Abstract
Infection of mice with Helicobacter hepaticus is common in research colonies, yet little is known about how this persistent infection affects immunologic research. The goal of this study was to determine whether H. hepaticus infection status can modulate immune responses specific to herpes simplex virus type 1 (HSV1) and the phenotypic and functional characteristics of dendritic cells (DC) of mice. We compared virus-specific antibody and T cell-mediated responses in H. hepaticus-infected and noninfected mice that were inoculated intranasally with HSV1. The effect of H. hepaticus on the HSV1-specific antibody and T cell-mediated immune responses in superficial cervical and tracheobronchal lymph nodes (LN) did not reach statistical significance. Surface expression of the maturation-associated markers CD40, CD80, CD86, and MHC II and percentages of IL12p40- and TNFα-producing DC from spleen and colic LN in H. hepaticus-infected mice and noninfected mice were measured in separate experiments. Expression of CD40, CD86, and MHC II and percentages of IL12p40- and TNFα-producing DC from colic LN were decreased in H. hepaticus-infected mice. In contrast, H. hepaticus infection did not reduce the expression of these molecules by splenic DC. Expression of CD40, CD80, CD86, and MHC II on splenic DC from H. hepaticus-infected mice was increased after in vitro lipopolysaccharide stimulation. These results indicate that H. hepaticus infection can influence the results of immunologic assays in mice and support the use of H. hepaticus-free mice in immunologic research.
Abbreviations: DC, dendritic cells; HSV1, herpes simplex virus type 1; LN, lymph nodes; MHC II, major histocompatibility complex class II; MHV, mouse hepatitis virus; OVA, ovalbumin peptide SIINFEKL; PE, phycoerythrin
Helicobacter hepaticus is a gram-negative, microaerophilic, curved to spiral-shaped bacterium with bipolar, sheathed flagella. H. hepaticus was described for the first time in 1994 as the cause of chronic active hepatitis associated with a high incidence of hepatocellular neoplasms in mice on a long-term toxicology study.39 Since then, H. hepaticus has been identified as a common contaminant of mouse colonies at a variety of research institutions. Although commercial breeders produce H. hepaticus-free animals, many mouse colonies at public and private research institutions still harbor H. hepaticus. A recent survey found H. hepaticus-infected mice in 59% of commercial and academic institutions in Canada, Europe, Asia, Australia, and the United States.35
H. hepaticus persistently colonizes the hepatic bile canaliculi and the cecal and colonic mucosa of mice.9,39 Infection can cause chronic active hepatitis, hepatocellular neoplasms, and typhlocolitis, which vary in severity depending on the strain, age, gender, and immune status of the mouse.5,9,11,39 In adult immunocompetent mice, H. hepaticus infection is usually asymptomatic. However, immune-dysregulated mice can develop inflammatory bowel disease, which may present as rectal prolapse or diarrhea.16
Mice initiate immune responses against H. hepaticus primarily through its interaction with Toll-like receptor 2 on antigen-presenting cells.21 Both systemic and local (at the site of infection) H. hepaticus-specific Th1-type immune responses are induced in immunocompetent mice.26,40 Systemic antibody and cell-mediated immunity against the bacteria persist for at least 46 wk after experimental inoculation.40 Gene expression profiles of cecal tissue of H. hepaticus-infected mice have shown that inflammatory responses differ depending on the mouse strain. For example, A/JCr mice had significant and prolonged expression of the Th1-type cytokines IFNγ and IL12p40 in cecal mucosa, and these expression levels persisted for at least 3 mo after H. hepaticus infection. However, C57BL/6 mice had a lesser elevation of IFNγ gene expression without an effect on IL12p40. IFN γ expression waned by 1 mo after inoculation in C57BL/6 mice.25 In addition, H. hepaticus-specific secretory IgA antibodies are persistently detected in the feces of mice.40 How these immune responses in H. hepaticus-infected mice might affect immunologic research is unknown.
The goal of this study was to determine whether immune responses to herpes simplex virus type 1 (HSV1) and the phenotypic and functional characteristics of dendritic cells (DC) are altered in H. hepaticus-infected mice. The intranasal HSV1 infection model is used widely to study immune mechanisms in mice. Immunity to HSV1 consists of virus-neutralizing antibodies in the serum and virus-specific T cells in the draining LN. Superficial cervical and mediastinal LN have been described as draining LN for intranasal HSV1 infection.2 The response to HSV1 infection peaks at 7 d after infection and leads to clearance of the viral load.2 In this study, we compared levels of HSV1-specific antibody and T cell-mediated immune responses between H. hepaticus-infected and noninfected mice.
Dendritic cells are important components of the immune system that play a role in antigen processing and presentation. On exposure to foreign antigen, DC mature and express increased levels of major histocompatibility complex class II proteins (MHC II), CD40, CD80, and CD86 on the cell surface. These maturation-associated cell surface markers interact with naive T and B cells to initiate antibody- and cell-mediated immune responses against foreign antigens.27 In addition, mature DC secrete proinflammatory cytokines, including TNFα and IL12p40. These cytokines lead to increased vascular permeability, complement activity, lymphocyte activation, lymphocyte proliferation, and increased antibody production.27 To determine whether infection with H. hepaticus affects characteristics of DC, we measured the expression of the maturation-associated cell surface markers CD40, CD80, CD86, and MHC II and proinflammatory cytokines IL12p40 and TNFα by DC derived from the spleen and colic LN of H. hepaticus-infected and noninfected mice. Our findings indicate that H. hepaticus infection can influence the various aspects of immune responsiveness and, therefore, must be considered as a potential variable in studies in which immune function is a measurable outcome.
Materials and Methods
Animals.
Specific-pathogen-free C57BL/6 mice (male; age, 3 to 4 wk; Jackson Laboratory, Bar Harbor, ME) were housed in individually ventilated microisolation cages with sterile food, bedding, and water in an AAALAC -accredited animal facility. All use of animals was approved by the Penn State Hershey Institutional Animal Care and Use Committee according to standards put forth in the Guide for the Care and Use of Laboratory Animals.14 All cage changes and animal manipulations were conducted in a class II biosafety cabinet with strict precautions to prevent cross-contamination. All of the mice were maintained in a specific-pathogen-free environment and were confirmed to be serologically negative for mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse norovirus, Sendai virus, Mycoplasma pulmonis, Theiler murine encephalomyelitis virus, mouse rotavirus, pneumonia virus of mice, reovirus 3, lymphocytic choriomeningitis virus, and ectromelia virus at the conclusion of the experiments.
H. hepaticus infection.
H. hepaticus ATCC 51449 was grown on chocolate agar or in brain–heart infusion broth with 10% (v/v) FBS under microaerobic conditions obtained by flushing sealed jars with anaerobic gas (90% N2, 5% CO2, and 5% H2). Mice were gavaged with 1 × 109 CFU H. hepaticus in 0.2 mL PBS. Control mice received 0.2 mL PBS alone. Feces were collected at 1 wk after inoculation and at the time of euthanasia to confirm the infection status of the mice by using Helicobacter fecal PCR as previously described3 with primers 5′ CTA TGA CGG GTA TCC GGC 3′ and 5′ ATT CCA CCT ACC TCT CCC A 3′.29
HSV1 infection.
Stocks of HSV1 strain McIntyre were prepared by infection of Vero cells as previously described1 and stored at −70 °C. Viral titer of the stocks was assessed by plaque assay using Vero cells. At 4 wk after H. hepaticus infection, isoflurane-anesthetized mice were infected intranasally with 1 × 107 PFU HSV1 in 20 μL PBS containing 1% (v/v) FBS.
Isolation of cells from LN.
At 1 wk after HSV1 infection, mice were euthanized by exsanguination after sedation with isoflurane. Superficial cervical LN were collected from the upper poles of the submandibular salivary glands,24,36 and tracheobronchal LN were collected from the tracheal bifurcation.38 The collected LN were placed in IMDM (Gibco, Carlsbad, CA) supplemented with 10% (v/v) FBS, 50 µM 2-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin sulfate (supplemented IMDM). As described previously,2 single-cell suspensions of the collected LN were prepared by mechanical dissociation and passage through a 70-µm nylon cell strainer (BD Biosciences, San Jose, CA). Trypan blue exclusion was used to determine the number of viable cells.
HSV1- specific T cell assay.
To quantify the HSV1-specific CD8+ T cells in the LN of HSV1-infected mice (H. hepaticus-infected and noninfected mice), cells isolated from LN were incubated with a phycoerythrin (PE)-labeled peptide comprising 4 HSV1 glycoprotein B amino acid residues (gB498–505) coupled to 4 H2Kb class I molecules and linked to each other by streptavidin–biotin, as described previously.2 The gB498–505 peptide is an HSV1-encoded immunodominant epitope in C57BL/6 mice,4 and mice that were noninfected with either agent were used as negative controls. Briefly, CD16 and CD32 Fcγ receptors on isolated mononuclear cells were blocked with antibody from a 2.4G2 hybridoma cell culture supernatant supplemented with 20% (v/v) mouse serum (Sigma–Aldrich, St Louis, MO). Cell surface expression of CD8 was detected by using Alexa Fluor 647-conjugated antiCD8 antibody (clone 53-6.7; eBioscience, San Diego, CA). After washes with FACS buffer (PBS supplemented with 1% [v/v] FBS, 0.02% [w/v] sodium azide), cells were resuspended in 2% (w/v) paraformaldehyde (prepared in PBS) prior to analysis by flow cytometry (FACSCalibur, Becton Dickinson, San Diego, CA). Data from flow cytometry were analyzed by using CellQuest software (Becton Dickinson).
Staining of intracellular IFNγ.
To detect IFNγ-producing, HSV1-specific CD8+ T cells in the LN of HSV1-infected mice (H. hepaticus-infected and noninfected mice) as described previously,2 LN-derived cells were resuspended in supplemented IMDM and incubated with 1 µM gB498–505 peptide for 2 h at 37 °C. As a control, cells were stimulated with the peptide corresponding to ovalbumin amino acid residues 257 to 264 (OVA257–264; SIINFEKL). Cells were treated (Golgi Plug, BD Biosciences; final concentration 1 µg/mL) to prevent the secretion of cytokines and incubated for an additional 4 h at 37 °C. Cells then were washed twice with FACS buffer, and CD16 and CD32 Fcγ receptors were blocked with 2.4G2 cell culture supernatant supplemented with mouse serum. To identify CD8+ T lymphocytes, cells were incubated with Alexa Fluor 647-conjugated antiCD8 antibody. After CD8 staining, cells were permeabilized (Cytofix/Cytoperm, BD Biosciences) and incubated on ice for 20 min in the dark. Cells then were washed twice with 1× Perm/Wash buffer (BD Biosciences) and incubated with FITC-conjugated antiIFNγ antibody (clone XMG1.2; eBioscience, San Diego, CA) diluted in 1× BD perm/wash buffer. Subsequently, cells were washed with 1× BD Perm/Wash buffer and then with FACS buffer. Finally, cells were fixed in 2% paraformaldehyde and analyzed by flow cytometry (FACSCalibur, Becton Dickinson); resulting data were analyzed by using CellQuest software (Becton Dickinson).
CD107a degranulation assay.
To detect and quantify HSV1-specific cytotoxic CD107+CD8+ T cells in HSV1-infected mice (H. hepaticus-infected and noninfected mice) as described previously,2 LN-derived cells were incubated in 96-well plates (5 × 105 cells per well) in the presence of 10 µM gB498–505 or OVA257–264 peptide at 37 °C. Wells also contained FITC-conjugated antiCD107a (1D4B; BD Pharmingen, San Jose, CA). After 1 h, 10 mM NH4Cl was added to cells to prevent endosome acidification; 3 h after the addition of NH4Cl, cells were stained with PE-conjugated antiIAb (AF6-120.1; BD Pharmingen) and Alexa Fluor 647-conjugated antiCD8 antibody. Cells were stained for IAb to exclude CD8α+ DC, which constitutively undergo endocytosis and take up antiCD107a.2 Flow cytometry was performed using a FACSCalibur (Becton Dickinson); data from the CD8+ IAbdim population of cells were analyzed by using CellQuest software (Becton Dickinson).
Virus neutralization assay.
Blood samples were collected by cardiocentesis of anesthetized mice 1 wk after HSV1 infection. The amount of serum-neutralizing antibody against HSV1 was determined by plaque reduction assay as described previously with minor modifications.6,10 Briefly, 2-fold serial dilutions of serum from HSV1-infected mice (H. hepaticus-infected and noninfected mice) were prepared in PBS containing 1% (v/v) FBS, and incubated with an equal volume of HSV1 suspension (112 PFU) for 30 min at 37 °C. Sera from mice that were not infected with either HSV1 or H. hepaticus were used as the negative controls. The mixture then was used to infect monolayers of Vero cells. The cells were incubated with the mixture at 37 °C for 1 h and then overlaid with 1% (w/v) methylcellulose in 1× Eagle medium without phenol red and supplemented with 5% (v/v) heat-inactivated FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin sulfate. The plates were incubated at 37 °C for 4 to 5 d. At the end of the incubation period, cells were stained and fixed with 5% formaldehyde (v/v) and 0.5% (w/v) crystal violet solution. Viral plaques were counted.
Isolation of cells from spleen and preparation of colic LN.
At 4 wk after H. hepaticus infection or PBS inoculation, mice were sedated with isoflurane for blood collection and euthanized by cervical dislocation for collection of spleens and colic LN. Colic LN, a subset of mesenteric LN, were collected from the mesocolon at the transition between the ascending and transverse colons.38 Homogenates of spleens and colic LN were enriched for DC as described previously with minor modifications.13 Spleen and colic LN were placed in Hanks-buffered salt solution without calcium and magnesium (Invitrogen, Carlsbad, CA) and supplemented with 0.1% (w/v) bovine serum albumin (Roche, Indianapolis, IN) at room temperature. Colic LN from 3 mice were pooled and treated as a single sample, whereas the spleen from each mouse was a single sample. Spleens and colic LN were treated with 1 mg/mL collagenase D (Roche, Indianapolis, IN) in calcium-free, magnesium-free Hanks-buffered salt solution for 30 min at 37 °C in a 5% CO2 incubator. To stop collagenase D digestion, tissues were suspended in IMDM supplemented with 10% (v/v) FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin sulfate. Single-cell suspensions were obtained by mechanical dissociation and passage through a 40-µm nylon cell strainer (BD Biosciences). Splenocytes then were treated with ACK lysis buffer (Lonza BioWhittaker, Walkersville, MD) for RBC lysis. The number of viable cells was determined by trypan blue dye exclusion.
Lipopolysaccharide treatment of cells for DC assay.
Splenocytes and colic LN cells (1 × 106 of each preparation) from H. hepaticus-infected and noninfected mice were stimulated with 1 μg/mL Escherichia coli 055:B5 lipopolysaccharide (Sigma) added to medium. Cells were incubated with lipopolysaccharide for 16 h at 37 °C, 5% CO2. As a control, an equal number of cells were incubated for the same period in medium only.
Phenotypic analysis of surface markers on DC.
As described previously with minor modifications,8 DC derived from spleen and colic LN of H. hepaticus-infected and noninfected mice were analyzed for maturation-associated surface markers both before and after in vitro lipopolysaccharide stimulation. Fc receptors were blocked with antimouse CD16+CD32 (eBioscience) antibody in FACS buffer. Cells were stained with various combinations of FITC-conjugated antiCD40 (HM40-3), PE–Cy7-conjugated antiCD90.1 (HIS51), and PerCP–Cy5.5-conjugated antiCD11c (N418) from eBioscience and FITC–conjugated antiB7.2 (CD86, GL1), PE-conjugated antiIAb (MHC II, AF6-120.1), allophycocyanin-conjugated antiB7.1 (CD80, 16-10A1), PE–Cy7-conjugated antiNK-1.1 (PK136), and PE–Cy7-conjugated antiCD19 (1D3) from BD Biosciences. Flow cytometry was performed on a FACSCanto (Becton Dickinson), and data were analyzed by using FACSDiva (Becton Dickinson) or FlowJo (TreeStar, Ashland, OR) software. For analysis, DC were defined as CD11c+, NK1.1low, CD19low, and CD90.1low.
The CD11c+ DC population was recovered from spleen and colic LN after collagenase treatment as described previously.13 Because the CD11c+ DC population obtained by this method can include natural killer cells and immature and mature B cells, the contaminating cells were removed by negative selection of CD19+ mature B cells, CD90.1+ immature B cells, and NK1.1+ natural killer cells.
Staining of intracellular proinflammatory cytokines produced by DC.
The proinflammatory cytokines produced by DC after lipopolysaccharide stimulation were labeled as described for IFNγ. To prevent secretion of cytokines into the culture supernatant, spleen and colic LN cells were treated (Golgi Plug, BD Biosciences; final concentration, 1 µg/mL) for the last 8 h of stimulation. Cells staining as CD11c+, NK1.1low, CD19low, CD90.1low by using PerCP–Cy5.5-conjugated antiCD11c, PE-Cy7-conjugated antiNK1.1, PCy7-conjugated antiCD19, and PE–Cy7-conjugated antiCD90 antibodies were defined as DC. For detection of proinflammatory cytokines, PE-conjugated antiIL12+IL23p40 (C17.8; eBioscience) and allophycocyanin-conjugated antiTNFα (MP6-XT22; BD Biosciences) antibodies were used. Data from flow cytometry (FACSCanto, BD Biosciences) were analyzed by using FACSDiva (Becton Dickinson) or FlowJo (TreeStar) software.
Statistical analysis.
Statistical significance was determined with the 2-tailed, unequal variance, unpaired t test. Comparisons between groups were performed, and differences associated with P values less than 0.05 were considered to be significant. Microsoft Office Excel 2007 (Microsoft corporation, Redmond, WA) was used for statistical analysis.
Results
Immune responses against intranasal HSV1 infection.
HSV1-specific antibody and T cell-mediated immune responses were compared between H. hepaticus-infected and noninfected mice 1 wk after intranasal HSV1 infection. To determine the magnitude of HSV1-specific antibody immune responses, plaque reduction assays were used on sera from mice. All sera from HSV1-infected mice contained antiHSV1 antibodies, as indicated by a decrease (P < 0.05) in the number of viral plaques when compared with sera from HSV1-naive control mice (data not shown). The number of plaques did not differ between sera from H. hepaticus-infected and noninfected mice (data not shown), indicating that H. hepaticus infection did not alter the magnitude of the neutralizing antibody response to HSV1.
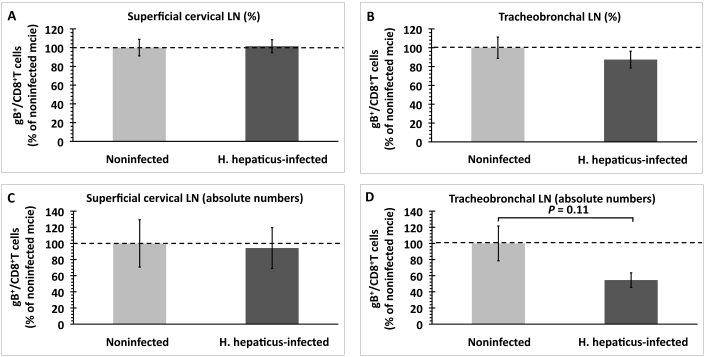
To evaluate cell-mediated immune responses to HSV1 infection, we determined the number of HSV1-specific CD8+ T cells in the draining LN of mice. These cells were detected and quantified based on their ability to bind to the HSV1 gB498–505–H2Kb tetramer. In superficial cervical LN, neither the percentage nor absolute number of gB498–505-specific CD8+ T cells differed between H. hepaticus-infected and noninfected mice (Figure 1 A, C). In tracheobronchal LN, the percentage of gB498–505 -specific CD8+ T cells did not differ between the 2 groups of mice (Figure 1 B), but a trend (P = 0.11) was found toward a decrease in the absolute number of gB498–505-specific CD8+ T cells in H. hepaticus-infected mice (Figure 1 D).
Figure 1.
Quantification of HSV1-specific CD8+ T cells in superficial cervical and tracheobronchal LN of H. hepaticus-infected and noninfected mice 1 wk after intranasal HSV1 infection. (A and B) Percentage of gB498–505-specific CD8+ T cells expressed in terms of total population of CD8+ T cells and (C and D) absolute numbers of gB498–505-specific CD8+ T cells. Data (mean ± SE) were normalized to the percentage or absolute number of gB498–505-specific CD8+ T cells in LN from noninfected mice within each experiment (n = 5 mice per group). Results were combined from 2 independent experiments. P value was determined by using the unpaired t test.
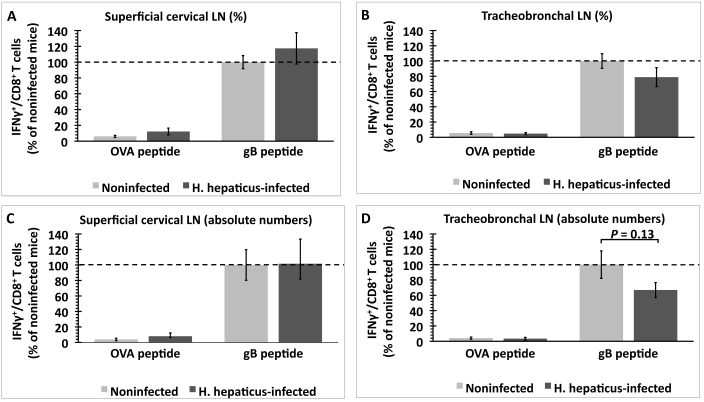
To compare the function of these HSV1 gB498–505-specific T cells between H. hepaticus-infected and noninfected mice, we quantified IFNγ-producing HSV1 gB498–505-specific CD8+ T cells in draining LN. Both the percentage and absolute number of IFNγ-producing CD8+ T cells from the superficial cervical and tracheobronchal LN were higher (P < 0.05) when cells were stimulated with gB498–505 peptide as compared with OVA257–264 peptide, indicating that this response was HSV1-specific (Figure 2). In superficial cervical LN, neither the percentage nor absolute number of IFNγ-producing gB498–505 peptide-specific CD8+ T cells differed between the 2 groups (Figure 2 A, C). In tracheobronchal LN, the percentage of IFNγ-producing gB498–505 peptide-specific CD8+ T cells did not differ between the 2 groups of mice (Figure 2 B); however, we noticed a decreasing (P = 0.13) trend in the absolute number of these cells in H. hepaticus-infected mice (Figure 2 D).
Figure 2.
Quantification of IFNγ-producing, HSV1-specific CD8+ T cells in the superficial cervical and tracheobronchal LN of H. hepaticus-infected and noninfected mice after in vitro stimulation with either OVA257–264 or gB498–505 peptide. (A and B) Percentage of IFNγ-producing, CD8+ T cells expressed in terms of total population of CD8+ T cells and (C and D) absolute number of IFNγ-producing CD8+ T cells. Data (mean ± SE) were normalized to the percentage or absolute number of IFNγ-producing CD8+ T cells after gB498–505 peptide stimulation of lymphoid cells from noninfected mice within each experiment (n = 5 mice per group). Results were combined from 2 independent experiments. P value was determined by using the unpaired t test.
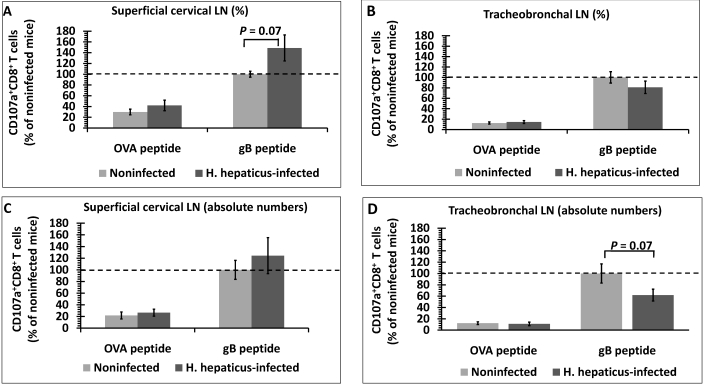
To further evaluate the functional activity of HSV1-specific T cells, we used a CD107a degranulation assay. During T cell degranulation in response to antigen exposure, the membranes of cytotoxic granules and T cells fuse, and CD107a (previously on the inner membrane of granules) is exposed on the T-cell surface.34 Therefore, surface expression of CD107a can be used as a marker of antigen-specific cytotoxic T cells. Both the percentage and absolute number of CD107a+CD8+ T cells in the draining LN was higher (P < 0.05) when cells were stimulated with gB498–505 peptide as compared with OVA257–264, indicating that this immune response was HSV1-specific (Figure 3). Superficial cervical LN showed a trend (P = 0.07) toward a higher percentage of gB498–505-specific CD107a+CD8+ T cells in H. hepaticus-infected mice compared with noninfected mice (Figure 3 A). However, the absolute number of these cells in the superficial cervical LN did not differ between the 2 groups of mice (Figure 3 C). In tracheobronchal LN, the percentage of gB498–505-specific CD107a+CD8+ T cells did not differ between H. hepaticus-infected and noninfected mice (Figure 3 B), but we found a trend (P = 0.07) toward a decrease in the absolute number of these cells in H. hepaticus-infected mice (Figure 3 D).
Figure 3.
Quantification of HSV1-specific CD107+CD8+ cytotoxic T cells in the superficial cervical and tracheobronchal LN of H. hepaticus-infected and noninfected mice after in vitro stimulation with OVA257–264 or gB498–505 peptide. (A and B) Percentage of CD107+CD8+ T cells expressed in terms of total population of CD8+ T cells and (C and D) absolute number of CD107+CD8+ T cells. Data (mean ± SE) were normalized to the percentage or absolute number of CD107+CD8+ T cells after gB498–505 peptide stimulation of lymphoid cells from noninfected mice within each experiment (n = 5 mice per group). Results were combined from 2 independent experiments. P value was determined by using the unpaired t test.
To summarize these findings, H. hepaticus infection had no statistically significant effect on HSV1-specific antibody and T cell-mediated immune responses in the superficial cervical and tracheobronchal LN of HSV1-infected mice. Nonsignificant trends toward reduced HSV1-specific T cell-mediated immune responses and increased HSV1-specific cytotoxic T cells were observed in tracheobronchal LN and superficial cervical LN, respectively.
Phenotypic and functional characteristics of DC from colic LN.
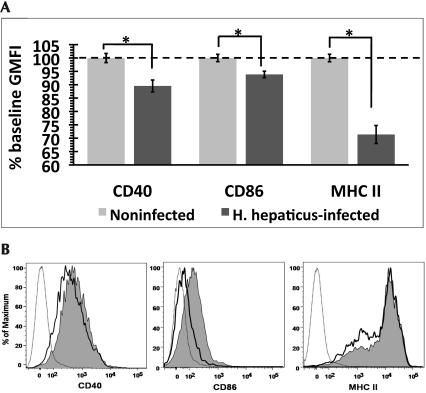
We used fluorescent flow cytometry to measure the expression of the maturation-associated surface markers CD40, CD80, CD86, and MHC II on the DC derived from colic LN of H. hepaticus-infected and noninfected mice. Surface expression of CD40, CD86, and MHC II was significantly (P < 0.05) lower on DC derived from H. hepaticus-infected mice than noninfected mice (Figure 4 A). Representative histograms showing the surface staining profile of CD11c+ DC population are presented in Figure 4 B. Because the expression of these markers increases with maturation, these data suggest that H. hepaticus infection inhibits the maturation of DC in the colic LN of mice. Surface expression of CD80 did not differ between the 2 groups of mice.
Figure 4.
Expression of maturation-associated surface markers on DC derived from colic LN. (A) Geometric mean (± SE) fluorescence intensity (GMFI) was normalized to the level of surface expression on DC from noninfected mice (% baseline GMFI) within each experiment (n = 5 to 6 mice per group). Results were combined from 3 independent experiments. (B) Representative histograms depicting the staining profile for surface markers on DC of H. hepaticus-infected (bold line) and noninfected (shaded area) mice. Dotted line represents unstained control. *, significant difference (P < 0.05) between indicated groups.
We also measured the expression of maturation-associated surface markers on the DC after in vitro lipopolysaccharide-induced maturation. After lipopolysaccharide exposure, DC from both H. hepaticus-infected and noninfected mice showed an increase (P < 0.05) in surface expression of CD40, CD80, and CD86 compared with that after exposure to medium alone (data not shown), but expression of these markers did not differ between H. hepaticus-infected and noninfected mice (data not shown). Surface expression of MHC II did not increase in either group of mice when cells were stimulated with lipopolysaccharide (data not shown).
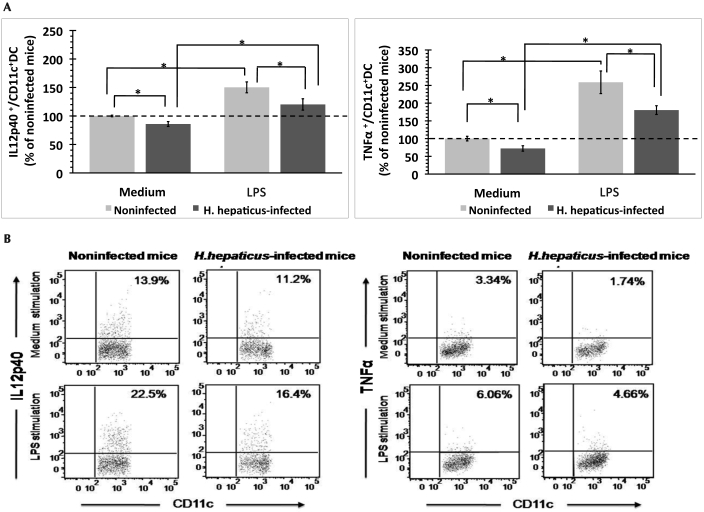
We compared the expression of the proinflammatory cytokines IL12p40 and TNFα by the DC derived from the colic LN of H. hepaticus-infected and noninfected mice after in vitro exposure to medium or lipopolysaccharide. The fluorescence intensity of IL12p40- and TNFα-positive DC did not differ between the 2 groups of mice (data not shown). The percentage of IL12p40- and TNFα-producing DC was higher after lipopolysaccharide exposure as compared with exposure to medium only (Figure 5 A). However, the percentages of IL12p40- and TNFα-producing DC in the colic LN were lower (P < 0.05) in H. hepaticus-infected mice than in noninfected mice after exposure to both lipopolysaccharide and medium only (Figure 5 A). Representative dot plots depicting the IL12p40- and TNFα-producing DC are shown in Figure 5 B.
Figure 5.
Intracellular proinflammatory cytokine expression by DC in the colic LN of H. hepaticus-infected and noninfected mice after in vitro exposure to medium or lipopolysaccharide (LPS). (A) The percentage (mean ± SE) of cytokine-producing DC was normalized to the percentage of cytokine-producing cells from noninfected mice after incubation with medium within each experiment (n = 5 or 6 mice per group). Results were combined from 3 independent experiments. (B) Representative dot plots depicting the intracellular staining profile for IL12p40 and TNFα in CD11c+ DC population. *, significant difference (P < 0.05) between indicated groups.
Phenotypic and functional characteristics of the DC from the spleen.
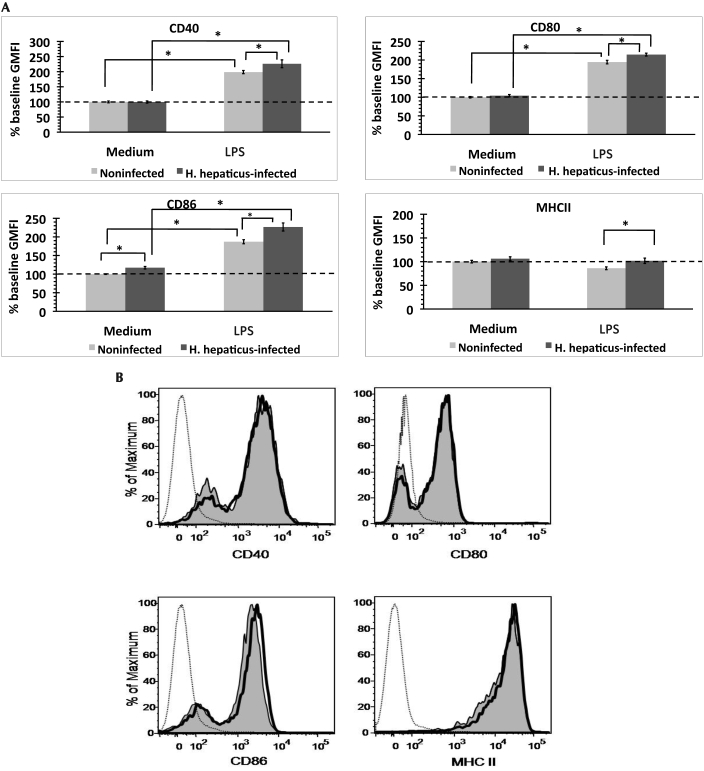
In contrast to colic LN-derived DC, splenic DC showed no difference in levels of CD40, CD80, CD86, and MHC II between H. hepaticus-infected and noninfected mice (data not shown). In vitro lipopolysaccharide exposure increased (P < 0.05) surface expression of CD40, CD80, and CD86 on DC from both groups of mice; MHC II expression did not increase (Figure 6 A). Surface expression of CD40, CD80, CD86 and MHC II reached significantly (P < 0.05) higher levels on lipopolysaccharide-exposed splenic DC from H. hepaticus-infected mice than noninfected mice (Figure 6 A). Expression of CD86 was similarly higher (P < 0.05) in H. hepaticus-infected mice after exposure to media (Figure 6 A). Representative histograms of the expression of these surface markers on DC are shown in Figure 6 B.
Figure 6.
Expression of maturation-associated surface markers on DC derived from the spleens of H. hepaticus-infected and noninfected mice after in vitro exposure to medium or lipopolysaccharide (LPS). (A) Geometric mean (± SE) fluorescence intensity (GMFI) was normalized to the level of surface expression on DC from noninfected mice after medium exposure (% baseline GMFI) within each experiment (n = 5 or 6 mice per group). Results were combined from 3 independent experiments. (B) Representative histograms show DC from H. hepaticus-infected (bold line) and noninfected mice (shaded area) after lipopolysaccharide stimulation. Dotted line represents unstained controls. *, significant difference (P < 0.05) between indicated groups.
Lipopolysaccharide increased (P < 0.05) the percentage of TNFα-producing DC in the spleens of both groups of mice (data not shown). However neither the percentage of TNFα-producing DC nor their fluorescence intensity differed between H. hepaticus-infected and noninfected mice (data not shown).
Discussion
Although the first reports of H. hepaticus were published 15 y ago, infections with H. hepaticus or related enterohepatic Helicobacter spp. remain common in mouse colonies at research facilities. Commercial breeders produce Helicobacter-free animals, but noncommercial facilities often still have many strains of Helicobacter-infected mice. The primary method of monitoring for Helicobacter—PCR of feces—is more costly than serologic monitoring that is used for common viral infections. Elimination of Helicobacter by rederivation, even by a nonsurgical method such as neonatal transfer, is time-consuming and expensive.
We initiated this study to determine whether and how H. hepaticus infection could influence immunologic studies at our institution. We used flow cytometry-based assays to measure specific markers and functional characteristics of cells of the immune system. We chose a mouse strain that is commonly used in immunologic research (C57BL/6) to increase the general applicability of our findings. When infected with H. hepaticus, C57BL/6 mice are considered to be relatively resistant to development of lesions.39
In the present study, H. hepaticus infection did not have a significant effect on either antibody- or T cell-mediated immune responses against HSV1 infection (Figure 1 through 3). After intranasal inoculation, HSV1 replicates in the nasal mucosa and then travels by means of peripheral nerves to the brain and spinal cord. C57BL/6 mice are resistant to HSV1-induced lesions, and the infection is cleared in 7 d by virus-specific, cell-mediated immunity.2 The advantage of using the HSV1 model is that it is a very well characterized model of T cell-mediated immune responses. However, if a viral infection model that infected the gut or liver had been used (for example, parvovirus or mouse hepatitis virus), virus-specific antibody- and T cell-mediated immune responses might have differed between H. hepaticus-infected and noninfected mice. In fact, coinfection of mice with both mouse hepatitis virus and H. hepaticus has been shown to increase the severity of pathology associated with mouse hepatitis virus.7 Another possible explanation for the results obtained is that pathogen-associated molecular patterns released from H. hepaticus may not drain from the primary site of infection through the lymphatics in sufficient quantity to affect DC priming of the response in the superficial cervical and tracheobronchal LN. We were unable to confirm that these 2 lymph nodes receive drainage from the liver or gut, which are primary sites of H. hepaticus infection.
Although statistical significance was not achieved, there was a trend for lower HSV1-specific T cell-mediated immune responses in the tracheobronchal LN of the H. hepaticus-infected mice. Absolute numbers of HSV1-specific CD8+ T cells, IFNγ-producing CD8+ T cells, and CD107+CD8+ cytotoxic T cells were lower in H. hepaticus-infected as compared with noninfected mice (Figures 1 D, 2 D, and 3 D), with the numbers of CD107+CD8+ cytotoxic T cells approaching statistical significance (P = 0.07; Figure 3 D). If more mice had been used per group, the differences might have achieved statistical significance. Such a trend was not seen for the percentage of HSV1-specific CD8+ T cells relative to the total number of CD8+ T cells counted. This finding suggests that the overall number of CD8+ T cells in the tracheobronchal LN of H. hepaticus-infected mice may have decreased. In fact, a previous study demonstrated that portal, celiac, and mediastinal LN receive lymphatic drainage from the liver of the mice.28 The mediastinal LN shown in reference 28 correspond to cranial mediastinal LN according to the nomenclature of others.38 Having found decreased levels of maturation-associated surface markers on DC obtained from LN that drain the colon of H. hepaticus-infected mice, we consider the possibility that the cells we collected from tracheobronchal LN might have been affected by some component of H. hepaticus in the liver, either by the unintentional collection of some cranial mediastinal LN, which are anatomically very close, or if tracheobronchal LN receive some degree of lymphatic drainage from the liver.
Superficial cervical LN demonstrated a trend (P = 0.07) for an increasing percentage of HSV1-specific CD107a+CD8+ cytotoxic T cells in H. hepaticus-infected than noninfected mice (Figure 3 A). Given that it involved the analysis of a single parameter, we view this finding as less compelling than the trend in tracheobronchal LN. The superficial cervical LN do not receive lymphatic drainage from tissues colonized by H. hepaticus.
DC derived from the colic LN of H. hepaticus-infected mice had lower expression of maturation-associated surface markers CD40, CD86, and MHC II as compared with those from noninfected mice (Figure 4). This effect is not permanent and is overcome when the DC were exposed to proinflammatory stimuli in vitro. The percentages of IL12p40- and TNFα-producing DC were lower in the colic LN of H. hepaticus-infected mice than noninfected mice after in vitro lipopolysaccharide or medium treatment (Figure 5). This shows that in the case of these proinflammatory cytokines, unlike the maturation-associated surface markers, the effect persists after the cells are collected from the mice and stimulated in vitro. These findings indicate that H. hepaticus has the potential to inhibit functional responses of the immune system. Our findings are supported by an in vitro study33 that demonstrated that treatment of mouse intestinal crypt epithelial cells with H. hepaticus lysate and the soluble component lipopolysaccharide reduced secretion of the proinflammatory chemokine macrophage inflammatory protein 2. Furthermore, both the lysate and lipopolysaccharide antagonized the stimulatory effect of E. coli lipopolysaccharide and flagellin on the immune responses of mouse intestinal crypt epithelial cells.33 Costimulation of CD86 by DC is very important for the generation of cytotoxic T cells.32 Therefore, in H. hepaticus-infected mice, decreased CD86 expression by DC in colic LN might lead to local immunosuppression by reduction in the generation of cytotoxic T cells. The effects on DC that we observed may be important factors that allow H. hepaticus to evade the host immune response and persistently colonize the mouse gut. The inhibition of DC in colic LN could underlie the reported increased severity of pathology from mouse hepatitis virus in mice coinfected with H. hepaticus.7 In contrast to our findings, other authors have reported upregulation of costimulatory molecules on DC from mesenteric LN of H. hepaticus-infected C57BL/6 mice.22 However, that study was published in abstract form only, thus precluding full comparison of the reported findings with our present data.
In the current study, the inhibitory cytokine IL10 may have played a role in the inhibition of DC from colic LN of H. hepaticus-infected mice. IL10 inhibits surface expression of CD80 and CD86 on murine bone marrow-derived DC19 as well as MHC II trafficking to the plasma membrane of antigen-presenting cells.17Therefore, decreased expression of CD86 and MHC II on DC derived from the colic LN of H. hepaticus-infected mice (Figure 4) might be because of IL10 production. Surface expression of CD80 was below the limit of detection by flow cytometry and thus prevented further analysis. Increased IL10 production might also have accounted for the decreased percentage of IL12p40-producing DC in the colic LN of H. hepaticus-infected mice (Figure 5)—IL10 inhibits IL12p40 gene transcription.42 The kinetics of IL10 expression in the colic LN of H. hepaticus-infected mice are unknown, but compared with those in uninfected mice, cecal mucosal levels of IL10 mRNA are increased in H. hepaticus-infected C57BL/6 mice for as long as 14 d after infection.25 In addition, IL10 was important in maintaining low levels of IL12p40 in the colon of H. hepaticus-infected C57BL/6 mice and in preventing the development of colitis.18 In contrast, H. hepaticus-infected C57BL/6 mice deficient in IL10 had higher expression of IL12p40 and developed colitis. Intestinal inflammation subsided when H. hepaticus-infected mice were treated with antiIL12p40, demonstrating the importance of IL12p40 in induction and maintenance of colitis.18 The inhibitory effect of IL10 on IL12p40 was mediated by p50/p105 subunit of NFκβ.37
Unlike DC derived from the colic LN, splenic DC from H. hepaticus-infected mice showed no decrease in the expression of maturation-associated surface markers (data not shown). Furthermore, after in vitro lipopolysaccharide stimulation, DC from the spleens of H. hepaticus-infected mice had higher levels of CD40, CD80, CD86, and MHC II than did noninfected mice (Figure 6). Possible reasons for the different responses of DC from these 2 sites may be related to the different routes by which antigens reach these sites and the effect of dose. Antigens reach the spleen only through the blood stream.27 Although we know of no reports of H. hepaticus bacteremia in mice, perhaps small numbers of H. hepaticus intermittently become bloodborne to stimulate a response in the spleen. Bacteremia with enterohepatic Helicobacter spp. in human patients has been reported,12,20 and 1 team of scientists isolated a novel Helicobacter from the spleen and other tissues of a mouse 10 d after intraperitoneal inoculation.23 Bacteremia has been proposed as the mechanism for the presence of amplifiable Helicobacter DNA in the reproductive organs of mice infected with H. typhlonius.30,31 Low-dose exposure to Helicobacter organisms may stimulate splenic DC, whereas DC in the colic LN are inhibited by chronic exposure to greater amounts of H. hepaticus or its products present in lymphatic drainage from the large intestine, the major site of H. hepaticus colonization. The related human pathogen H. pylori translocates from the stomach to the draining gastric LN,15 so it is possible that H. hepaticus travels to the colic LN to directly exert an inhibitory effect on DC.
Another possible reason for the different responses in our current study is that the populations of DC themselves differ between the LN and spleen.41 In the spleen, 100% of the DC are blood-derived or resident and are fairly immature, with only low levels of expression of MHC II. However, approximately 50% of the DC in the LN are blood-derived; the other half are migratory DC, which travel from peripheral tissues to the draining LN, where their maturation is triggered by encountering pathogen products at the periphery. Although the presence of a different DC population in itself would not account for increased expression of maturation-associated markers on splenic-derived DC from H. hepaticus-infected mice (Figure 6), H. hepaticus may induce a proinflammatory mediator that travels from the site of infection through blood to the spleen. Such a process could account for the differing effects of H. hepaticus infection we observed on DC at the local level of the draining LN and the systemic level of the spleen. This possibility will require further investigation. It would be interesting to determine whether a stimulatory effect on DC occurs in LN that receive no lymphatic drainage from tissues colonized by H. hepaticus, such as superficial cervical or popliteal LN.
Stimulation with medium only led to high MHC II expression on DC from both the colic LN and spleen, but upregulation after in vitro lipopolysaccharide stimulation did not occur (Figure 6 A). If the expression level of MHC II did in fact increase after lipopolysaccharide stimulation, we likely were unable to detect it because the expression levels were already near the upper limit of detection by flow cytometry.
In conclusion, we used a variety of assays to show that H. hepaticus infection of mice changes several immunologic parameters. Future studies will determine the mechanisms by which this modulation occurs. The findings we present here emphasize the importance of using H. hepaticus-free mice in studies in which immunologic function is a factor in the results.
Acknowledgments
We thank Nate Sheaffer and Dave Stanford of the Cell Science–Flow Cytometry Core Facility (Section of Research Resources, Penn State College of Medicine) for assistance with flow cytometry. We also thank Kathleen Ashcraft, Michael Elftman, John Hunzeker, Jennifer C Mellinger, Janet Sei, Erica Granger, and Raghu Tadagavadi for their technical assistance. This project was funded by grant K01 RR017552 from the National Center for Research Resources to C Beckwith and the Department of Comparative Medicine, Penn State Hershey College of Medicine.
References
- 1.Anglen CS, Truckenmiller ME, Schell TD, Bonneau RH. 2003. The dual role of CD8+ T lymphocytes in the development of stress-induced herpes simplex encephalitis. J Neuroimmunol 140:13–27 [DOI] [PubMed] [Google Scholar]
- 2.Ashcraft KA, Hunzeker J, Bonneau RH. 2008. Psychological stress impairs the local CD8+ T cell response to mucosal HSV1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology 33:951–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckwith CS, Franklin CL, Hook RR, Jr, Besch-Williford CL, Riley LK. 1997. Fecal PCR assay for diagnosis of Helicobacter infection in laboratory rodents. J Clin Microbiol 35:1620–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. 1993. Epitope specificity of H2Kb-restricted, HSV1-, and HSV2-crossreactive cytotoxic T lymphocyte clones. Virology 195:62–70 [DOI] [PubMed] [Google Scholar]
- 5.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. 1997. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun 65:3126–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahlavi A, Rabkin S, Todo T, Sundaresan P, Martuza R. 1999. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther 6:1751–1758 [DOI] [PubMed] [Google Scholar]
- 7.Compton SR, Ball-Goodrich LJ, Zeiss CJ, Johnson LK, Johnson EA, Macy JD. 2003. Pathogenesis of mouse hepatitis virus infection in gamma interferon-deficient mice is modulated by coinfection with Helicobacter hepaticus. Comp Med 53:197–206 [PubMed] [Google Scholar]
- 8.Elftman MD, Norbury CC, Bonneau RH, Truckenmiller ME. 2007. Corticosterone impairs dendritic cell maturation and function. Immunology 122:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Jr, Gorelick PL, Ward JM. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol 32:1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiasi H, Nesburn AB, Kaiwar R, Wechsler SL. 1991. Immunoselection of recombinant baculoviruses expressing high levels of biologically active herpes simplex virus type 1 glycoprotein D. Arch Virol 121:163–178 [DOI] [PubMed] [Google Scholar]
- 11.Hailey JR, Haseman JK, Bucher JR, Radovsky AE, Malarkey DE, Miller RT, Nyska A, Maronpot RR. 1998. Impact of Helicobacter hepaticus infection in B6C3F1 mice from 12 National Toxicology Program 2-year carcinogenesis studies. Toxicol Pathol 26:602–611 [DOI] [PubMed] [Google Scholar]
- 12.Holst H, Andresen K, Blom J, Højlyng N, Kemp M, Krogfelt KA, Christensen JJ. 2008. A case of Helicobacter cinaedi bacteraemia in a previously healthy person with cellulitis. Open Microbiol J 2:29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G. 1998. Isolation of dendritic cells, p 3.7.1–3.7.15. Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. New York (NY): John Wiley and Sons [Google Scholar]
- 14.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 15.Ito T, Kobayashi D, Uchida K, Takemura T, Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N, Furukawa A, Muraoka H, Ikeda S, Sekine M, Ando N, Suzuki Y, Yamada T, Suzuki T, Eishi Y. 2008. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest 88:664–681 [DOI] [PubMed] [Google Scholar]
- 16.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–120. Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press [Google Scholar]
- 17.Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. 1997. Interleukin 10 downregulates MHC class II αβ peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7:861–871 [DOI] [PubMed] [Google Scholar]
- 18.Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, Cheever AW, Sher A. 2001. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun 69:4232–4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lateef Z, Fleming S, Halliday G, Faulkner L, Mercer A, Baird M. 2003. Orf virus-encoded interleukin 10 inhibits maturation, antigen presentation and migration of murine dendritic cells. J Gen Virol 84:1101–1109 [DOI] [PubMed] [Google Scholar]
- 20.Leemann C, Gambillara E, Prod'hom G, Jaton K, Panizzon R, Bille J, Francioli P, Greub G, Laffitte E, Tarr PE. 2006. First case of bacteremia and multifocal cellulitis due to Helicobacter canis in an immunocompetent patient. J Clin Microbiol 44:4598–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun 72:6446–6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBee ME, Iweala OI, Nagler-Anderson C, Schauer DB. 2005. Subclinical Helicobacter hepaticus infection alters immune response to soluble luminal antigen. In: 13th International Workshop on Campylobacter, Helicobacter, and related organisms. 4–8 September 2005, Gold Coast, Queensland, Australia [Google Scholar]
- 23.McCathey SN, Shomer NH, Schrenzel MD, Whary MT, Taylor NS, Fox JG. 1999. Colonization and tissue tropism of Helicobacter pylori and a novel urease-negative Helicobacter species in ICR mice are independent of route of exposure. Helicobacter 4:249–259 [DOI] [PubMed] [Google Scholar]
- 24.Melancon MP, Wang Y, Wen X, Bankson JA, Stephens LC, Jasser S, Gelovani JG, Myers JN, Li C. 2007. Development of a macromolecular dual-modality MR–optical imaging for sentinel lymph node mapping. Invest Radiol 42:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myles MH, Dieckgraefe BK, Criley JM, Franklin CL. 2007. Characterization of cecal gene expression in a differentially susceptible mouse model of bacterial-induced inflammatory bowel disease. Inflamm Bowel Dis 13:822–836 [DOI] [PubMed] [Google Scholar]
- 26.Myles MH, Livingston RS, Livingston BA, Criley JM, Franklin CL. 2003. Analysis of gene expression in ceca of Helicobacter hepaticus-infected A/JCr mice before and after development of typhlitis. Infect Immun 71:3885–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parham P. 2000. The immune system, p 1–372. New York (NY): Garland Publishing–Elsevier Science [Google Scholar]
- 28.Rashidi B, Gamagami R, Sasson A, Sun FX, Geller J, Moossa AR, Hoffman RM. 2000. An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin Cancer Res 6:2556–2561 [PubMed] [Google Scholar]
- 29.Riley LK, Franklin CL, Hook RR, Jr, Besch-Williford C. 1996. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol 34:942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scavizzi F, Raspa M. 2006. Helicobacter typhlonius was detected in the sex organs of 3 mouse strains but did not transmit vertically. Lab Anim 40:70–79 [DOI] [PubMed] [Google Scholar]
- 31.Sharp JM, Vanderford DA, Chichlowski M, Myles MH, Hale LP. 2008. Helicobacter infection decreases reproductive performance of IL10-deficient mice. Comp Med 58:447–453 [PMC free article] [PubMed] [Google Scholar]
- 32.Sigal LJ, Reiser H, Rock KL. 1998. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J Immunol 161:2740–2745 [PubMed] [Google Scholar]
- 33.Sterzenbach T, Lee SK, Brenneke B, von Goetz F, Schauer DB, Fox JG, Suerbaum S, Josenhans C. 2007. Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect Immun 75:2717–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suni MA, Maino VC, Maecker HT. 2005. Ex vivo analysis of T-cell function. Curr Opin Immunol 17:434–440 [DOI] [PubMed] [Google Scholar]
- 35.Taylor NS, Xu S, Nambiar P, Dewhirst FE, Fox JG. 2007. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol 45:2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilney NL. 1971. Patterns of lymphatic drainage in the adult laboratory rat. J Anat 109:369–383 [PMC free article] [PubMed] [Google Scholar]
- 37.Tomczak MF, Erdman SE, Davidson A, Wang YY, Nambiar PR, Rogers AB, Rickman B, Luchetti D, Fox JG, Horwitz BH. 2006. Inhibition of Helicobacter hepaticus-induced colitis by IL10 requires the p50/p105 subunit of NF-kappa B. J Immunol 177:7332–7339 [DOI] [PubMed] [Google Scholar]
- 38.Van den Broeck W, Derore A, Simoens P. 2006. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods 312:12–19 [DOI] [PubMed] [Google Scholar]
- 39.Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ, Jr, Gorelick PL, Nagashima K, Gonda MA, Gilden RV, Tully JG, Russell RJ, Benvensite RE, Paster BJ, Dewhirst FE, Donovan JC, Anderson LM, Rice JM. 1994. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst 86:1222–1227 [DOI] [PubMed] [Google Scholar]
- 40.Whary MT, Morgan TJ, Dangler CA, Gaudes KJ, Taylor NS, Fox JG. 1998. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun 66:3142–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. 2003. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102:2187–2194 [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Nazarian AA, Smale ST. 2004. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol 24:2385–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]