Abstract
This study examines the effects of intravenous infusion of adenosine and sublingual nitroglycerin on coronary angiograms obtained by current-generation multidetector computed tomography. We assessed coronary vasodilation at baseline and after intravenous adenosine (140 µg/kg/min) or sublingual nitroglycerin spray (800 µg) in 7 female swine (weight, 40.9 ± 1.4 kg) by using electrocardiogram-gated coronary angiography with a 64-detector scanner (rotation time, 400 ms; 120kV; 400 mA) and intravenous contrast (300 mg/mL iohexol, 4.5 mL/s, 2 mL/kg). Cross-sectional areas of segments in the left anterior descending, circumflex, and right coronary arteries were evaluated in oblique orthogonal views. Images were acquired at an average heart rate of 73 ± 11 beats per minute. Changes in aortic pressure were not significant with nitroglycerin but decreased (approximately 10%) with adenosine. Of the 76 segments analyzed (baseline range, 2 to 39 mm2), 1 distal segment could not be assessed after adenosine. Segment cross-sectional area increased by 11.3% with nitroglycerin but decreased by 9.6% during adenosine infusion. The results of the present study are consistent with the practice of using sublingual nitroglycerin to enhance visualization of epicardial vessels and suggest that intravenous adenosine may hinder coronary artery visualization. This study is the first repeated-measures electrocardiogram-gated CT evaluation to use the same imaging technology to assess changes in coronary cross-sectional area before and after treatment with a vasodilator. The nitroglycerin-associated changes in our swine model were modest in comparison with previously reported human studies.
Abbreviations: LAD, left anterior descending artery; MDCT, multidetector computed tomography
Recent advances in multidetector computed tomography (MDCT) for coronary angiography, their increased availability, and the pending release of a new generation of CT scanners contribute to this methodology's potential for revolutionizing the early diagnosis and functional assessment of coronary artery disease.15,16,25 However, the benefits of methodologic advances do not diminish the need to validate and assess the safety, efficacy, and costs of technology.15 In this regard, the present study uses an animal model with coronary anatomy analogous to that of humans11,43 to assess the effects of sublingual nitroglycerin and intravenous adenosine on coronary epicardial vessel visualization by using baseline imaging as a control. The radiation exposure associated with CT procedures precludes human studies that involve repeated measures on the same subjects.
Sublingual nitroglycerin is hypothesized to enhance the visualization of epicardial coronary vessels due to its vasodilatory properties.8-10 Nitroglycerin acts as a nitric oxide generator to induce relaxation of vascular smooth muscle independent of endothelial function.1,18,20 Current evidence supporting improved coronary visualization with sublingual nitroglycerin is derived from clinical cross-sectional studies that compare results in different groups of patients with heterogeneous coronary lesions.5,6,16,27,44 A recent survey reported that more than 80% of facilities in the United States routinely use nitroglycerin in cardiac MDCT angiography.23
In contrast to the action of nitroglycerin on epicardial vascular smooth muscle, intravenous adenosine frequently is used as a pharmacologic stress to affect vasodilatation within the coronary microcirculation.20 Although current-generation CT scanners have limited capability to assess myocardial tissue perfusion, research efforts from advanced imaging centers suggest that future-generation CT scanners soon will permit myocardial perfusion imaging.13,14,29 No studies to date have assessed epicardial vessel changes by MDCT with intravenous doses of adenosine appropriate for myocardial perfusion imaging.
The objective of this study was to provide an animal model for MDCT studies that permits repeated measures of epicardial vessels without the limitations of cross-sectional designs or the complications of heterogeneity of vascular lesions within clinical populations. This study used routes of administration of nitroglycerin and adenosine that are consistent with clinical practice.
Materials and Methods
Seven female American Yorkshire–Landrace–Duroc swine (weight, 40.9 ± 1.4 kg; Sus scrofa; Midwest Research Swine, Gibbon, MN) were used in this study. On arrival from the source facility, animals were acclimated for a minimum of 72 h and housed in an AAALAC-accredited facility with environmental enrichment. Before being released from quarantine, the animals were screened for zoonotic diseases and normal blood chemistries. All animals were pathogen-free. In this protocol, the animals were fasted, with the exception of access to water 12 h prior to anesthesia. All animals were premedicated with buprenorphine hydrochloride (0.05 mg/kg IM) and sedated with tiletamine–zolazepam (4 to 6 mg/kg IM; Telazol, Fort Dodge Animal Health, Fort Dodge, IA). Once sedation was achieved, 5% isoflurane was administered by mask for intubation and urinary bladder catheter placement. The swine then were mechanically ventilated to maintain an end-tidal CO2 pressure of 40 ± 3 mm Hg. Vascular access was established by percutaneous cannulation of a right ear vein for contrast infusion, left ear vein for administration of adenosine, left femoral vein for intravenous anesthesia during CT imaging, and right femoral artery for continuously monitoring arterial blood pressure. Access was established while animals were maintained on 2% to 3% isoflurane and 100% oxygen. In addition, arterial oxygen saturation and the electrocardiogram were monitored continuously.
After preparation for physiologic monitoring, an intravenous regimen consisting of ketamine (1000 μg/kg/min) and midazolam (5 mg/h) was established and swine were weaned from isoflurane anesthesia. Metoprolol tartrate (5 mg IV; Mospira, Lake Forest, IL) was given as necessary to achieve baseline heart rates that were as comparable as possible to those of clinical protocols.
The study was approved by the Institutional Animal Care and Use Committee at Brooke Army Medical Center and was carried out in accordance with the Guide for the Care and Use of Laboratory Animals.19
Imaging protocol.
With all swine in the dorsal–recumbent position, cardiac CT coronary angiography was performed at baseline, after more than 3 min of intravenous infusion of 140 µg/kg/min adenosine (Sigma Aldrich, St Louis, MO), and after sublingual administration of 800 µg (2 sublingual sprays) nitroglycerin (Nitrolingual Pumpspray, Sciele Pharma, Atlanta, GA). Before each pharmacologic intervention, swine returned to baseline hemodynamic conditions.
All CT scans were obtained by using a 64-detector MDCT scanner (Aquilion 64, Toshiba Medical Systems, Otawara, Japan) with a gantry rotation time of 400 ms, tube voltage of 120 kV, tube current of 400 mA, detector collimation of 0.5 mm × 64, and with a variable helical pitch dependent on heart rate. The optimal spatial resolution of 3D volume-rendered and multiplanar images with this system is 0.35 mm. After initial scans to define optimal field of view for cardiac volumes and coronary angiography were completed, a region of interest in the descending thoracic aorta superior to the tracheal carina was selected for initiation of bolus tracking by using a threshold of +100 HU. Intravenous contrast (iohexol, 300 mg/mL; GE Healthcare, Princeton, NJ) was injected (2 mL/kg at 4.5 mL/s; Mark V, Medrad, Warrendale, PA) by means of an ear vein. Images were obtained during 15 s of suspended ventilation at end-expiration.
Image analysis.
Phase Exact software (Toshiba Medical Systems) was used to identify images with minimal motion artifact at baseline. In this study, the best phase images consistently were obtained at end-diastole at or near the onset of the Q wave of the electrocardiogram. Retrospective electrocardiogram-gating permitted comparisons between baseline and intervention in the same diastolic phase of the cardiac cycle. Upon completion of image acquisition, and preview of electrocardiography gate, data were transferred to a workstation (Vitrea DICOM, Vital Images, Minnetonka, MN) for offline analysis. To reduce imaging artifacts, images were acquired during periods of suspended ventilation (end-expiration), when heart rates were stable (± 10% of mean rate).
The CT angiograms of the 3D volume-rendered coronary arteries of the left anterior descending (LAD), left circumflex, and the right coronary artery in each animal were viewed in multiple orthogonal views to facilitate reproducibly identifying vessel segments, as defined in the 1999 Guidelines for Coronary Angiography.40 The maximal intensity projections were adjusted for visualization, and cross-sectional area calculations were performed at the same maximal intensity projection. Curved multiplanar reconstruction was used to confirm consistent vascular locations relative to vessel branch locations for measurement of cross-sectional area after each intervention. Because repeated scans were performed with animals in the same position, once baseline image segments were selected, baseline image ‘snapshots’ were obtained, and the oblique projections angles recorded at each site to reproduce measurement sites after interventions. A single investigator obtained 3 cross-sectional area measurements at each site and the mean reported as the site measurement.
Statistical analysis.
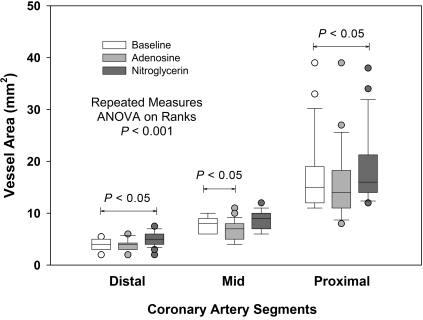
In this study, repeated-measures ANOVA was used to assess hemodynamic changes and differences in vessel segment cross-sectional areas between baseline and 2 pharmacologic challenges (adenosine infusion and sublingual nitroglycerin). Parametric data are presented as mean ± 1 SD; Dunn test was used for post hoc comparisons with baseline values. Differences with P values less than 0.05 were considered significant. Pharmacologic changes in vessel cross-sectional area were assessed as a function of baseline vessel size by regression analysis. Data were analyzed by using commercially available software (Sigma Stat version 3.11 for Windows, Systat Software, Chicago, IL). Slopes of regression lines for nitroglycerin and adenosine responses were compared with the line of identity by using methods of Zar.45 In addition, changes in cross-sectional area changes were assessed according to the size of the baseline conduit vessel, with areas of 2 to 5.5 mm2, 6 to 10 mm2, and larger than 10 mm2 representing the distal, mid, and proximal segments of coronary arteries, respectively. Data lacking normal distribution are presented as box plots.
Results
Coronary angiograms were obtained from all 7 female swine. Table 1 describes the hemodynamic status of animals immediately prior to CT angiography. Images were acquired at an average heart rate of 73 ± 11 bpm. Although the sample size of this study was not sufficient to detect hemodynamic differences with use of vasodilators, little difference was noted in heart rates or blood pressure with the administration of nitroglycerin. However, a trend (P < 0.07) was noted for decreased (by approximately 10%) aortic diastolic blood pressure after adenosine infusion.
Table 1.
Hemodynamic parameters measured (mean ± 1 SD; n = 7)
| Baseline | Adenosine (% change relative to baseline) | Nitroglycerin (% change relative to baseline) | P (ANOVA) | |
| Heart rate (bpm) | 70 ± 3 | 76 ± 18 (8.5) | 72 ± 5 (2.4) | 0.55 |
| Systolic aortic pressure (mm Hg) | 101 ± 10 | 91 ± 11 (–9.9) | 97 ± 9 (–3.8) | 0.22 |
| Diastolic aortic pressure (mmHg) | 55 ± 6 | 47 ± 7 (–13.8) | 53 ± 5 (–4.2) | 0.07 |
| Mean aortic pressure (mm Hg) | 70 ± 7 | 62 ± 10 (–11.0) | 68 ± 7 (–2.2) | 0.18 |
CT angiograms were obtained successfully from all 7 swine and were timed to occur during drug interventions to achieve maximal pharmacologic efficacy. Images were obtained more than 3 min after adenosine infusion was started and at 5.2 ± 0.9 min after sublingual administration of nitroglycerin. A total of 76 coronary segments were analyzed. The area of coronary segments at baseline ranged from 2 mm2 to 39 mm2. Segments included the proximal, mid, and distal right coronary artery as well as the posterior descending artery. In 1 animal, the distal segment of the posterior descending artery could not be visualized during adenosine infusion. The left main coronary artery; the proximal, mid, and distal segments of the LAD; and the left circumflex arteries were identified and assessed in all animals.
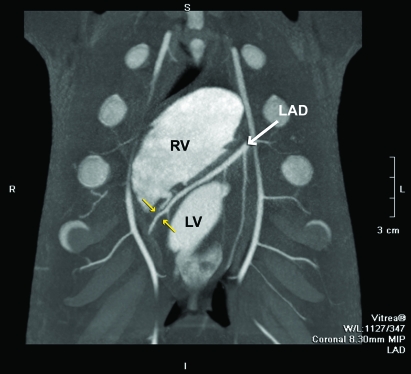
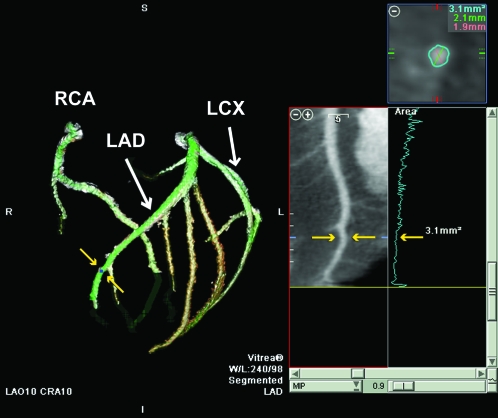
Figure 1 is an example maximal intensity projection (8.3 mm) of the interventricular septal course of the LAD coronary artery in a coronal view. Figure 2 is a 3D volume-rendered image of the coronary vasculature from the same animal with a 10° left anterior oblique and cranial projection. The cross-sectional area at the site of measurement (yellow arrows in both Figures 1 and 2) was 3.1 mm2; the major (2.1 mm) and minor (1.9 mm) dimensions for this measurement location are presented in the transverse multiplanar image (Figure 2, upper right corner).
Figure 1.
Coronal view of an 8.3-mm maximum intensity projection image of the LAD as it courses through the anterior interventricular sulcus. In this view, the right ventricle (RV) is superior to the left ventricle (LV) and the diagonal branches of the LAD are seen traversing inferiorly to the anterior LV wall. In this projection, the internal mammary arteries (unlabeled) with distributions to the anterior chest wall also are visible. The yellow arrows depict an example of a distal site for measurement of LAD cross-sectional area.
Figure 2.
Three-dimensional volume-rendered view of the right coronary artery (RCA), LAD, and left circumflex (LCX) coronary arteries in a 10° left anterior oblique and cranial projection. The yellow arrows illustrate the location of a distal LAD measurement site (same site as Figure 1) in 3D and curved multiplanar maximal intensity projection views of the LAD. At this site, the cross-sectional area of the LAD was 3.1 mm2. The upper right panel presents measurements of cross-sectional area from the transverse multiplanar reformatted image. The middle right panel is an example of the anatomical tapering of the LAD (blue line) as a plot of transverse multiplanar reformatted areas versus vessel length.
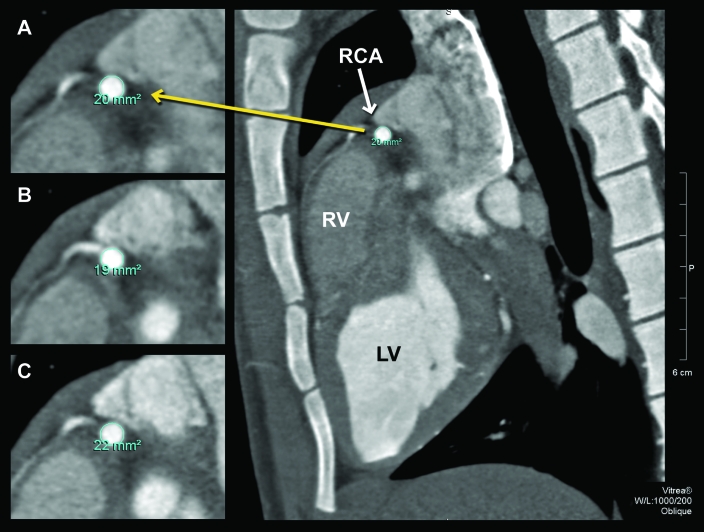
When compared with baseline data, overall cross-sectional areas after pharmacologic intervention differed. The mean cross-sectional area at baseline of all measurement sites was 10.0 ± 6.9 mm2, compared with 9.0 ± 6.5 mm2 during adenosine infusion and 11.0 ± 7.2 mm2 after sublingual nitroglycerin (ANOVA; P < 0.001). The average overall increase in vessel cross-sectional area with nitroglycerin was 11.3% ± 14.1% (P < 0.05), and the average decrease in vessel cross-sectional area after adenosine was 9.6% ± 14% (P < 0.05). Figure 3 illustrates the representative changes in the cross-sectional area with pharmacologic interventions at a site in the proximal right coronary artery (white arrow) of 1 animal. Note in the expanded views (left) that cross-sectional area at baseline was 20 mm2 (Figure 3 A) but 19 mm2 after adenosine (Figure 3 B) and 22 mm2 after nitroglycerin (Figure 3 C).
Figure 3.
Oblique 0.5-mm maximal intensity projections images of the proximal right coronary artery (RCA) as it traverses the anterior right atrioventricular sulcus. Magnified views demonstrate changes in vessel cross-sectional area (A) at baseline (area, 20 mm2), (B) after adenosine (19 mm2), and (C) after nitroglycerin (22 mm2).
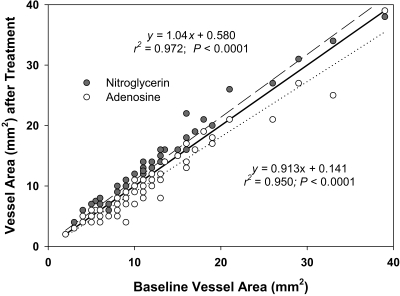
Figure 4 presents the nitroglycerin and adenosine responses as a function of baseline vessel cross-sectional area. These data demonstrate a significant upward shift in vessel cross-sectional areas with nitroglycerin throughout the measurement range (y = 1.039x + 0.580; r2 = 0.972; P < 0.0001), favoring only modest enhanced visualization. In contrast, a downward shift in the regression line (y = 0.913x + 0.141; r2 = 0.950; P < 0.0001) was noted with intravenously administered adenosine. The slopes of both the nitroglycerin and adenosine responses differed (P < 0.05) from that of the line of identity. When data were grouped by vessel size at baseline (2 to 5.5 mm2, 6 to 10 mm2, and greater than 10 mm2; Figure 5), nitroglycerin increased cross-sectional area by 17.7% ± 18.7%, 6.4% ± 11.3%, and 11.4% ± 10.0%, respectively. In contrast, adenosine decreased cross-sectional area in distal, mid, and proximal vessels by 3.0% ± 12.6%, 15.6% ± 15.5%, and 9.1% ± 10.8%, respectively.
Figure 4.
Plot of nitroglycerin- and adenosine-associated changes in coronary cross-sectional area versus baseline measurements. An upward shift in vessel cross-sectional area with nitroglycerin (long dashed line with gray circles) from the line of identity (dark line) favors enhanced visualization. The downward shift in the regression line for intravenously adenosine (dotted line and open circles) suggests poorer visualization.
Figure 5.
Box plots compare the distribution of distal (2 to 5.5 mm2), mid (6 to 10 mm2), and proximal (larger than 10 mm2) coronary vessels for baseline data and after adenosine and nitroglycerin treatment. The line within each box plot represents median cross-sectional area, and the box plot whiskers represent 10% and 90% of range values.
Discussion
Our study was stimulated by recent clinical case-control studies suggesting a benefit for sublingual nitroglycerin in MDCT protocols to enhance visualization of coronary arteries.27,39 Concerns regarding radiation exposure and the effects of contrast agent with MDCT procedures have precluded definitive studies in humans. Therefore, we sought to use an animal model that allowed a repeated-measures study design. Our swine model also permitted assessment of the effects of intravenous adenosine on epicardial vessel size with MDCT imaging.
Previous conventional angiographic studies8-10 demonstrated a dose–response relationship for sublingual nitroglycerin and coronary artery dilation. In these studies, sublingual nitroglycerin produced changes in coronary artery diameters ranging from 10% to 20%. In another study,36 sublingual nitrate spray was more effective than an equivalent dose of sublingual capsules and as effective as 0.2 mg intracoronary nitroglycerin in vasodilating normal coronary arteries (mean diameter increased by at least 13%). Sublingual and intracoronary nitroglycerin often are given during cardiac catheterization to “decrease coronary artery spasm, decrease coronary vascular tone, allow better visualization of coronary lesions, and allow better visualization of coronary collaterals.”21
Given the established history of nitroglycerin use in cardiac catheterization laboratories, many institutions empirically adopted the use of nitroglycerin in protocols assessing coronary arteries as MDCT imaging evolved for the study of coronary artery disease. This approach appears warranted by cross-sectional and case-control clinical studies,5-7,16,27,44 given the caveats of the heterogeneity of the vasculature within patient populations, variations in image gating procedures, and inconsistent timing of image acquisition with regard to the time course of action of sublingual nitroglycerin.39 A study using 64-slice CT in patients with coronary artery disease suggested that nitroglycerin improved visualization of branching vessels 5 min after administration of 0.5 mg of a nitroglycerin aerosol preparation.44 A retrospective review reported a 16% increase in the diameter of proximal coronary artery segments in response to nitroglycerin in 10 patients who had repeat studies for clinical indications over a mean interstudy interval of 1.9 y.7 The authors of a randomized, prospective case-control study (total n = 42) using 64-row MDCT reported that sublingual nitroglycerin spray increased (by at least 28%) the cross-sectional area of proximal right and left coronary artery segments and enhanced visualization of septal branches.6 Others reported improved diagnostic accuracy (by approximately 5%) and enhanced visualization of branch vessels with nitroglycerin when they retrospectively compared patients with and without nitroglycerin incorporated into a clinical MDCT protocol using conventional angiography as the reference standard.5 More recently other investigators have reported enhanced coronary visualization with sublingual nitroglycerin through vessel enlargement and improved intraluminal contrast density in patient populations matched for gender and body mass index.27
Each of the studies cited above suggested the benefit of improved coronary artery visualization in MDCT protocols with nitroglycerin given limitations in their study designs. However, offsetting these benefits are longer procedure times for patients and a 10% incidence of side effects (mild headache or dizziness).5 We employed an animal model with coronary anatomy analogous to man,11,43 that allowed repeated measures of vessel cross-sectional area at the same anatomical site, using the same image gating protocol and uncomplicated by heterogeneity associated with coronary artery disease3,24,32 to assess the effects of sublingual nitroglycerin and intravenous adenosine on epicardial vessel cross-sectional area.
The present study demonstrated an approximately 11% average increase in the cross-sectional area of coronary arteries by MDCT angiography after administration of 800 µg sublingual nitroglycerin spray. These results are consistent with conclusions cited by clinical trials8-10,36 and offer the first such evidence of these benefits in which subjects served as their own controls. In addition, our model extends observations of nitroglycerin-induced dilatation to smaller coronary conduit vessels than have been reported in human studies.5-7,16,27,44
The magnitude of change in vessel cross-sectional area with nitroglycerin in the present study is consistent with the 10% to 20% reported previously8-10 with conventional angiography. Conventional angiography provides greater spatial resolution than do current-generation CT scanners.27 However, our observed changes in vessel area were somewhat less than that reported in case-control studies. This difference may reflect either a limitation in the previously reported case-control studies or our selection of an anesthetic regimen that achieved consistently low baseline heart rates with minimal use of beta blockade for optimal imaging but that depresses vascular smooth muscle activity28 and lowers perfusion pressures.14
Although the current clinical focus of MDCT coronary angiography in patients with coronary artery disease has been the assessment of lesions in epicardial vessels,8,33 rapid advances in technology will soon permit dynamic perfusion imaging of the entire heart.12,13,29 Investigations at various institutions have already demonstrated the feasibility of myocardial perfusion imaging with MDCT in both acute and chronic ischemic models.12,13,29 Adenosine is the agent used most frequently for inducing hyperemic responses for myocardial perfusion studies.20 The effects of adenosine on epicardial vessels are endothelium-dependent and secondary to increased shear stress associated with increased blood flow through the microvasculature.4,30 Although intracoronary adenosine can induce flow-mediated dilatation of conduit vessels,4,42 intravenous adenosine leads to no or minimal dilation of these vessels.26,37,38 In addition intravenous infusion of adenosine has a systemic effect that induces a transient decrease in blood pressure and therefore a decrease in the perfusion pressure of coronary arteries.
In the present study, intravenous adenosine led to a significant 9% decrease in epicardial conduit vessel cross-sectional area. Absence of or decreased flow-mediated dilatation generally is clinically associated with pathologic epithelial dysfunction.41 Because this was not the case in our young healthy swine, an alternative explanation is that systemic effects of intravenous adenosine (10% decrease in blood pressure) reduced perfusion pressures and offset evidence of a flow-mediated dilation. The observations of smaller epicardial vessels after adenosine treatment suggest that new MDCT imaging protocols evaluating myocardial perfusion may have an impact on the visualization of epicardial coronary lesions and small branching vessels.
Because of their coronary anatomic and physiologic similarities to humans, swine have emerged as the large animal model of choice for studies of chronic cardiac ischemia, angiogenesis, revascularization, coronary blood flow and anatomy, and myocardial metabolism.17 Advanced imaging modalities35 including electrocardiogram-gated MDCT imaging are now well established for assessing coronary anatomy and ventricular function in swine.2 Although the effects of sublingual nitroglycerin on coronary vessel diameter in swine have not been reported previously, the vasoreactivity of intracoronary nitroglycerin34 and intravenous adenosine31 are well reported in studies using advanced imaging modalities and are similar to those in humans. Compared with previous studies, advantages of our study design included better controlled methodologies and an animal model that permitted repeated measures between baseline and pharmacologic interventions at well-defined anatomic sites; however we did not examine the reliability of CT measures of cross-sectional area as a function of vessel size. Reliability measures are particularly important when assessing small conduit vessels, which are identified as the critical “battlefield” for the clinical use of MDCT coronary angiography.27 In addition, reliability measures are important because typical heart rates in animal models are often suboptimal for coronary angiography with the current generation of MDCT scanners.
In summary, the present study is the first to use repeated measures in coronary vasculature without heterogeneous disease processes to support the use of intravenous nitroglycerin in MDCT protocols. Although the current results regarding sublingual nitroglycerin are consistent with the increased cross-sectional area reported by using standard angiography, the magnitude of the response observed (+11%) was more modest than reported in less well-controlled MDCT cross-sectional studies. In contrast to the benefits of sublingual nitroglycerin in improving visualization through increased cross-sectional area, no improvement in visualization of epicardial coronary vessels was noted after treatment of our swine model with intravenous adenosine.
Acknowledgments
We express our appreciation to Dr Daniel Vargas and Frank Goerner (University of Texas Health Science Center, San Antonio, Texas) for their contributions during protocol development. We also thank Alisa Leon, Belinda Meyers, Christopher Bell, and Suzy Kai for their assistance in this protocol.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Abrams J. 1995. The role of nitrates in coronary heart disease. Arch Intern Med 155:357–364 [PubMed] [Google Scholar]
- 2.Ahn YK, Ryu JM, Jeong HC, Kim YH, Jeong MH, Lee MY, Lee SH, Park JH, Yun SP, Han HJ. 2008. Comparison of cardiac function and coronary angiography between conventional pigs and micropigs as measured by multidetector row computed tomography. J Vet Sci 9:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselbergs FW, Monnink SH, Veeger NJ, van Boven AJ, van Haelst PL, Jessurun GA, van Gilst WH, Tio RA. 2004. Coronary vasomotor response is related to the angiographic extent of coronary sclerosis in patients with stable angina pectoris. Clin Sci 106:115–120 [DOI] [PubMed] [Google Scholar]
- 4.Bache RJ. 1998. Vasodilator reserve: a functional assessment of coronary health. Circulation 98:1257–1260 [DOI] [PubMed] [Google Scholar]
- 5.Chun EJ, Lee W, Choi YH, Koo BK, Choi SI, Jae HJ, Kim HC, So YH, Chung JW, Park JH. 2008. Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr 32:86–92 [DOI] [PubMed] [Google Scholar]
- 6.Decramer I, Vanhoenacker PK, Sarno G, Van Hoe L, Bladt O, Wijns W, Parizel PM. 2008. Effects of sublingual nitroglycerin on coronary lumen diameter and number of visualized septal branches on 64-MDCT angiography. AJR Am J Roentgenol 190:219–225 [DOI] [PubMed] [Google Scholar]
- 7.Dewey M, Hoffmann H, Hamm B. 2006. Multislice CT coronary angiography: effect of sublingual nitroglycerine on the diameter of coronary arteries. Rofo 178:600–604 [DOI] [PubMed] [Google Scholar]
- 8.Feldman RL, Marx JD, Pepine CJ, Conti CR. 1982. Analysis of coronary responses to various doses of intracoronary nitroglycerin. Circulation 66:321–327 [DOI] [PubMed] [Google Scholar]
- 9.Feldman RL, Pepine CJ, Conti CR. 1981. Magnitude of dilatation of large and small coronary arteries of nitroglycerin. Circulation 64:324–333 [DOI] [PubMed] [Google Scholar]
- 10.Feldman RL, Pepine CJ, Curry RC, Jr, Conti CR. 1979. Coronary arterial responses to graded doses of nitroglycerin. Am J Cardiol 43:91–97 [DOI] [PubMed] [Google Scholar]
- 11.Gal D, Isner JM. 1992. Atherosclerotic Yucatan microswine as a model for novel cardiovascular interventions and imaging, p 118–140. : Swindle MM, Moody DC, Phillips LD. Swine as models for biomedical research. Ames (IA): Iowa State University Press [Google Scholar]
- 12.George RT, Jerosch-Herold M, Silva C, Kitagawa K, Bluemke DA, Lima JA, Lardo AC. 2007. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol 42:815–822 [DOI] [PubMed] [Google Scholar]
- 13.George RT, Silva C, Cordeiro MA, DiPaula A, Thompson DR, McCarthy WF, Ichihara T, Lima JA, Lardo AC. 2006. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol 48:153–160 [DOI] [PubMed] [Google Scholar]
- 14.Hannon JP, Bossone CA, Wade CE. 1990. Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci 40:293–298 [PubMed] [Google Scholar]
- 15.Hoffmann U, Butler J. 2006. MDCT-based coronary angiography: a Rosetta stone for understanding coronary disease? Eur J Radiol 57:329–330 [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann U, Ferencik M, Cury RC, Pena AJ. 2006. Coronary CT angiography. J Nucl Med 47:797–806 [PubMed] [Google Scholar]
- 17.Hughes GC, Post MJ, Simons M, Annex BH. 2003. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol 94:1689–1701 [DOI] [PubMed] [Google Scholar]
- 18.Ignarro LJ. 2002. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 53:503–514 [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 20.Iskandrian AE. 2007. A new generation of coronary vasodilators in stress perfusion imaging. Am J Cardiol 99:1619–1620 [DOI] [PubMed] [Google Scholar]
- 21.Johnson MR. 1991. Part III. Coronary angiography, chapter 12. Clinical aspects of data acquisition in coronary angiography, p 182–210. : Marcus ML, Schelbert HR, Skorton DJ, Wolf GL. Cardiac imaging. A companion to Braunwald's heart disease. Philadelphia (PA): WB Saunders [Google Scholar]
- 22.Janne d'Othee B, Siebert U, Cury R, Jadvar H, Dunn EJ, Hoffmann U. 2008. A systematic review on diagnostic accuracy of CT-based detection of significant coronary artery disease. Eur J Radiol 65:449–461 [DOI] [PubMed] [Google Scholar]
- 23.Johnson PT, Eng J, Pannu HK, Fishman EK. 2008. 64-MDCT angiography of the coronary arteries: nationwide survey of patient preparation practice. AJR Am J Roentgenol 190:743–747 [DOI] [PubMed] [Google Scholar]
- 24.Jost S, Nolte CW, Sturm M, Hausleiter J, Hausmann D. 1998. How to standardize vasomotor tone in serial studies based on quantitation of coronary dimensions? Int J Card Imaging 14:357–372 [DOI] [PubMed] [Google Scholar]
- 25.Kalra MK, Brady TJ. 2008. Current status and future directions in technical developments of cardiac computed tomography. J Cardiovasc Comput Tomogr 2:71–80 [DOI] [PubMed] [Google Scholar]
- 26.Kern MJ, Deligonul U, Tatineni S, Serota H, Aguirre F, Hilton TC. 1991. Intravenous adenosine: continuous infusion and low dose bolus administration for determination of coronary vasodilator reserve in patients with and without coronary artery disease. J Am Coll Cardiol 18:718–729 [DOI] [PubMed] [Google Scholar]
- 27.Klass O, Mutlu S, Hohl K, Feuerlein S, Jeltsch M, Brambs HJ, Hoffmann MH. 2009. Multidetector computed tomography coronary angiography: sublingual nitroglycerine improves image quality significantly because of peripheral coronary vasodilatation. J Comput Assist Tomogr 33:199–203 [DOI] [PubMed] [Google Scholar]
- 28.Klockgether-Radke AP, Pawlowski P, Neumann P, Hellige G. 2005. Mechanisms involved in the relaxing effect of midazolam on coronary arteries. Eur J Anaesthesiol 22:135–139 [DOI] [PubMed] [Google Scholar]
- 29.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, Halperin HR, Wu KC, Hare JM, Lima JA. 2006. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation 113:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupi A, Buffon A, Finocchiaro ML, Conti E, Maseri A, Crea F. 1997. Mechanisms of adenosine-induced epicardial coronary artery dilatation. Eur Heart J 18:614–617 [DOI] [PubMed] [Google Scholar]
- 31.Malik JA, Rubal BJ, Clarke GD, Dick EJ, Jr, Ward JA, Harris RA. 2005. Use and limitations of magnetic resonance phase-contrast assessment of coronary flow reserve in a model of collateral dependence. Comp Med 55:317–325 [PubMed] [Google Scholar]
- 32.McLeod AL, Newby DE, Northridge DB, Fox KA, Uren NG. 2003. Influence of differential vascular remodeling on the coronary vasomotor response. Cardiovasc Res 59:520–526 [DOI] [PubMed] [Google Scholar]
- 33.Miller J, Rochitte C, Dewey M. Coronary artery evaluation using 64-row multidetector computed tomography angiography (CORE-64): results of a multicenter, international trial to assess diagnostic accuracy compared with conventional coronary angiography. PS-03. Presented at American Heart Association's 30th Annual Scientific Sessions, 2007 November 3–7, in Orlando, Florida [Google Scholar]
- 34.Molloi S, Berenji GR, Dang TT, Kassab G. 2003. Assessment of vasoreactivity using video densitometry coronary angiography. Int J Cardiovasc Imaging 19:271–279 [DOI] [PubMed] [Google Scholar]
- 35.Olsen AK, Zeidler D, Pedersen K, Sorensen M, Jensen SB, Munk OL. 2007. Imaging techniques: CT, MRI, and PET scanning, p 387–395. : Swindle M. 2nd ed Swine in the laboratory. Surgery, anesthesia, imaging, and experimental techniques. Boca Raton (FL): CRC Press [Google Scholar]
- 36.Pfister M, Seiler C, Fleisch M, Gobel H, Luscher T, Meier B. 1998. Nitrate induced coronary vasodilatation: differential effects of sublingual application by capsule or spray. Heart 80:365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redberg RF, Sobol Y, Chou TM, Malloy M, Kumar S, Botvinick E, Kane J. 1995. Adenosine-induced coronary vasodilation during transesophageal Doppler echocardiography. Rapid and safe measurement of coronary flow reserve ratio can predict significant left anterior descending coronary stenosis. Circulation 92:190–196 [DOI] [PubMed] [Google Scholar]
- 38.Rossen JD, Quillen JE, Lopez AG, Stenberg RG, Talman CL, Winniford MD. 1991. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol 18:485–491 [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Isobe S, Sugiura K, Mimura T, Yotsudake Y, Meno C, Kato M, Harada K, Murohara T. 2009. Optimal starting time of acquisition and feasibility of complementary administration of nitroglycerin with intravenous β-blocker in multislice computed tomography. J Comput Assist Tomogr 33:193–198 [DOI] [PubMed] [Google Scholar]
- 40.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A, Jr, Russell RO, Jr, Ryan TJ, Smith SC., Jr 1999. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology–American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 33:1756–1824 [DOI] [PubMed] [Google Scholar]
- 41.Schindler TH, Hornig B, Buser PT, Olschewski M, Magosaki N, Pfisterer M, Nitzsche EU, Solzbach U, Jost H. 2003. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol 23:495–501 [DOI] [PubMed] [Google Scholar]
- 42.Shiode N, Morishima N, Nakayama K, Yamagata T, Matsuura H, Kajiyama G. 1996. Flow-mediated vasodilation of human epicardial coronary arteries: effect of inhibition of nitric oxide synthesis. J Am Coll Cardiol 27:304–310 [DOI] [PubMed] [Google Scholar]
- 43.Smith CP. 2000. Information resources on swine in biomedical research 1990-2000. AWIC Resource Series No. 11. Beltsville (MD): United States Department of Agriculture [Google Scholar]
- 44.Wang Y, Jin ZY, Kong L, Zhang Z, Song L, Lin S. 64-slice coronary CT angiography with use of nitroglycerine, p 552. Program and abstracts of the Radiological Society of North America 91st Scientific Assembly and Annual Meeting, 2005 November 27–December 2 in Chicago, Illinois [Google Scholar]
- 45.Zar JH. 1974. Biostatistical analysis. Englewood Cliffs (NJ): Prentice-Hall [Google Scholar]