine. Fig. 2A shows the MRM LC/MS/MS chromatogram for N-arachidonoyl glycine standard in the negative ion mode where the mass of the parent ion (m/z 360) is paired with the mass of glycine ion (m/z 74). Analysis of the enzymatic assays containing anandamide, NAD+ and ADH7 using the above MRM method gave chromatograms with identical retention times as the synthetic N-arachidonoyl glycine standard (Fig. 2B). This peak was not present in the absence of enzyme indicating that the product was formed by ADH7-catalyzed reaction. The identity of N-arachidonoyl glycine from the ADH7-catalyzed reaction mixtures was further confirmed by complete mass spectral fragmentation analysis using ESI MS/MS. Fig. 2C represents the product ion spectrum of N-arachidonoyl glycine standard in the negative ion mode and the characteristic fragments in the spectrum include arachidonoyl glycine parent ion (m/z 360), the glycine ion (m/z 74) and the fragments due to loss of water (m/z 342) and CO2 (m/z 316). Fig. 2D shows the product ion spectrum for the ADH7 synthesized N-arachidonoyl glycine and this spectrum is qualitatively identical to that of the synthetic standard. These results show that ADH7 oxidizes anandamide to N-arachidonoyl glycinal which is further metabolized in a sequential oxidation reaction to N-arachidonoyl glycine.
Figure 2.
Chromatographic and mass spectral fragmentation analysis of standard and ADH7-synthesized N-arachidonoyl glycine. LC/MS/MS MRM chromatograms of standard and ADH7-synthesized N-arachidonoyl glycine (A and B). MS/MS spectra of standard and ADH7-synthesized N-arachidonoyl glycine (C and D).
To further confirm the results, we carried out experiments with deuterated anandamide. Substitution of anandamide with d8-anandamide (8 olefinic hydrogens on the acyl chain are replaced by 8 deuteriums) or d4-anandamide (4 methylene hydrogens on the ethanolamine portion of anandamide are replaced by 4 deuteriums) in the reaction mixtures produced d8-arachidonoyl glycine and d2-arachidonoyl glycine respectively that are eight and two mass units heavier than the unlabeled N-arachidonoyl glycine confirming unequivocally that ADH7 metabolizes anandamide to N-arachidonoyl glycine.
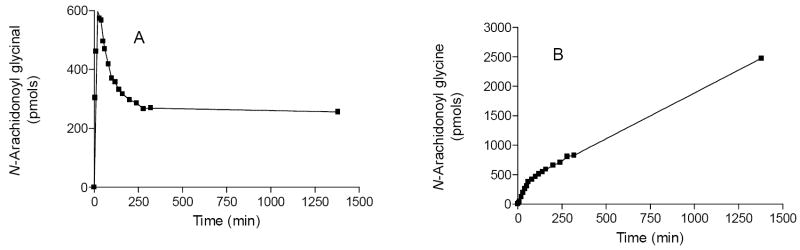
In an effort to determine the kinetic parameters for the reaction, we carried out time course experiments by varying the concentrations of anandamide. A representative time course data for the formation and decay of N-arachidonoyl glycinal is shown in Fig. 3A while Fig. 3B shows the time course for the formation of N-arachidonoyl glycine using 8 μM anandamide concentration. Initially there is a linear and time-dependent rise in the formation of N-arachidonoyl glycinal which reaches to a maximum at 20 min followed by a slow exponential decay to a steady concentration. The fall in the concentration is due to its sequential oxidation to N-arachidonoyl glycine in a time- and concentration-dependent manner that is linear for at least 30 min of the reaction progress. The peak due to N-arachidonoyl glycine is observed even at 2 min following the addition of enzyme, suggesting that there is a very short lag time before the formation of N-arachidonoyl glycine begins. This confirms that ADH7 metabolizes anandamide in two sequential oxidation reactions, implying that the system cannot be analyzed using simple Michaelis-Menton assumptions. Our preliminary results showed that the concentrations of N-arachidonoyl glycinal and N-arachidonoyl glycine increased with increase in anandamide concentrations (1 to 8 μM) but substrate inhibition was observed at higher concentrations (>9 μM) and concentrations lower than 1 μM were difficult to analyze using the MRM approach. Thus, a Km value could not be estimated with the present approach but appears to be below 1 μM. The initial velocity for the formation of N-arachidonoyl glycinal is 0.35 μM/min/μM protein at 8 μM anandamide concentration. This initial velocity (0.35 min-1) is about 275-fold lower than that determined for retinol oxidation by ADH7 suggesting anandamide to be a high affinity, but slow substrate.21 The slow rate is probably due to the ability of the product to remain bound to the substrate binding tunnel in ADH as the enzyme exchanges coenzyme for the next catalytic cycle, making product glycinal release the most probable rate-limiting step. This is consistent with other ADH enzyme:substrate combinations that show sequential oxidation and dismutation of aldehyde substrates.30 As mentioned above, ADH7 also catalyzed the reduction of N-arachidonoyl glycinal to anandamide, but this reaction was prone to dismutation which complicates its analysis and requires considerably more detailed kinetic studies to determine the kinetic parameters and compare them with the kinetic parameters of other anandamide metabolizing enzymes to gain insights into possibilities of ADH pathway of anandamide metabolism occurring in vivo.
Figure 3.
Time course for the formation of N-arachidonoyl glycinal (A) and N-arachidonoyl glycine (B) by human ADH7. Anandamide (8 μM) and NAD+ (2.4 mM) were incubated with human ADH7 (56 nM) in 100 mM sodium phosphate buffer, pH 7.4 (3 ml) at 37 °C for the times indicated. Aliquots (100 μL) were withdrawn, diluted with acetonitrile (100 μl) and analyzed by LC/MS/MS using MRM methods.
We investigated the affinity of anandamide for another human alcohol dehydrogenase, ADH5. The ADH5 isoenzyme, which is ubiquitously expressed, was essentially inactive. Our preliminary experiments also showed that the ADH7 isoenzyme metabolized other N-acyl ethanolamides like N-linoleoyl ethanolamide to N-linoleoyl glycinal and N-linoleoyl glycine. Although these results show that ADH7 can metabolize fatty acyl ethanolamides in vitro, the biological significance of this pathway of metabolism remains to be fully determined and the studies presented here represent an effort in that direction. Cannabinoid receptors and anandamide are present in the gastrointestinal tract and play a major role in several physiological and pathophysiological processes including obesity, feeding behavior, liver disease and alcoholism.1 Indeed the levels of anandamide are reported to be elevated in the gastrointestinal tract during pathological conditions31; therefore the presence of alcohol dehydrogenase isoenzymes in the same tissue may contribute to the regulation of local anandamide concentrations. Additionally the role of ADHs may not be limited to the metabolism of anandamide or N-acyl ethanolamides in general, they may also be important for the biosynthesis of a wide range of fatty acyl glycines from fatty acyl ethanolamides in these tissues.
In summary, we have successfully developed a simple and high yielding method for the synthesis of a series of long chain fatty N-acyl glycinals which can be potential intermediates in enzymatic oxidation reactions of N-acyl ethanolamides to N-acyl glycines in vivo. Although aldehydes bearing α-nitrogen are known to be inhibitors of hydrolytic enzyme like leucine aminopeptidase32, the biological effects of these compounds remain to be explored. We have also shown that anandamide is a substrate for human ADH7 and is metabolized to N-arachidonoyl glycinal, a new intermediate which is then sequentially metabolized by ADH7 to N-arachidonoyl glycine, which is emerging as an important lipid mediator and is an endogenous ligand for GPR18 and GPR92. This ADH7-catalyzed conversion of anandamide to N-arachidonyl glycine is analogous to other ADH-catalyzed oxidations of alcohols to acids like the ADH7-catalyzed oxidation of all trans-retinol to all trans-retinoic acid in human gastric mucosa.33 As our studies suggest that anandamide is a high affinity substrate for ADH in vitro, the presence of both anandamide and alcohol dehydrogenases in the gastrointestinal tract may make anandamide a potential substrate for alcohol dehydrogenases and one of the plausible pathways for deactivation of anandamide in vivo.
Supplementary Material
Acknowledgments
The authors are grateful to National Institute on Drug Abuse (Grant DA018224 to JMW), The Gill Center for Biomolecular Science, Indiana University, Bloomington, IN and the Lilly Foundation Inc., Indianapolis, IN for financial support.
Footnotes
This work is dedicated to the memory of Dr. J. Michael Walker
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fowler CJ. Mol Neurobiol. 2007;36:15. doi: 10.1007/s12035-007-0001-6. [DOI] [PubMed] [Google Scholar]; Fride E, Gobshtis N. Immunol Endocr & Metab Agents in Med Chem. 2007;7:157. [Google Scholar]; Mackie K. Annu Rev Pharmacol Toxicol. 2006;46:101. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]; Di Marzo V, Bifulco M, De Petrocellis L. Nat Rev Drug Discovery. 2004;3:771. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 2.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A. Science. 1992;258:1946. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 3.Schmid PC, Kuwae T, Krebsbach RJ, Schmid HHO. Chem Phys Lipids. 1997;87:103. doi: 10.1016/s0009-3084(97)00032-7. [DOI] [PubMed] [Google Scholar]; Lin S, Khanolkar AD, Fan P, Goutopoulos A, Qin C, Papahadjis D, Makriyannis A. J Med Chem. 1998;41:5353. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- 4.Di Tomaso E, Beltramo M, Piomelli D. Nature. 1996;382:677. doi: 10.1038/382677a0. [DOI] [PubMed] [Google Scholar]
- 5.Bachur NR, Masek K, Melmon KL, Udenfriend S. J Biol Chem. 1965;240:1019. [PubMed] [Google Scholar]
- 6.Natarajan V, Reddy PV, Schmid PC, Schmid HH. Biochim Biophys Acta. 1982;712:342. doi: 10.1016/0005-2760(82)90352-6. [DOI] [PubMed] [Google Scholar]; Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Nature. 1994;372:686. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]; Cadas H, di Tomaso E, Piomelli D. J Neurosci. 1997;17:1226. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; Simon GM, Cravatt BF. J Biol Chem. 2006;281:26465. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]; Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. Proc Natl Acad Sci USA. 2006;103:13345. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Simon GM, Cravatt BF. J Biol Chem. 2008;283:9341. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giang DK, Cravatt BF. Proc Natl Acad Sci USA. 1997;94:2238. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu M, Ives D, Ramesha CS. J Biol Chem. 1997;272:21181. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]; Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. J Biol Chem. 2002;277:44877. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 9.Hampson AJ, Hill WA, Zan-Phillips M, Makriyannis A, Leung E, Eglen RM, Bornheim LM. Biochem Biophys Acta. 1995;1259:173. doi: 10.1016/0005-2760(95)00157-8. [DOI] [PubMed] [Google Scholar]; Ueda N, Yamamoto K, Kurahashi Y, Yamamoto S, Ogawa M, Matsuki N, Kudo I, Shinkai H, Shirakawa E, Tokunaga T. Adv Prostaglandin Thromboxane Leukotriene Res. 1995;23:163. [PubMed] [Google Scholar]; Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Mol Pharmacol. 1998;54:180. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- 10.Bornheim LM, Kim KY, Chen B, Correia MA. Biochem Pharmacol. 1995;50:677. doi: 10.1016/0006-2952(95)00177-2. [DOI] [PubMed] [Google Scholar]; Snider NT, Kornilov AM, Kent UM, Hollenberg PF. J Pharmacol Exp Ther. 2007;321:590. doi: 10.1124/jpet.107.119321. [DOI] [PubMed] [Google Scholar]
- 11.Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. J Biol Chem. 2001;276:42639. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 12.Merkler DJ, Chew GH, Gee AJ, Merkler KA, Sorondo JP, Johnson ME. Biochemistry. 2004;43:12667. doi: 10.1021/bi049529p. [DOI] [PubMed] [Google Scholar]; Chaturvedi S, Driscoll WJ, Elliot BM, Faraday MM, Grunberg NE, Mueller GP. Prostaglandin Other Lipid Mediat. 2006;81:136. doi: 10.1016/j.prostaglandins.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimmerman N, Bradshaw HB, Hughes HV, Chen, Jay S-C, Hu SS-J, McHugh D, Vefring E, Jahnsen JA, Thompson EL, Masuda K, Cravatt BF, Burstein S, Vasko MR, Prieto AL, O’Dell DK, Walker JM. Mol Pharmacol. 2008;74:213. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. Biochem Biophy Res Commun. 2006;347:827. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 15.Oh DY, Yoon JM, Moon MJ, Hwang J-I, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O’Dell DK, Walker JM, Na HS, Lee MG, Kwon HB, Kim K, Seong JY. J Biol Chem. 2008;283:21054. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burstein SH, Rossetti RG, Yagen B, Zurier RB. Prostaglandins other lipid mediat. 2000;61:29. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]; Bradshaw HB, Hu SS-J, Rimmerman N, O’Dell DK, Masuda K, Cravatt BF, Walker JM. Abstract of papers, 16th annual symposium on the cannabinoids. Tihany, Hungary: International Cannabinoid Research Society (ICRS); 2006. [Google Scholar]
- 17.Muller GP, Driscoll WJ. J Biol Chem. 2007;282:22364. doi: 10.1074/jbc.M701801200. [DOI] [PubMed] [Google Scholar]
- 18.McCue JM, Driscoll WJ, Mueller GP. Biochem Biophys Res Commun. 2008;365:322. doi: 10.1016/j.bbrc.2007.10.175. [DOI] [PubMed] [Google Scholar]
- 19.Edenberg HJ, Bosron WF. In: Comprehensive Toxicology. Guengerich FP, editor. Vol. 3. Pergamon; New York: 1997. pp. 119–131. [Google Scholar]; Li T-K, Bosron WF. Ann NY Acad Sci. 1987;492:1. doi: 10.1111/j.1749-6632.1987.tb48648.x. [DOI] [PubMed] [Google Scholar]; Bosron WF, Li T-K. Enzyme. 1987;37:19. doi: 10.1159/000469238. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS. Clin Toxicol. 1994;32:631. doi: 10.3109/15563659409017974. [DOI] [PubMed] [Google Scholar]
- 21.Boleda MD, Saubi N, Farres J, Pares X. Arch Biochem Biophys. 1993;307:85. doi: 10.1006/abbi.1993.1564. [DOI] [PubMed] [Google Scholar]; Yang ZN, Davis GJ, Hurley TD, Stone CL, Li T-K, Bosron WF. Alcsm Clin Exp Res. 1994;18:587. doi: 10.1111/j.1530-0277.1994.tb00914.x. [DOI] [PubMed] [Google Scholar]; Sellin S, Holmquist B, Mannervik B, Vallee BL. Biochemistry. 1991;30:2514. doi: 10.1021/bi00223a031. [DOI] [PubMed] [Google Scholar]; Chou CF, Lai CL, Chang YC, Duester G, Yin SJ. J Biol Chem. 2002;277:25209. doi: 10.1074/jbc.M201947200. [DOI] [PubMed] [Google Scholar]
- 22.Kedishvili NY, Bosron WF, Stone CL, Hurley TD, Peggs CF, Thomasson HR, Popov KM, Carr LG, Edenberg HJ, Li T-K. J Biol Chem. 1995;270:3625. doi: 10.1074/jbc.270.8.3625. [DOI] [PubMed] [Google Scholar]
- 23.Vallee BL, Bazzone TJ. Isozymes Curr Top Biol Med Res. 1983;8:219. [PubMed] [Google Scholar]
- 24.Parés X, Moreno A, Cederlund E, Höög J-O, Jörnvall J. FEBS Lett. 1990;277:115. doi: 10.1016/0014-5793(90)80822-z. [DOI] [PubMed] [Google Scholar]
- 25.Stone CL, Thomasson HR, Bosron WF, Li T-K. Alcohol Clin Exp Res. 1993;17:911. doi: 10.1111/j.1530-0277.1993.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 26.Easton CJ, Xia L, Pitt MJ, Ferrante A, Poulos A, Rathjen DA. Synthesis. 2001:451. [Google Scholar]
- 27.Mancuso AJ, Huang S-L, Swern D. J Org Chem. 1978;43:2480. [Google Scholar]
- 28.Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994;7:639. [Google Scholar]; Hasan M, Musawir M, Davey NP, Kozhevnikov IV. J Mol Catal. 2002;180:77. [Google Scholar]
- 29.Synthesis, spectral data and enzyme assays are given in the supporting information.
- 30.Henehan GT, Oppenheimer NJ. Biochemistry. 1993;32:735. doi: 10.1021/bi00054a001. [DOI] [PubMed] [Google Scholar]; Svensson S, Lundsjo A, Cronholm T, Hoog JO. FEBS Lett. 1996;394:217. doi: 10.1016/0014-5793(96)00954-4. [DOI] [PubMed] [Google Scholar]
- 31.Capasso R, Izzo AA. J Neuroendocrinol. 2008;20:39. doi: 10.1111/j.1365-2826.2008.01686.x. [DOI] [PubMed] [Google Scholar]; Izzo AA, Mascolo N, Capasso F. Curr Opin Pharmacol. 2001;1:597. doi: 10.1016/s1471-4892(01)00102-3. [DOI] [PubMed] [Google Scholar]; Sanger GJ. Br J Pharmacol. 2007;152:663. doi: 10.1038/sj.bjp.0707422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson L, Isley TC, Wolfenden R. Biochemistry. 1982;21:4177. doi: 10.1021/bi00260a040. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto M, Yokoyama H, Suzuki H, Shiraishi-Yokoyama H, Hibi T. Am J Physiol Gastrointest Liver Physiol. 2005;289:G429–G433. doi: 10.1152/ajpgi.00502.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.