Abstract
We recently described a genetically engineered mouse model that develops ovarian granulosa cell tumors (GCTs) that mimic many aspects of the advanced human disease, including distant dissemination. However, because the primary tumors killed their hosts before metastases were able to form, the use of these mice to study metastatic disease required the development of a simple, reliable, and humane surgical protocol for the excision of large GCTs from debilitated mice. Here we describe a protocol involving multimodal anesthesia, tumor removal through ventral midline celiotomy and perioperative fluid therapy, and analgesia that led to the postoperative survival of more than 90% of mice, despite the removal of tumors representing as much as 10% of the animal's body weight. Intraabdominal recurrence of the GCT did not occur in surviving animals, but most developed pulmonary or adrenal metastases (or both) by 12 wk after surgery. We propose that this mouse model of metastatic GCT will serve as a useful preclinical model for the development of novel treatment modalities and diagnostic techniques. Furthermore, our results delineate anesthetic and surgical principles for the removal of large abdominal tumors from mice that will be applicable to other models of human cancers.
Abbreviation: GCT, granulosa cell tumor
The ovarian granulosa cell tumor (GCT) is the most prevalent sex cord tumor in women and is thought to represent up to 5% of all ovarian cancers.8 It is generally considered a low-grade malignancy, but a small percentage of GCTs are considered aggressive, and a large proportion of patients develop recurrent disease postoperatively.10 Few therapeutic options are available for the recurrent disease, and fastidious long-term follow-up is required in most patients, given that recurrent lesions have been diagnosed as long as 40 y after removal of the original tumor.4
One of the key factors that has limited progress in the development of therapeutic approaches for ovarian cancer is the dearth of relevant animal models.9 Our laboratory has recently developed a genetically engineered mouse model (Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+) that develops an aggressive form of GCT in animals of both sexes.1 In female mice, the disease is characterized by perinatal onset and rapid tumor development in both ovaries, causing death by approximately 8 wk of age. Histopathologic analyses of 6- to 8-wk-old Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+; Amhr2tm3(cre)Bhr/+ tissues revealed the presence of tumor cell embolisms in the lungs, suggesting metastatic potential for GCTs.6 However, the mice invariably died or required euthanasia for humane reasons before true metastases were able to form. Surgical excision of the primary tumors allowed for the postoperative survival of the mice for several months, and permitted the development of metastases in several tissues including lung and adrenal gland, thereby confirming the metastatic potential of the tumor cells.6
Because of its mimicry of advanced human disease from the molecular to tumor biology level, we have proposed that the Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ model could represent a powerful tool for the development of new therapeutic strategies for metastatic GCT. However, because the GCTs kill their hosts before metastases are able to form, their use as a model of the metastatic disease requires the development of a simple, reliable, and humane surgical protocol for the excision of the primary tumors. Such a protocol would need to take into account that pulmonary tumor cell embolisms have not been noted in these mice before the age of 6 wk, necessitating that surgery be performed in debilitated animals with tumor burdens approaching 10% of their body weight. A review of the existing literature on anesthesia, fluid therapy, pain management, and surgical techniques in mice yielded little information applicable to our model, necessitating that we develop a novel protocol that addressed both the medical needs of the mice and the scientific objectives of the research program.
Materials and Methods
Mice.
Female Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice were derived by selective breeding of the Ptentm1Hwu, Ctnnb1tm1Mmt, and Amhr2tm3(cre)Bhr parental strains as previously described.6 Mice were housed in ventilated microisolation caging at no more than 4 mice per cage in a specific-pathogen-free facility with a 12:12-h light:dark cycle and were fed a standard rodent chow ad libitum. The mice were 42 ± 3 d of age at the time of surgical intervention. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Université de Montréal and were in accordance with the Canadian Council on Animal Care (CCAC) policy on humane care and use of laboratory animals.2
Anesthesia.
The mice were fasted for no more than 2 h prior to premedication. Warm sterile 0.9% saline (1 mL SC) was administered in the interscapular region 30 min prior to premedication with medetomidine (0.15 mg/kg SC; Domitor, Pfizer Santé Animal, Kirkland, Quebec, Canada). Approximately 10 min later, anesthesia was induced with ketamine (37 mg/kg SC; Vetalar, Bioniche, Belleville, Ontario, Canada). Anesthesia was maintained by using a nonrebreathing Bain system to deliver isoflurane (0.5% to 1.5% in 100 mL/min oxygen) through an adapted mask (item MMA, Viking Medical, Birmingham, AL). Mice were kept warm throughout the anesthetic period by using a circulating warm-water pad topped with a moldable heating bag (Sac Magique, Sac Santé, Montréal, Québec, Canada) on which the animal was placed in dorsal recumbency. Monitoring during anesthesia included evaluation of respiratory rate and mucous membrane color. After abdominal closure (approximately 10 to 15 min into the surgical procedure), warm saline containing 0.5% dextrose (1 mL IP) was injected. Isoflurane was stopped, and 1 or 2 drops of diluted atipamezole (0.5 mg/mL; Antisedan, Pfizer Santé Animal) solution was administered on the oral mucous membranes. Morphine (2 mg/kg SC ) then was administered as previously recommended.3 Oxygen supplementation by mask was continued until the mice were able to move around. Once fully awake, the animals were given access to water and food.
Surgical procedure.
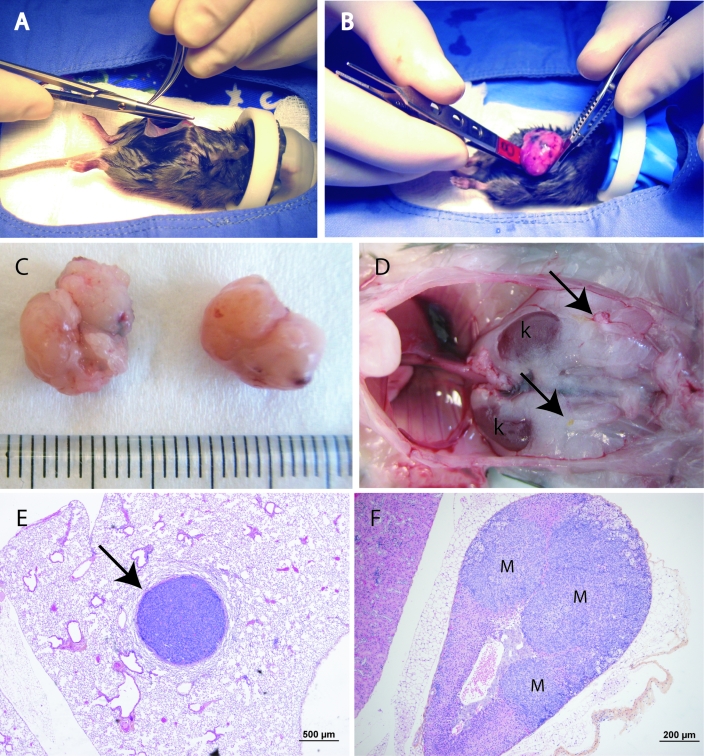
Dorsal recumbency was maintained with the help of a moldable heating bag as well as an adapted mask that allowed for insertion of the front limbs with the head to stabilize the mouse. The surgical site was disinfected by using gauzes saturated with chlorhexidine (2%) or isopropyl alcohol (70%) 3 times each in alternation, and care was taken to keep nonsurgery areas of the mice as dry as possible. No shaving was performed mainly due to risk of injury and hypothermia. A median celiotomy was performed from the xyphoid process to a few millimeters cranial to the pubic rim, allowing for complete exploration of the abdominal cavity (Figure 1 A). The GCTs were carefully exteriorized 1 at a time and isolated from the rest of the abdominal organs (Figure 1 B, C). By using a bipolar electrocautery system (Elleman International, Hewlett, NY), each GCT was dissected from its adhesions (if present) and its vessels were cauterized simultaneously. Care was taken to minimize blood loss and to cauterize as close to the tumor as possible to avoid inadvertent damage to the kidney and ureter. The abdominal cavity then was explored for evidence of abdominal hemorrhage, and the linea alba was closed with a tightly sealed simple continuous pattern by using 5-0 polydioxanone suture (PDS, Ethicon, Johnson and Johnson, Langhorne, PA) to allow intraperitoneal fluid therapy. The skin and subcutaneous tissue also were closed as a single layer using a simple continuous pattern of 5-0 polydioxanone suture.
Figure 1.
(A) Celiotomy is performed first by incising the skin with a scalpel blade from the xyphoid process to a few millimeters cranial to the pubic rim. The abdominal cavity then is opened by cutting through the abdominal wall by using small Metzenbaum scissors. Note that the forelimbs were inserted into the anesthesia mask along with the head to stabilize the mouse and facilitate surgery. (B) The first ovarian tumor is exteriorized carefully by using small ophthalmology forceps. The tumors are friable and bleed easily, so careful manipulations are necessary to prevent hemorrhage. (C) Two GCTs isolated from a 39-d-old mouse. The scale is in millimeters. (D) The abdominal cavity of a Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse euthanized 12 wk after surgery (digestive tract and liver removed). Arrows indicate well-healed uterine stumps with no evidence of tumor recurrence. Also note the lack of intraabdominal dissemination. k, Kidney. (E) A pulmonary GCT metastasis (arrow) in the mouse shown in panel D. (F) Adrenal GCT metastases (M) in the mouse shown in panel D.
Monitoring and analgesia after surgery.
The mice were monitored continuously after anesthesia until they ate. They then were assessed by observation, weighing, and subjective monitoring of food and water consumption twice daily until 5 d after surgery. Mice were observed for approximately 10 min once in the morning between 0700 and 1000 and once in the late afternoon between 1600 and 1900. They were subjectively evaluated without the use of a scoring system for the presence of pain as manifested by inactivity or abnormal body positioning during assessments. If pain was judged to be present, meloxicam (0.1 mg/kg SC; Metacam, Boehringer Ingelheim, Burlington, Ontario, Canada) was administered on an as-needed basis for a maximum of twice daily for 5 d. The development of surgical complications including infection and hemorrhage was monitored for the 5 d following surgery by evaluating respiratory rate and mucous membrane color and the incision for bruising, inflammation, bleeding, or discharge.
Necropsy, tissue collection, and histopathology.
Mice were anesthetized with isoflurane prior to cervical dislocation. The lungs were inflated with 10% buffered formalin infused through the trachea prior to excision. The heart, thymus, kidneys, adrenals, liver, and spleen were excised and fixed in 10% buffered formalin for 24 h before embedding, sectioning (7 µm), and staining with hematoxylin–phloxin–saffron.
Results
Large ovarian GSTs were removed surgically from 21 Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ female mice. The average duration of the procedure including anesthesia and surgery was 52 min (range, 45 to 75 min), with an average recovery time of 24.5 min (range, 10 to 45 min). The preoperative body weight (mean ± SEM) of the mice was 18.9 ± 0.6 g, and the mean total tumor burden was 1.78 g, representing 9.4% of total body weight. No complications were noted during induction and maintenance of anesthesia or surgery for any of the mice. Death occurred during the anesthetic recovery phase in 2 mice. The cause was undetermined in 1 mouse; intraabdominal bleeding was identified as the cause of death in the other. Swelling of the surgical site occurred in 1 mouse and lasted for 3 d postoperatively. Bleeding was suspected in 3 mice in light of pale mucous membranes the day after surgery, and was treated with subcutaneous fluids (1 mL warm saline), which resulted in rapid recovery. No infection or dehiscence of the surgical wounds was noted in any of the mice.
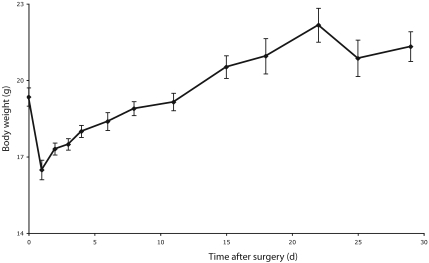
Decreased activity was noted in 4 mice, and generally lasted no longer than the third day after surgery. Among these 4 mice, pain was identified in 1 and treated with meloxicam. The other 3 mice each received warm 0.9% saline (1 mL SC) and recovered uneventfully. An initial weight loss during the immediate postoperative period (primarily due to excision of tumors) was followed by a gradual weight gain, corroborating the subjective impression of adequate postoperative food intake (Figure 2).
Figure 2.
Postoperative body weight. Values are presented as means (points) ± SEM (error bars), n = 5 to 10 animals per time point.
Overall 19 mice recovered from surgery, representing a 90.5% survival rate. These 19 mice were euthanized 12 wk after surgery, and necropsies were performed. No animals showed any signs of surgical complications, and all had well-healed uterine stumps with no evidence of local or intraabdominal recurrence of the GCT (Figure 1 D). As expected, histopathologic analysis identified pulmonary (Figure 1 E) and adrenal (Figure 1 F) GCT metastases in 9 and 13 animals, respectively.
Discussion
A key factor that has limited progress in the development of therapeutic options for metastatic cancers is the lack of practical and biologically relevant animal models. In this report, we describe a novel model for GCT that replicates many aspects of the advanced human disease, including the formation of distant metastases after surgical removal of the primary tumors. Our ability to perform invasive surgical procedures in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice with acceptable morbidity and mortality, by using widely available equipment and with relatively little expense or surgical expertise, indicates that this model will be practical for studying the biologic and clinical aspects of metastatic GCT disease. Furthermore, the anesthetic and surgical protocols that we have described likely will be useful for the development of other mouse models of human cancers that require the removal of large tumors from debilitated animals.
The use of multimodal anesthesia in small rodents has rarely been reported, and generally either injectable or volatile anesthetics are used in mice. The reasons for this approach rarely are stated but likely include the lack of available information regarding methods and advantages of multimodal anesthesia in small rodents as well as economic considerations. In addition, the constraints of particular research projects may limit the anesthetic and analgesic options available.5 Given the weakened state of 6-wk-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice and the volume of the tumors to be excised, we chose to develop a multimodal anesthetic protocol with the specific aims of minimizing the duration of the anesthesia and obtaining a rapid recovery to avoid hypoglycemia and hypothermia.3 Medetomidine and ketamine were used to produce sedation, analgesia, and loss of consciousness. The doses used were approximately half of the lowest doses reported,5 a choice made in light of the likely hemodynamic changes due the presence of the large tumors. The maintenance of anesthesia with decreased concentrations of isoflurane was possible due to the use of premedication and induction agents to balance the anesthesia. At the end of the procedure, medetomidine was antagonized with atipamezole to induce rapid recovery. We believe that this multimodal anesthesia approach, combined with aggressive fluid therapy, was a key contributing factor to the high postoperative survival rate and relative lack of postoperative complications.
Pain management is known to affect postprocedural mortality in small rodents.7 Manifestations of pain often include hypo- or adipsia as well as hypo- or anorexia which then cause hypoglycemia, dehydration, and death. Given the invasive nature of the surgery to be performed in our mouse model, careful consideration was given to the methods of analgesia. Because atipamezole reverses analgesia produced by medetomidine, analgesia was continued postoperatively with morphine and occasionally meloxicam. These analgesic agents were chosen for their availability, low cost, and established effectiveness in rodents. Opioids such as buprenorphine (which has a longer duration of action than morphine) may have represented a better option but were not used due to issues of local availability. The efficacy of administration of analgesics should always be evaluated by pain assessment, which is difficult in small rodents because no rigorously validated pain scoring schemes have been developed for mice. However, some behavioral changes have been associated with pain in mice, including arching of the back, reduction in normal activities such as climbing, contraction of the abdominal muscles, and twitching of the skin overlying the back and abdomen.5 The mice in the present study were evaluated regularly and subjectively for these behavioral changes, but the development of a more comprehensive pain assessment and scoring protocol to improve pain management and outcome may be warranted.
In summary, the present study demonstrates that the removal of large ovarian tumors from debilitated mice can achieve a high rate of survival. We propose that the surgical Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ model represents an important new tool for the study of the metastatic phenotype that is often the cause of death in GCT patients. In addition, we hope this model will contribute to the development of novel treatment modalities and diagnostic techniques.
Acknowledgments
The authors thank Céline Forget for assistance with mouse colony management and genotyping analyses. This work was funded by the Canada Research Chair in Ovarian Molecular Biology and Functional Genomics (to DB) and an operating grant from the Canadian Institutes of Health Research (to DB).
References
- 1.Boyer A, Paquet M, Lague MN, Hermo L, Boerboom D. 2009. Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in testicular stromal cells causes granulosa cell tumor of the testis. Carcinogenesis 30:869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Council on Animal Care 1984. Chapter XIX, laboratory mice. : Guide to the care and use of experimental animals. Ottawa (Canada): Canadian Council on Animal Care [Google Scholar]
- 3.Cantwell SL. 2001. Ferret, rabit, and rodent anesthesia. Vet Clin North Am Exot Anim Pract 4:169–191 [DOI] [PubMed] [Google Scholar]
- 4.East N, Alobaid A, Goffin F, Ouallouche K, Gauthier P. 2005. Granulosa cell tumor: a recurrence 40 years after initial diagnosis. J Obstet Gynaecol Can 27:363–364 [DOI] [PubMed] [Google Scholar]
- 5.Flecknell PA, Richardson CA, Popovic A. 2007. Laboratory animals, p 765–784. In: Tranquilli WJ, Thurmon JC, Grimm KA. Lumb and Jones' veterinary anesthesia and analgesia. Ames (IA): Blackwell Publishing Professional [Google Scholar]
- 6.Laguë MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, Behringer RR, Fuller PJ, Mitchell A, Doré M, Huneault LM, Richards JS, Boerboom D. 2008. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis 29:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langlois I, Mercier B, Kona-Boun JJ. 2006. Principes d'analgésie chez les nouveaux animaux de compagnie. Le Médecin Vétérinaire du Québec 36:22–30 [Google Scholar]
- 8.Schumer ST, Cannistra SA. 2003. Granulosa cell tumor of the ovary. J Clin Oncol 21:1180–1189 [DOI] [PubMed] [Google Scholar]
- 9.Vanderhyden BC, Shaw TJ, Ethier JF. 2003. Animal models of ovarian cancer. Reprod Biol Endocrinol 1:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villella J, Herrmann FR, Kaul S, Lele S, Marchetti D, Natiella J, Odunsi K, Mhawech-Fauceglia P. 2007. Clinical and pathological predictive factors in women with adult-type granulosa cell tumor of the ovary. Int J Gynecol Pathol 26:154–159 [DOI] [PubMed] [Google Scholar]