Abstract
After seeing a scene containing an emotional component (e.g., a snake in a forest) people often demonstrate a “trade-off” in memory, where memory for the emotional component (e.g., the snake) is good, but memory for the nonemotional elements (e.g., the forest) is poor. The result is an incomplete memory retaining central emotional information at the expense of neutral background information. Though almost everyone demonstrates the trade-off, there may be individual differences in the magnitude of the effect. We investigated whether differences in the strength of the trade-off would correlate with anxiety levels, working memory capacity, and executive functioning abilities. Sixty-four participants studied scenes comprised of a negative or neutral item placed on a neutral background, and memory was later tested for items and backgrounds separately. The magnitude of the trade-off correlated positively with anxiety and negatively with visuospatial working memory and executive function. These results suggest that greater anxiety, poor visuospatial working memory, and poor executive function may inhibit formation of complete mental representations of these complex emotional scenes.
Keywords: individual differences, emotion, memory, trade-off
Although memories often are retained with a great degree of clarity, it is well known that humans do not create truly photographic quality memories. Most of the time, we are unable to retain in memory all features of an event with vividness and accuracy. Rather, some features of an experience are recalled with vividness and accuracy, while others are less clear and may become distorted over time.
These effects have been noted many times in laboratory studies of memory for complex visual scenes. Although participants usually can remember at least some aspects of the scene, they often cannot remember all the scene’s details (Burke, Heuer, & Reisberg, 1992; Kensinger, Garoff-Eaton, & Schacter, 2007a). Interestingly, several factors impact the amount of detail remembered within a complex visual image, including the nature of the features contained within the image and individual differences in the types of information prioritized for processing and retention (Calvo & Avero, 2005; Easterbrook, 1959; Rohner, 2004).
In general, emotional content in an image is more likely to be detected and attended to at the outset and also better remembered than neutral information (Ohman, Flykt, & Esteves, 2001; Phelps, 2006). Aversive information in particular has the ability to automatically attract attention (Li, Li, & Luo, 2006), which may increase the likelihood that negative visual features will later be remembered (Kensinger, Garoff-Eaton, & Schacter, 2006). However, not all features in a complex emotional stimulus are better remembered than neutral stimuli, as the additional attention allocated to emotional stimuli can come at the expense of attention toward peripheral or contextual details (Kensinger et al., 2006, 2007a; Kensinger, Piguet, Krendl, & Corkin, 2005; Mathews & Mackintosh, 2004, but see Libkuman, Nichols-Whitehead, Griffith, & Thomas, 1999; Wessel, van der Kooy, & Merckelbach, 2000). This narrowing of attentional focus may create an enhancement in memory for emotional items with an accompanying deficit in memory for a scene’s background or context, where emotionally arousing critical details are remembered at the expense of their contextual elements (Easterbrook, 1959; Safer, Christianson, Autry, & Osterlund, 1998). The advantage in memory for emotional items in a scene, at the expense of memory for more background details, is called an emotion-induced memory trade-off (Buchanan & Adolphs, 2002; Kensinger et al., 2007a).
The trade-off seems to be particularly pronounced when emotion is elicited visually, with an emotionally arousing element acting as an “attention magnet” (reviewed by Reisberg & Heuer, 2004). By contrast, the trade-off seems to be evoked less often when emotion is induced thematically, through the development of a story (Laney, Campbell, Heuer, & Reisberg, 2004; Libkuman et al., 1999; Wessel et al., 2000). In these cases, arousal often leads to a universal improvement in memory for both central and peripheral information. These data suggest that there may be an aspect of how complex visual scenes are processed that makes them particularly likely to elicit the trade-off. Although it is unclear exactly which properties may lead to a pronounced trade-off, likely possibilities are that visual scenes usually contain more information than can be processed simultaneously (e.g., not all aspects of the forest can be viewed within the allotted time), the source of the emotional arousal is easily localizable (e.g., is a snake), and the emotional and nonemotional elements are usually distinct entities that are presented concurrently (e.g., the snake is presented concurrently with the other details of the forest). By contrast, most of these properties do not hold true of thematically-induced emotion.
Although a number of studies have confirmed the presence of emotion-related memory trade-offs, particularly when emotion is elicited through a visual element (Kensinger, Garoff-Eaton, & Schacter, 2007a, 2007b; Reisberg & Heuer, 2004; Safer et al., 1998; but see Laney et al., 2004; Libkuman et al., 1999; Wessel et al., 2000), past studies have not examined whether individual differences influence the magnitude of the trade-off. Yet, growing evidence suggests that while there may be many similarities in how individuals process information, there also may be systematic individual differences. In particular, a person’s dispositional characteristics, sex, or neuropsychological processing can influence emotional processing (reviewed by Hamann & Canli, 2004). Thus, to more completely understand emotional processing and emotional memory, it is essential to investigate these individual differences, as well as the commonalities shared among individuals.
To date, most of the studies examining individual differences have focused upon emotional processing rather than emotional memory (Canli, 2004; Hamann & Canli, 2004; Mathews, Yiend, & Lawrence, 2004; Wager, Phan, Liberzon, & Taylor, 2003; but see Cahill, Gorski, Belcher, & Huynh, 2004; Canli, Desmond, Zhao, & Gabrieli, 2002 for studies examining effects of sex on emotional memory). However, there is good reason to believe that differences in emotional processing would have subsequent effects on emotional memory. The types of information that are processed most efficiently and effectively are also those that are most likely to be retained over the short- and long-term, as has been shown using emotionally arousing stimuli (Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000; Schmidt & Saari, 2007; for review see Phelps, 2006). Thus, the present study extends prior research by examining the relationship of individual differences to emotional memory. Specifically, we investigated how individual differences relate to the magnitude of the emotion-induced memory trade-off. We chose to focus on three specific types of individual differences: level of anxiety, working memory capacity, and executive functioning ability.
Anxiety is one dispositional characteristic that affects processing of emotional stimuli. Compared to lower anxiety persons, those with high anxiety show an attentional bias and increased vigilance towards threatening information, which is independent of response bias (Calvo & Avero, 2005; Williams, Watts, MacLeod, & Mathews, 1999, for review). They also attend disproportionately to even mildly negative stimuli, possibly because they interpret them as more threatening than do lower trait-anxious individuals (Calvo & Avero, 2005; Rohner, 2004). Highly anxious individuals not only orient their attention toward threatening information more often than less anxious individuals (e.g., Rohner, 2004), they also have difficulty disengaging attention from threat-related stimuli (e.g., Fox, Russo, Bowles, & Dutton, 2001). These anxiety-induced changes in attention appear to have downstream effects upon the way in which information is remembered; for example, anxious individuals are more likely than are non-anxious individuals to remember pictures as being “zoomed in” on the emotional elements (Mathews & Mackintosh, 2004). Further evidence of this narrowed memorial focus is found in spider phobics; when confronted with a large spider, they show no difference from controls in memory for details central to the experience, yet they have poor memory for peripheral experiential details (Wessel & Merckelbach, 1997). The present study examined whether there would be mnemonic effects of sub-clinical anxiety, and in particular whether higher anxiety levels would enhance the magnitude of the emotion-induced memory trade-off. Because even individuals with sub-clinical anxiety demonstrate a mood-congruent attentional bias toward negative information (Calvo & Avero, 2005; Rohner, 2004), it seemed likely that they also would display a greater trade-off than less anxious individuals.
Cognitive factors, as well as affective ones, are likely to influence how people attend to, and later remember, presented information. In particular, working memory–the ability to actively maintain and update information stored in mind (for review see Courtney, 2004; Munakata, Morton, & O’Reilly, 2007) - is correlated with a person’s ability to attend to select aspects of visual scenes (Conway, Jarrold, Kane, Miyake, & Towse, 2007) and to flexibly engage and disengage attention as required by task demands (Hasher, 2007). Although the existing literature mainly has investigated the application of working memory resources to the processing of neutral arrays of stimuli (Lepsien & Nobre, 2006; Smith & Jonides, 1999), it seems likely that individual differences in working memory would impact the emotion-induced trade-off as well. Specifically, individuals with better working memory ability should show more flexible guidance of attention and therefore a reduced emotion-induced memory trade-off.
Working memory typically is divided into four components: a central executive (or set of executive functions) - allowing a person to plan, initiate, modify, and carry through with goal-oriented behavior and to inhibit task-irrelevant behaviors; two maintenance systems, one which stores and updates verbal information and the other which stores and updates visuospatial information; and an episodic memory buffer (Baddeley, 1996, 2000). The present study focuses on the executive and visuospatial maintenance components, as they are most relevant to the task at hand.
Visuospatial maintenance and updating is essential to visual scene processing, and tends to have stronger correlations with general “fluid intelligence” (the ability to acquire new skills and to respond in a flexible manner; Horn & Cattell, 1966) than does the verbal component (Hale, Myerson, Emery, Lawrence, & Dufault, 2007). Executive functioning, or cognitive control, also is essential for scene processing. It conveys the top-down processes that act to overcome bottom-up capture of attention and to focus attention in a flexible and goal-relevant manner (Henderson, 2007; Kane, Conway, Hambrick, & Engle, 2007). Together, visuospatial working memory and executive control may permit processing of both the central emotional features automatically attracting attention and the more peripheral details requiring cognitively guided application of attentional resources, thereby reducing the magnitude of the emotion-induced memory trade-off.
To examine the relationship between individual differences and the emotion-induced memory trade-off, we showed young adults photographic scenes containing either neutral or highly arousing negative items presented within the context of a neutral background (e.g., a chipmunk by a river or a snake by a river). At a later recognition memory test, participants viewed items and backgrounds separately. The trade-off in memory occurred when participants remembered negative arousing items better than neutral items but remembered backgrounds previously associated with negative arousing items more poorly than those associated with neutral items. These trade-off values were correlated with individual differences in performance on measures of anxiety, executive functioning, and visuospatial working memory ability. We assessed the magnitude of trade-off for two different types of memory: memory for the specific visual details of items and backgrounds (e.g., remembering exactly what the snake or the river looked like) and memory for the general features of items but not necessarily their precise visual details (e.g., remembering that a snake or a river was in the scene, but not necessarily remembering its exact visual details). We focused on these two trade-off scores because prior research has shown that emotion can influence the visual specificity with which information is remembered (e.g., Kensinger, et al., 2006) and that the trade-off in memory can be greater for specific visual details than for general item information (e.g., Kensinger et al., 2007a).
Methods
Participants
The data reported here are from sixty-four participants (31 men and 33 women, mean age = 20.67; range = 18–30 years) who participated in an experiment for payment or class credit. All participants were native English speakers, had normal or corrected to normal vision, had no history of psychiatric disorder or prior neurological trauma, and were not currently taking centrally-acting medications. Participants scored within the normal range on a measure of depression level (Koenig, Meador, Cohen, & Blazer, 1988). Informed consent was obtained from each person in a method approved by the Institutional Review Boards of Harvard University and Boston College.
Materials
Stimuli included images of negative arousing (e.g., snake) or neutral (e.g., chipmunk) items, and neutral background scenes (e.g., a river). A separate group of eight young adult participants previously had rated the stimuli for valence and arousal, and stimuli were selected for inclusion when there was agreement about the classifications across participants. Negative stimuli were low in valence, rated less than 3 on a scale of 1–7 (where lower numbers signify a more negative image and higher values signify a more positive image), and high in arousal, rated greater than 4 on a scale of 1–7 (where higher numbers describe more exciting or agitating images and lower numbers indicate more calming or soothing images). Neutral stimuli were in the mid-range for valence (rated 3–5, i.e., neither positive nor negative) and were low in arousal (ratings less than 4). Negative items were more negative than neutral items and were higher in arousal (p<.001). Composite images were created by placing an item onto a plausible background scene. Two versions of each item (e.g., 2 snakes) and background scene (e.g., 2 rivers) were chosen that differed in visual detail, but shared the same semantic label (see Fig. 1A). Eight versions of each scene theme were created to vary the emotion type and identity of the central item as well as the identity of the peripheral background viewed across participants (i.e., snake 1 or 2 with river 1 or 2; chipmunk 1 or 2 with river 1 or 2). Scenes were all of comparable size and presented against a white background on a computer screen directly in front of the participant.
Figure 1.
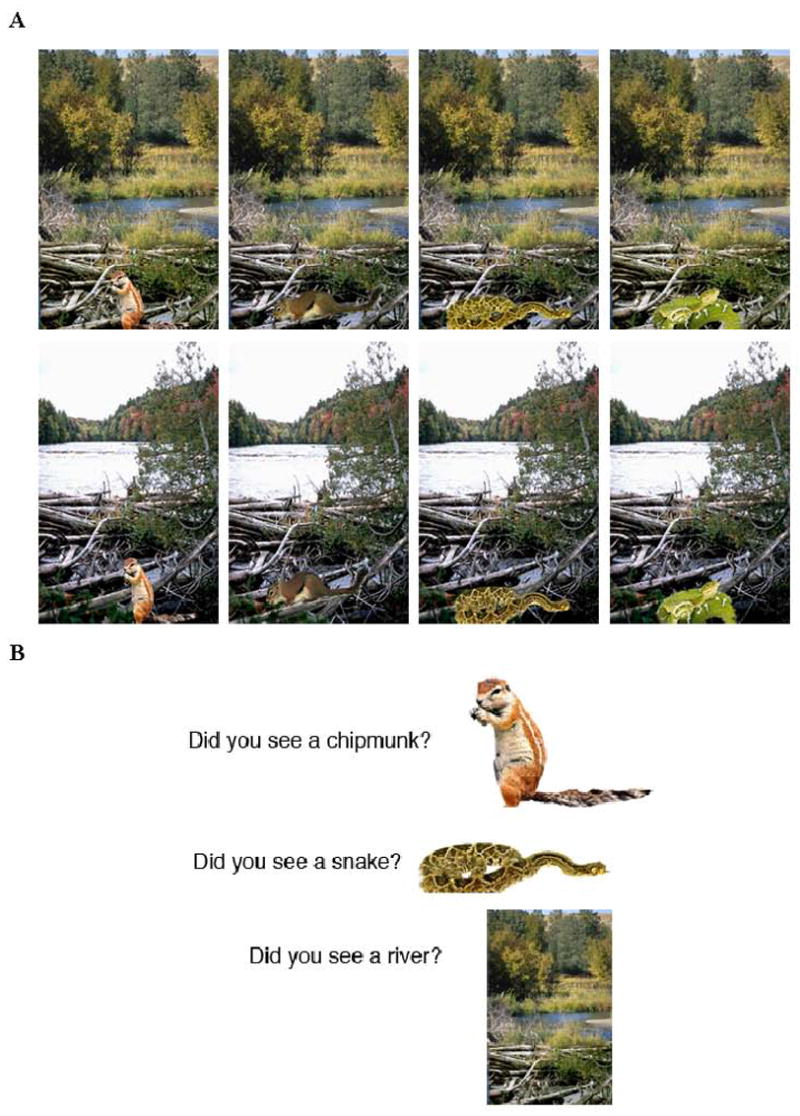
Participants studied one of eight possible versions of each scene theme, consisting of an item (either negative or neutral) placed on a neutral background. Scene versions were created by placing of two possible versions of either a negative or neutral item onto one of two versions of a neutral background (A). At test, items and backgrounds were presented separately and participants were asked to indicate whether each item or background was the same identical stimulus that had been previously studied, was one that was similar in theme to one that was studied although not identical, or was new (B).
For the purposes of this experiment (as in Kensinger et al., 2007a, 2007b,), the term “central” refers to the item (e.g., snake or chipmunk) and the term “peripheral” refers to the background (e.g., river). This terminology is consistent with prior studies that have defined these terms in relation to the spatial or thematic link to the emotional component of the stimulus (Burke et al., 1992; Heuer & Reisberg, 1990). In other words, “central” defines the aspects tied to the emotionality of the scene and “peripheral” defines those aspects more tangential to those emotional elements.
Procedure
During the study session, participants viewed 64 composite images, half with neutral items and half with negative arousing items displayed on neutral backgrounds. They performed an incidental encoding task where they were asked to indicate if they preferred to approach or retreat from each scene, using a 1–7 scale (1=move extremely close, 4=stay at present location, 7=move extremely far away). After a delay period, a surprise recognition test assessed memory for items and backgrounds presented independently (see Fig. 1B). Because the experimental task data analyzed here originally were gathered in two previous studies (portions reported in Kensinger et al., 2007a; and in Payne, Stickgold, Swanberg, & Kensinger, in press), there were some procedural variations across participants: 34 participants performed the recognition memory task after a short delay (30 minutes), while 30 participants performed the task after a longer delay (12 waking hours). Of those in the short delay condition, 16 participants viewed each scene for 2 sec., and 18 participants viewed each scene for 5 sec. The results of Kensinger et al., (2007a) and Payne et al., (in press) reported no significant differences, for the subset of data used here, of either presentation rate or delay period upon the magnitude of the trade-off in emotional memory. For this reason, we did not expect to find an effect of delay or presentation rate when data from these two studies were collapsed. Indeed, these different procedural conditions were analyzed together in the present study because they did not significantly influence relationships with magnitude of the memory trade-off 1.
In the test session, participants were asked whether each item or background shown was identical to one they had studied earlier (“same”), was one that shared the same verbal label but was not identical (“similar”), or was one that had not been studied (“new”). Test materials included 16 items and 16 backgrounds from each of four categories: same negative, same neutral, similar negative, and similar neutral. Additionally, the test included 32 new negative items, 32 new neutral items, and 64 new backgrounds, for a total of 256 scene components presented. Although same and similar items were included with equal frequency on the recognition memory task to prevent participants from being biased to give one response or the other, analyses were restricted to same items because such responses are more straightforward to interpret (see Kensinger et al., 2006 see Kensinger et al., 2007a for further discussion).
Recognition Memory Analyses
The trade-off in memory, resulting in enhancement for emotionally relevant items at the expense of other features, may be manifested in a general level of memory accuracy for the features of a scene, or in a more elaborate and specific detail-oriented memory. The effects of emotion on memory often differ for gist-based information versus for more specific details (Buchanan & Adolphs, 2002; Kensinger et al., 2007a) and therefore, the magnitude of trade-off was calculated separately for general recognition and specific recognition. “Same” responses to same items and backgrounds were used as a measure of specific recognition, memory for the exact visual detail of a studied item or background. The sum of “same” and “similar” responses to same items and backgrounds was used as a measure of general recognition memory, memory for at least some characteristic of the studied item or background, if not details of the exemplar studied. Recognition values were calculated separately for four scene component types: negative items, neutral items, backgrounds originally paired with negative items, and backgrounds originally paired with neutral items. The trade-off was calculated as the combined benefit in memory for negative items and deficit in memory for backgrounds presented with negative items. Thus, the largest trade-offs would occur when memory for negative items was much better than memory for neutral items, and when memory for backgrounds was much worse for those that had been studied with negative items than for those that had been studied with neutral items. These individual components of the tradeoff (i.e., the enhancement in memory for emotional items and the decrement in memory for the backgrounds paired with emotional items) also were analyzed.
Individual Differences Measures
The Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) was administered twice, once prior to the experiment, and again directly following the study portion of the experimental task. The administration prior to the study was designed to measure trait anxiety, where participants were asked to assess their level of anxiety experienced during the past month. The post-study assessment was intended to measure state of anxiety experienced after viewing the images. Visuospatial working memory ability was measured by performance on the Spatial Span task reverse portion (Wechsler, 1997), where participants must keep a layout of a visual arrangement in mind for a short time and use the information to guide future responses. Visuospatial working memory ability was also assessed by the number of total errors and number of trials with errors on the Self-Ordered Pointing of locations (Petrides & Milner, 1982), which requires participants to remember the spatial location of boxes chosen on a page. Results of Self-Ordered Pointing of locations were not included in final analyses because participants had difficulty following task instructions to choose locations randomly. Many participants later indicated they had used a selection strategy to perform the task. Visual working memory was measured as number of total errors and number of trials with errors made on Self-Ordered Pointing of patterns (Petrides & Milner, 1982), which requires participants to remember the identities of visual patterns regardless of spatial location. Executive functioning was assessed using number and percentage of perseverations on the verbal fluency to letters task (Monsch, Bondi, Butters, Salmon, Katzman, & Thal, 1992), and by the Dysexecutive questionnaire (Wilson, Alderman, Burgess, Emslie, & Evans, 1996). (Further description of tasks found in Table 1.) Performance on each of these tasks was correlated with the trade-off scores described above.
Table 1.
Descriptions of individual differences measures
| Construct | Measure | Instructions |
|---|---|---|
| Verbal Storage and Manipulation | Spatial Span, backwards | repeat digits in the opposite order as they were read |
| Nonverbal Storage and Manipulation | Self-Ordered Pointing of Patterns | On each of 12 trials, select a different visual pattern (from among 12 choices) so that no pattern is repeatedly selected |
| Self-Ordered Pointing of Locations | On each of 12 trials, select a different visual location (from among 12 choices) so that no location is repeatedly selected | |
| Inhibitory ability | Verbal fluency | Generate as many words beginning with a particular letter (F, A, or S) as possible in 1 min without repeating words |
Because eight correlations were performed, we applied a Bonferroni correction in order to avoid Type 1 error. We adjusted the α level from .05 to .05 divided by 8, or .006, meaning that we only considered correlations of p=.006 or less to be significant. Applying this threshold did not change the interpretation of results.
Results
Recognition Memory Performance
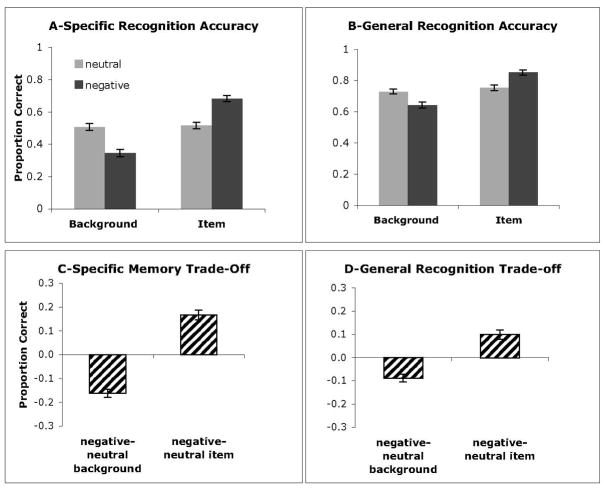
Separate two-way ANOVAs were calculated for the specific and general recognition scores, with factors of emotion (negative, neutral) and component (item, background). Results of both analyses revealed interactions between emotion and component (specific recognition, F(1,63)=162.09, p<.0005, partial η2=.72; general recognition, F(1,63)= 52.08, p<.0005, partial η2=.45). This interaction reflects the emotion-induced trade-off: memory is enhanced for the negative items as compared to the neutral items, but is reduced for the backgrounds presented with negative items as compared to the backgrounds presented with neutral items (see Fig. 2). Since the tradeoff in memory for emotional scenes persisted in general recognition memory and specific recognition memory, trade-off values were computed separately for each memory score. These trade-off values were calculated as follows: [negative item recognition − neutral item recognition] + [recognition for background paired with neutral item − recognition for background paired with negative item]. The advantage in memory for emotional compared to neutral items, and the decrement in memory for backgrounds paired with emotional compared to neutral stimuli, showed significant opposing differences (specific memory better for emotional than for neutral items, t(63) = 8.22, p<.0005, and worse for emotional than for neutral backgrounds, t(63) = 9.67, p<.0005; general memory better for emotional compared to neutral items t(63) = 5.06, p<.0005, and worse for emotional compared to neutral backgrounds, t(63) = 5.33, p<.0005). These results confirm that the emotional memory trade-off is due to both enhanced memory for emotional items and to reduced memory for emotional backgrounds. These trade-off values were then subjected to additional analyses to examine the effects of individual differences on the magnitude of the trade-off.
Figure 2.
Specific recognition (A) and general recognition (B) scores revealed similar patterns of results: Backgrounds previously presented with neutral items were better recognized than those presented with negative items. Negative items were remembered better than neutral items. Values representing the trade-offs in memory were calculated by subtracting recognition scores for neutral elements from those of negative elements (C & D). There was a deficit in memory for backgrounds presented with negative items, relative to those with neutral items, and an enhancement in memory for negative relative to neutral items. The trade-off was in memory was stronger for specific than general recognition memory.
Effects of Sex on Recognition Memory
There were no significant differences in magnitude of trade-off between sexes (specific recognition, (F(1,63)<1), Female M=.51 (SE=.06), Male M=.57 (SE=.10); general recognition, (F(1,63)<1), Female M=.17 (SE=.04), Male M=.21, (SE=.03)), so data from men and women were combined in all analyses.
Correlations Between Anxiety Levels and Recognition Memory
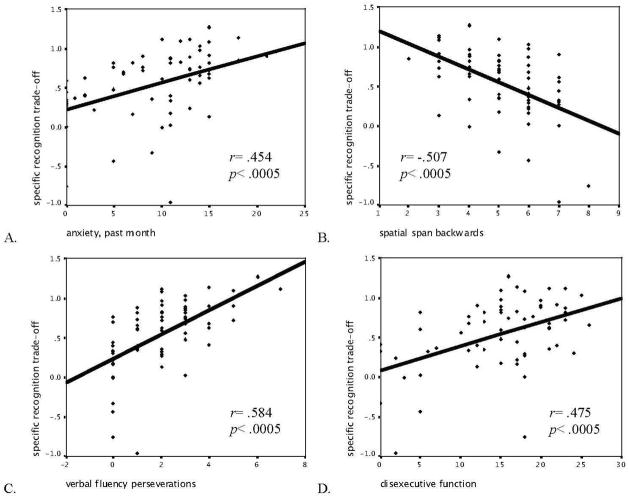
The specific recognition memory trade-off positively correlated with both versions of the anxiety questionnaire: the one taken prior to the study assessing trait anxiety (r=.45, p<.0005; see Fig. 3A), and the one assessing feelings of anxiety in response to viewing the stimuli (r=.42, p<.001; see Table 2). Responses on the two anxiety questionnaires also were highly correlated with one another (r=.43, p<.0005). When we examined whether anxiety correlated with the enhancement in memory for emotional items or with the decrement in memory for the backgrounds of emotional scenes, the results revealed that reported anxiety prior to testing was significantly related to the trade-off in memory for specific backgrounds; the correlation between anxiety after viewing the scenes and the memory decrement for backgrounds was in the same direction but did not reach significance (see Table 3). The general recognition memory trade-off did not correlate with scores on either administration of the anxiety questionnaire (all p>.3).
Figure 3.
Correlations between specific recognition memory trade-off and results of neuropsychological testing.
Table 2.
Correlations between trade-offs in memory and neuropsychological measures
| Test | Specific Recognition Trade-off | General Recognition Trade-off | |
|---|---|---|---|
| Anxiety Measures | |||
| Beck Anxiety Inventory, past month | r | 0.454 | 0.075 |
| (Beck et al., 1988) | p | **<.0005 | 0.554 |
| Beck Anxiety Inventory, post-study | 0.421 | 0.125 | |
| (Beck et al., 1988) | **0.001 | 0.327 | |
| Visuospatial Working Memory | |||
| Spatial Span, backwards | −0.507 | −0.069 | |
| (Wechsler, 1997) | **<.0005 | 0.589 | |
| Visual Working Memory | |||
| Self-Ordered Pointing, total number of errors | −0.048 | −0.140 | |
| (Petrides & Milner, 1982) | 0.705 | 0.269 | |
| Self-Ordered Pointing, number of trials with errors | −0.119 | −0.213 | |
| (Petrides & Milner, 1982) | 0.349 | 0.091 | |
| Executive Function | |||
| Verbal fluency, number of perseverations | 0.584 | 0.411 | |
| (Monsch et al., 1992) | **<.0005 | **0.001 | |
| Verbal fluency, percentage of perseverations | 0.569 | 0.430 | |
| (Monsch et al., 1992) | **<.0005 | **<.0005 | |
| Disexecutive questionnaire | 0.475 | 0.250 | |
| (Wilson et al., 1996) | **<.0005 | 0.047 | |
For all measures, higher scores indicate worse performance, except for Spatial Span
indicates correlation significant at p <.006
Table 3.
Correlations between individual differences variables and components of trade-off in emotional memory
| Test | specific recognition |
general recognition |
||||
|---|---|---|---|---|---|---|
| items | backgrounds | items | backgrounds | |||
| Anxiety Measures | ||||||
| Beck Anxiety Inventory, past month (BAI-M) | r | 0.15 | −0.37 | −0.01 | −0.13 | |
| (Beck et al., 1988) | p | 0.22 | **.003 | 0.96 | 0.32 | |
| Beck Anxiety Inventory, post-study (BAI-S) | 0.18 | −0.24 | 0.16 | −0.01 | ||
| (Beck et al., 1988) | 0.16 | 0.06 | 0.21 | 0.95 | ||
| Visuospatial Working Memory | ||||||
| Spatial Span, backwards (SS) | −0.14 | 0.32 | 0.27 | 0.14 | ||
| (Wechsler, 1997) | 0.27 | 0.01 | 0.03 | 0.28 | ||
| Visual Working Memory | ||||||
| Self-Ordered Pointing, total number of errors (SOP-E) | −0.02 | 0.01 | −0.18 | 0.12 | ||
| (Petrides & Milner, 1982) | 0.91 | 0.96 | 0.16 | 0.34 | ||
| Self-Ordered Pointing, number of trials with errors (SOP-T) | −0.09 | 0.00 | −0.18 | 0.01 | ||
| (Petrides & Milner, 1982) | 0.49 | 0.98 | 0.17 | 0.93 | ||
| Executive Function | ||||||
| Verbal fluency, number of perseverations (VF #) | 0.39 | −0.42 | 0.31 | −0.28 | ||
| (Monsch et al., 1992) | **.001 | **.001 | 0.01 | 0.02 | ||
| Verbal fluency, percentage of perseverations (VF %) | 0.38 | −0.41 | 0.32 | −0.30 | ||
| (Monsch et al., 1992) | **.002 | **.001 | 0.01 | 0.02 | ||
| Disexecutive questionnaire (DEX) | 0.34 | −0.20 | 0.34 | 0.01 | ||
| (Wilson et al., 1996) | **.006 | 0.12 | **.006 | 0.94 | ||
Trade-off in memory calculated separately for items and backgrounds as emotional minus neutral scene element
indicates significant at threshold of p=.006, when applying Bonferroni correction.
Correlations Between Working Memory and Recognition Memory
Visuospatial working memory, as assessed by performance on the Spatial Span backwards portion, correlated negatively (r=−.51, p<.0005) with the overall specific recognition trade-off (see Fig. 3B), but not with the memory enhancement for emotional items or the decrement in memory for backgrounds presented with emotional items when those constructs were measured in isolation (see Table 3). The number of errors and number of trials with errors on the Self-Ordered Pointing of patterns task, which are indicators of poor visual working memory, did not significantly correlate with specific recognition trade-off (p>.3). Finally, general recognition trade-off scores did not correlate significantly with performance on any tasks assessing working memory (all p>.09; see Table 2).
Correlations Between Executive Functioning and Recognition Memory
There was a positive correlation (r=.58, p<.0005, see Fig. 3C; r=.57, p<.0005) between specific recognition memory trade-off and the number and percentage of perseverations on the verbal fluency to letters task (with higher values signifying poorer performance). Number and percentage of perseverations significantly correlated with both components of the trade-off: the enhancement in memory for emotional items and the decrement in memory for backgrounds presented with emotional items (see Table 3). Specific recognition memory trade-off also correlated positively with scores on the Dysexecutive questionnaire (higher reported values on the questionnaire signify less executive function. r=.48, p<.0005, see Fig. 3D), and the Dysexecutive questionnaire was particularly related to the enhancement in specific recognition for emotional items (see Table 3). General recognition significantly correlated (r=.41, p<.001; r=.43, p<.0005) with the number and percentage of perseverations made on the verbal fluency to letters task (see Table 2), but not with the Dysexecutive questionnaire (r=.25, p=.05). However, although the Dysexecutive questionnaire did not correlate with the overall trade-off in general recognition, it did correspond with enhanced memory for emotional items (rs=.34, ps=.006, see Table 3), indicating that less executive functioning is related to greater item memory trade-off for both specific and general recognition
Partial correlations
Because there were significant correlations found between test measures (see Table 4), partial correlations were computed. Controlling for the influence of anxiety upon visuospatial working memory or executive function did not significantly change the pattern of results for the trade-off in emotional memory (all absolute values of r>.35, p<.006). Similarly, anxiety remained correlated with the trade-off even when controlling for effects of visuospatial working memory ability or executive function. In no case was the strength of the correlation significantly reduced by partialling out covariance of another factor (z(r) <1.17, p>.20).
Table 4.
Intercorrelations between neuropsychological measures
| Test | Anxiety |
V-S WM |
V WM |
Executive Function |
|||||
|---|---|---|---|---|---|---|---|---|---|
| BAI-M | BAI-S | SS | SOP-E | SOP-T | VF # | VF % | DEX | ||
| Anxiety Measures | |||||||||
| Beck Anxiety Inventory, past month (BAI-M) | r | 1.00 | 0.43 | −0.53 | −0.11 | −0.14 | 0.38 | 0.42 | 0.12 |
| (Beck et al., 1988) | p | * | **0.00 | **0.00 | 0.40 | 0.29 | **0.00 | **0.00 | 0.35 |
| Beck Anxiety Inventory, post-study (BAI-S) | 0.43 | 1.00 | −0.34 | −0.29 | −0.28 | 0.43 | 0.43 | 0.30 | |
| (Beck et al., 1988) | **0.00 | * | **0.00 | 0.02 | 0.02 | **0.00 | **0.00 | 0.02 | |
| Visuospatial Working Memory | |||||||||
| Spatial Span, backwards (SS) | −0.53 | −0.34 | 1.00 | 0.08 | 0.02 | −0.27 | −0.26 | −0.18 | |
| (Wechsler, 1997) | **0.00 | **0.00 | * | 0.54 | 0.86 | 0.03 | 0.04 | 0.15 | |
| Visual Working Memory | |||||||||
| Self-Ordered Pointing, total number of errors (SOP-E) | −0.11 | −0.29 | 0.08 | 1.00 | 0.79 | −0.15 | −0.17 | −0.04 | |
| (Petrides & Milner, 1982) | 0.40 | 0.02 | 0.54 | * | **0.00 | 0.24 | 0.17 | 0.73 | |
| Self-Ordered Pointing, number of trials with errors (SOP-T) | −0.14 | −0.28 | 0.02 | 0.79 | 1.00 | −0.08 | −0.11 | −0.09 | |
| (Petrides & Milner, 1982) | 0.29 | 0.02 | 0.86 | **0.00 | * | 0.52 | 0.41 | 0.51 | |
| Executive Function | |||||||||
| Verbal fluency, number of perseverations (VF #) | 0.38 | 0.43 | −0.27 | −0.15 | −0.08 | 1.00 | 0.98 | 0.25 | |
| (Monsch et al., 1992) | **0.00 | **0.00 | 0.03 | 0.24 | 0.52 | * | **0.00 | 0.05 | |
| Verbal fluency, percentage of perseverations (VF %) | 0.42 | 0.43 | −0.26 | −0.17 | −0.11 | 0.98 | 1.00 | 0.25 | |
| (Monsch et al., 1992) | **0.00 | **0.00 | 0.04 | 0.17 | 0.41 | **0.00 | * | 0.05 | |
| Disexecutive questionnaire (DEX) | 0.12 | 0.30 | −0.18 | −0.04 | −0.09 | 0.25 | 0.25 | 1.00 | |
| (Wilson et al., 1996) | 0.35 | 0.02 | 0.15 | 0.73 | 0.51 | 0.05 | 0.05 | * | |
indicates significant at p<.006 when applying Bonferroni correction.
False Alarm Rates
To consider the possibility that response bias may influence the trade-off effect, we examined (a) false alarm rate to negative items, (b) the difference in false alarm rate to negative items versus neutral items, and (c) the rate of false alarms to backgrounds. These false alarm rates were not significantly correlated with the individual differences measures (none of the correlations survived Bonferroni correction; all absolute values of r< .26, p>.04). These results suggest that individual differences in sensitivity to the trade-off effect do not result solely from individual differences in response bias.
Discussion
Results of this study demonstrate the predicted trade-off in specific and general recognition memory for emotional scenes, with memory enhancement for emotionally arousing negative items coming at the expense of memory for peripheral background scene details. Although the trade-off existed for both specific and general recognition scores, a greater trade-off was apparent for specific recognition scores. This finding is consistent with prior evidence that negative emotion may encourage more detail-oriented processing of information, while particularly impairing memory for specific details tangential to the emotional aspects of a scene or event (Adolphs, Denberg, & Tranel, 2001; Adolphs, Tranel, & Buchanan, 2005; Denberg, Buchanan, Tranel, & Adolphs, 2003; Kensinger et al., 2006, 2007a).
Although a robust trade-off was found here, particularly for the specific recognition scores, individual differences in anxiety, visuospatial working memory, and executive function were related to the magnitude of the trade-off. Higher levels of anxiety correlated with a larger trade-off in specific recognition. These correlations existed both with reports of current levels of state anxiety and also with more trait-based reports of levels of anxiety experienced over the past month.
Closer analyses revealed that feelings of anxiety in response to viewing the stimuli strongly correlated with the memory decrement for backgrounds presented with emotional items, perhaps suggesting that anxiety prevents participants from switching their attention to those nonemotional scene elements. This finding is consistent with prior studies demonstrating that anxiety increases a person’s focus on threat-related information (Calvo & Avero, 2005; Fox et al., 2001; Rohner, 2004), making it harder for the individual to divert attention away from that information (Fox et al., 2001), and to remember more peripheral details of the experience (Wessel & Merckelbach, 1997). Though prior research suggests that anxiety may be influencing attention allocation during encoding, this correlational study cannot identify whether anxiety influences the memory trade-off at the level of attention, consolidation, or retrieval.
The specific recognition trade-off also correlated significantly with poorer performance on measures of cognitive control processes, including visuospatial working memory and executive functioning. Cognitive control and working memory processes are coordinated to reduce the effects of distractors in selective attention tasks (Lavie, Hirst, Fockert, & Viding, 2004). Less attentional control leads to slower, more effortful focusing of visual attention upon a central goal-relevant stimulus when attempting to exclude competing information from the periphery, resulting in poorer performance upon working memory span tasks (Heitz & Engle, 2007). Therefore, it follows that people who are better able to use executive control mechanisms to disengage from an automatic attentional focus upon emotionally arousing stimuli would be better at monitoring the visual landscape, allowing a more complete representation of the scene within memory. Indeed, our results show a strong inverse correlation between measures of executive functioning and emotional memory trade-off, with both independent factors comprising the trade-off, as well as the overall trade-off increasing as executive functioning decreased.
Interestingly, however, neither anxiety nor visuospatial working memory scores correlated with general recognition memory trade-off scores. The fact that specific recognition scores showed stronger correlations with individual differences measures than general recognition scores could have a resulted for a few reasons. Specific recognition may demand greater visuospatial working memory resources and executive control ability than does general recognition. It is well known that the gist of a scene can be extracted rapidly, whereas detecting the details requires longer viewing time (reviewed by Henderson, 2007). Given this disparity in the amount of attention required to encode gist information versus visual details, it makes sense that specific recognition could be more related to individual differences in attention allocation than would be general recognition. It also is possible that our enhanced ability to detect correlations with specific recognition stems from the fact that the trade-off was elicited more readily for these precise details. Thus, the trade-off in specific recognition may simply have been a more sensitive measure of memory than the trade-off in general recognition. Future studies will do well to examine whether there are other types of individual differences–such as a person’s ability to extract semantic meaning from information - which would correspond more strongly with the general recognition trade-off. In contrast to the robust correlations between visuospatial working memory and the specific recognition trade-off, our measure of visual working memory (Self-Ordered Choosing of patterns) showed no relation to the trade-off. Though we do not want to put too much emphasis on a null result, it may be important to consider that neural networks supporting visual and visuospatial working memory are organized in fundamentally different ways within the brain. A great deal of evidence has suggested that there are separate “what” versus “where” pathways distinguishing object identity from spatial location (Levy & Goldman-Rakic, 2000; Wilson, Scalaidhe, & Goldman-Rakic, 1993) and it is possible that the ability to guide attention to disparate spatial locations and to maintain spatial information plays a particularly important role in the current task. Future research could follow up on this intriguing possibility. For now, we can conclude the trade-off correlates strongly with a person’s inability to maintain a large amount of visuospatial information, but that a person’s ability to maintain other types of information in mind may have less relevance to the trade-off.
Although so far we have discussed the effects of visuospatial working memory, cognitive control, and anxiety separately, it also is likely that there are interactions between the different processes. For example, proficient spatial working memory may rely upon executive control processes to sustain goal-consistent attention when there is interference from complex or multiple competing stimuli (Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001; Shackman, Sarinopoulos, Maxwell, Pizzagalli, Lavric, & Davidson, 2006). In fact, proficient executive functioning may be the most important factor in effective spatial working memory ability (Miyake et al., 2001), with many of the individual differences in working memory task performance relating to differences in cognitive control ability (Heitz & Engle, 2007; Gray, 2001).
Anxiety also may exert many of its effects through relationship with working memory. It can disrupt the application of working memory resources in verbal (Gray, 2001; Gray, Braver, & Raichle, 2002) and spatial working memory tasks (Lavric, Rippon, & Gray, 2003; Li et al., 2006; Shackman et al., 2006), although the effects of anxiety may be greater on spatial working memory processes. Shackman et al. (2006) hypothesized that this inverse relation between anxiety level and spatial working memory ability was caused by interference between mental resources applied to anxiety-related cognition and those necessary for appropriate allocation of spatial attention. Because these are the same skills needed to fully attend to and process complex visual scenes, it would not be surprising to find that anxiety would interfere with the processing of complete visual scenes, rather than only the emotionally arousing elements.
While several prior studies have probed the connections between executive functioning and working memory, or anxiety and working memory, the conjoint impact of these factors upon emotional processing has remained relatively unexplored. Cognitive processes may be impaired when multiple tasks place demands upon the same resources simultaneously. When resources are depleted by one cognitive demand, they are less available to support processes drawing from the same limited reservoir available for cognitive functioning (Hirst & Kalmar, 1987; Schmeichel, 2007). Models of limited attentional or cognitive resources propose that emotional states may take up mental resources, which are then unavailable for controlled cognition (Gray, 2001). Anxiety may operate in this fashion, by usurping limited brain resources, creating competition for resources necessary for the application of visuospatial working memory and executive functioning. It will be important for future research to examine the extent to which the factors discussed here as modulators of the trade-off make independent versus related contributions to influencing the effect.
It is possible that our choice of encoding task instructions had an impact upon the way in which the trade-off was expressed. We chose this encoding task, deciding whether to approach or back away from each image, since it would be a close approximation of the automatic decision one would make when confronting similar situations in everyday life. However, it is likely that the decision of whether to approach or back away from a scene depends upon an appreciation of the emotional salience within that scene. Thus, these instructions may have led to a greater trade-off than would have been found with other encoding instructions. Indeed, previous research has shown that encoding tasks can influence occurrence of the trade-off effect. While tasks that involve passive viewing of scenes tend to elicit strong trade-offs (e.g. Burke et al., 1992; Kensinger et al., 2007a; Wessel et al., 2000, expt. 2), tasks that require participants to closely attend to scene details can reduce or eliminate the trade-off (Kensinger et al., 2005; Kensinger et al., 2007a). Regardless of the precise reasons why the trade-off was elicited across all participants, however, the critical finding of this study is that the magnitude of the trade-off is strongly related to individual differences.
Conclusion
This study supports prior literature reporting a trade-off in memory for emotional scene information, and extends these findings by showing the relationship between this trade-off and responses on measures of individual differences. The majority of research investigating the effects of emotion on memory has been restricted to group level analyses. Results of this study emphasize the importance of moving beyond these group statistics to investigate individual differences. Although the trade-off in emotional memory was robust, individual differences in anxiety level, visuospatial working memory, and executive functioning correlated strongly with the magnitude of the tradeoff. As we found that these factors made somewhat independent contributions to the trade-off, future research should explore whether there are functional connections between them. Anxiety is thought to modulate many of the same prefrontal processes that are essential for cognitive control and for selection of task-relevant spatial information (e.g., Gray et al., 2002), thus making it plausible that interactions among these factors combine to alter the way in which emotional information is processed. In particular, the neural networks supporting emotional processing may experience competition from resources used in the experience of anxiety and those necessary for proficient visuospatial working memory and executive functioning.
Acknowledgments
This research was supported by a Harvard Mind, Brain, and Behavior postdoctoral fellowship to J.D.P, by National Science Foundation grant BCS 0542694 to E.A.K, and by National Institute on Aging grant NIA AG08441 to D.L.S. We thank Kelley Swanberg and Yuliya Nikolova for their assistance testing participants.
Footnotes
ANOVAs revealed no effect of presentation rate on the magnitude of the trade-off for specific recognition (F(1,39)=1.56, p=.22) or general recognition scores (F(1,39)< .1). ANOVAs also revealed no main effect of delay on the magnitude of the trade-off in general recognition scores (F(1,63)=2.08, p=.16) and only a trend for an effect of delay upon specific recognition scores (F(1,63)=3.62, p=.06). The strength of the correlations between the general and specific recognition trade-offs and cognitive scores also did not differ as a function of presentation rate or delay; using Fisher’s r-to-z transformed correlation coefficients revealed no differences in the strength of correlations between cognitive measures and trade-off magnitude that survived the Bonferroni correction (general, z(r)<2.27, p>.02; specific, z(r)<2.74, p>.006).
References
- Adolphs R, Denberg NL, Tranel D. The amygdala’s role in long-term declarative memory for gist and detail. Behavioral Neuroscience. 2001;115:983–992. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Buchanan TW. Amygdala damage impairs emotional memory for gist but not details of complex stimuli. Nature Neuroscience. 2005;8:512–518. doi: 10.1038/nn1413. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Exploring the central executive. The Quarterly Journal of Experimental Psychology. 1996;49A:5–28. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Adolphs R. The role of the human amygdala in emotional modulation of long-term declarative memory. In: Moore S, Oaksfold M, editors. Emotional Cognition: From Brain to Behavior. London: John Benjamins; 2002. [Google Scholar]
- Burke A, Heuer F, Reisberg D. Remembering emotional events. Memory and Cognition. 1992;20:277–290. doi: 10.3758/bf03199665. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Belcher A, Huynh Q. The influence of sex versus sex-related traits on long-term memory for gist and detail from an emotional story. Consiousness and Cognition. 2004;13:391–400. doi: 10.1016/j.concog.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: Gaze direction and duration. Cognition & Emotion. 2005;19:433–451. doi: 10.1080/02699930441000157. [DOI] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extroversion and neuroticism: learning from individual differences in emotion processing. Journal of Personality. 2004;72:1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences, USA. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN. Variations in working memory: An introduction. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variations in Working Memory. New York: Oxford University Press; 2007. [Google Scholar]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cognitive, Affective and Behavioral Neuroscience. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Denberg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal elderly persons. Emotion. 2003;3:239–254. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effects of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences, USA. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale S, Myerson J, Emery LJ, Lawrence BM, Dufault C. Variations in working memory across the life span. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variations in Working Memory. New York: Oxford University Press; 2007. [Google Scholar]
- Hamann S, Canli T. Individual differences in emotional processing. Current Opinion in Neurobiology. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hasher L. Inhibition: An attentional control mechanism. In: Roediger HL III, Dudai Y, Fitzpatrick SM, editors. Science of Memory: Concepts. New York: Oxford University Press; 2007. [Google Scholar]
- Heitz RP, Engle RW. Focusing the spotlight: Individual differences in visual attention control. Journal of Experimental Psychology: General. 2007;136:217–240. doi: 10.1037/0096-3445.136.2.217. [DOI] [PubMed] [Google Scholar]
- Henderson JM. Regarding scenes. Current Directions in Psychological Science. 2007;16:219–222. [Google Scholar]
- Heuer F, Reisberg D. Vivid memories of emotional events: the accuracy of remembered minutiae. Memory and Cognition. 1990;18:496–506. doi: 10.3758/bf03198482. [DOI] [PubMed] [Google Scholar]
- Hirst W, Kalmar D. Characterizing attentional resources. Journal of Experimental Psychology: General. 1987;116:68–81. doi: 10.1037//0096-3445.116.1.68. [DOI] [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Refinement and test of the theory of fluid and crystallized general intelligences. Journal of Educational Psychology. 1966;57:253–270. doi: 10.1037/h0023816. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Hambrick DZ, Engle RW. Variations in working memory capacity as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variations in Working Memory. New York: Oxford University Press; 2007. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007a;56:575–591. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion specificity in young and older adults. Journal of Gerontology. 2007b;62:208–215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Piguet O, Krendl AC, Corkin S. Memory for contextual details: Effects of emotion and aging. Psychology and Aging. 2005;20:241–250. doi: 10.1037/0882-7974.20.2.241. [DOI] [PubMed] [Google Scholar]
- Koenig HG, Meador KG, Cohen HJ, Blazer DG. Self-rated depression scales and screening for major depression in the older hospitalized patient with medical illness. Journal of the American Geriatrics Society. 1988;36:699–706. doi: 10.1111/j.1532-5415.1988.tb07171.x. [DOI] [PubMed] [Google Scholar]
- Laney C, Campbell HV, Heuer F, Reisberg D. Memory for thematically arousing events, Memory & Cognition. 2004;32:1149–1159. doi: 10.3758/bf03196888. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lavric A, Rippon G, Gray JR. Threat-evoked anxiety disrupts spatial working memory performance: an attentional account. Cognitive Therapy and Research. 2003;27:489–504. [Google Scholar]
- Lepsien J, Nobre AC. Cognitive control of attention in the human brain: Insights from orienting attention to mental representations. Brain Research. 2006;1105:20–31. doi: 10.1016/j.brainres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Li X, Li X, Luo Y. Differential influences of negative emotion on spatial and verbal working memory: Evidence from event-related potential and source current density analysis. NeuroReport. 2006;17:1555–1559. doi: 10.1097/01.wnr.0000234744.50442.2b. [DOI] [PubMed] [Google Scholar]
- Libkuman TM, Nichols-Whitehead P, Griffith J, Thomas R. Source of arousal and memory for detail. Memory & Cognition. 1999;27:166–190. doi: 10.3758/bf03201222. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. Take a closer look: Emotion modifies the boundary extension effect. Emotion. 2004;4:36–45. doi: 10.1037/1528-3542.4.1.36. [DOI] [PubMed] [Google Scholar]
- Mathews A, Yiend J, Lawrence AD. Individual differences in the modulation of fear-related brain activation by attentional control. Journal of Cognitive Neuroscience. 2004;16:1683–1694. doi: 10.1162/0898929042947810. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent variable analysis. Journal of Experimental Psychology: General. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Morton JB, O’Reilly RC. Developmental and computational approaches to variation in working memory. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variations in Working Memory. New York: Oxford University Press; 2007. [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;3:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. doi: 10.1111/j.1467-9280.2008.02157.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal and temporal lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual review of psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. Memory for emotional events. In: Reisberg D, Hertel P, editors. Memory and Emotion. Oxford: University Press; 2004. [Google Scholar]
- Rohner J. Memory-based attentional biases: Anxiety is linked to threat avoidance. Cognition & Emotion. 2004;18:1027–1054. [Google Scholar]
- Safer MA, Christianson S, Autry MW, Osterlund K. Tunnel memory for traumatic events. Applied Cognitive Psychology. 1998;12:99–117. [Google Scholar]
- Schmeichel BJ. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. Journal of Experimental Psychology: General. 2007;136:241–255. doi: 10.1037/0096-3445.136.2.241. [DOI] [PubMed] [Google Scholar]
- Schmidt SR, Saari B. The emotional memory effect: Differential processing or item distinctiveness? Memory & Cognition. 2007;35:1905–1916. doi: 10.3758/bf03192924. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wessel I, Merckelbach H. The impact of anxiety on memory for details in spider phobics. Applied Cognitive Psychology. 1997;11:223–231. [Google Scholar]
- Wessel I, van der Kooy P, Merckelbach H. Differential recall of central and peripheral details of emotional slides is not a stable phenomenon. Memory. 2000;8:95–109. doi: 10.1080/096582100387641. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive Psychology and Emotional Disorders. 2. New York: John Wiley & Sons; 1997. [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie HC, Evans JJ. The Behavioural Assessment of the Dysexecutive Syndrome. Thames Valley Test Company; Bury St. Edmunds, England: 1996. [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]