Abstract
Background/Aims
The vasculature has become an important target in the development of therapies for an increasing number of human diseases, yet there are few reliable markers available to identify and evaluate the endothelium in experimental rodent models. In the present study we have characterized the expression, sub-cellular localization and accessibility of the rat pan-endothelial marker podocalyxin using a newly developed monoclonal antibody, mAb G278.
Methods
Podocalyxin expression and accessibility to binding by mAb G278 was determined in the rat by Western blotting, immunohistochemistry, immunofluorescence, SPECT Imaging and biodistribution analysis.
Results
mAb G278 binds to the protein core of podocalyxin and reliably immunostained endothelial cells in blood vessels of all calibers in fresh frozen, fixed frozen and paraffin-embedded sections of all tissues examined, but did not stain lymphatic vessels. Western blotting, in vivo imaging and biodistribution analyses demonstrated that highest levels of endothelial podocalyxin were found in the lung and heart. We also determined that podocalyxin is not enriched in caveolae and that it’s expression can be modulated in the tumor microenvironment.
Conclusion
Our study shows that podocalyxin is a better identifier of rat endothelia than are some of the more commonly used marker proteins and that mAb G278 is a robust antibody for use not only in identifying rat blood vessels but also as a tool to elucidate the function of podocalyxin.
Keywords: Caveolae, immunofluorescence, Immunohistochemistry, Molecular imaging, Biodistribution, SPECT imaging
Introduction
The endothelium is the interface which separates circulating blood from underlying tissues and mediates the exchange of nutrients, metabolic waste products and other molecules to maintain normal physiological function [1]. In addition to its role in normal homeostasis, the vasculature is associated with an array of pathological conditions brought on by or supported by either excessive angiogenesis (eg. cancer, diabetic retinopathy, age-related macular degeneration, rheumatoid arthritis) or insufficient angiogenesis (eg. ischemic heart disease, delayed wound healing, osteoporosis) (for a review see [2]). Because of the strong relationship between disease and imbalances in angiogenesis, the vasculature has become an important therapeutic target for an increasing number of diseases. Developing molecules that can effectively target blood vessels relies heavily on the use of animals models and therefore requires reagents such as monoclonal antibodies that can reliably and accurately identify the vasculature in a variety of experimental methods. Unfortunately, there are few such antibodies, especially those that can identify the rat endothelium.
The focus of our research is to map the rat endothelial cell surface proteome and to identify accessible vascular targets using a variety of techniques including mass spectrometry-based proteomics analysis, and hybridoma and phage technologies [3–6]. During the course of this work we have produced a number of antibodies to selected tissue and tumor-induced protein targets. Here we utilize one of these antibodies, monoclonal antibody (mAb) G278, to characterize and to evaluate the expression and distribution of the pan-endothelial marker podocalyxin (podxl) in normal and tumor tissues in the rat.
Methods
Animals
All animal experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Sidney Kimmel Cancer Center (SKCC). Animals were housed in the SKCC animal care facility, and those animals which received radiolabeled antibodies were housed and imaged in a separate lead-shielded animal facility at SKCC. Fischer rats (150–250 g) were obtained from Charles River Laboratories, and BALB/C mice (6–8 wks) were purchased from Jackson Laboratories.
Rat tumor models
The rat sarcoma cell line, FRcJ4 was a kind gift from Dr. Bernard Binétruy (Director of Research, INSERM, University of Nice). Lung tumors were initiated by injecting rats via the tail vein with FRcJ4 cells or the breast carcinoma cell line, 13762, as previously described [7,8]. Tumor-bearing lungs were prepared for sectioning as described below.
Hybridomas, monoclonal and polyclonal antibodies
BALB/C mice were immunized with luminal endothelial cell membranes (P) isolated from rat lungs using the silica coating technique [7–11]. Each mouse was injected sc with 300 µg of P suspended in Freund’s complete adjuvant, followed by two boosts with 300 µg of P in Freund’s incomplete adjuvant. Serum was collected from tail bleeds taken after the first and second boosts, diluted in block (5% FBS in PBS) by serial doubling dilution and the titers were measured by P ELISA (see below). The mouse with the highest titer after the second boost was given a final injection of 300 µg of soluble P (ie no Freund’s) and the spleen was harvested five days later. Splenocytes were fused with P3X.63-Ag8.653 myelomas using PEG and selected in HAT medium following standard protocols [12].
At ten days and 12 days after the fusion, half of the medium (ie 100 µL) was removed from the wells in the fusion plates and replaced with fresh HAT medium. The fusion plates were then screened by P ELISA on the 14th day after fusion to identify wells containing hybridomas producing antibodies against rat endothelial cell antigens. Selected parent hybridomas were expanded in culture and the culture supernatants were tested on Western blots of rat lung homogenate and rat lung P. Parent hybridomas producing antibodies that recognized an antigen which was significantly enriched in P were subjected to two rounds of subcloning to ensure clonality.
Rabbit anti-rat podoplanin was purchased from Sigma-Aldrich (St. Louis, MO). Goat polyclonal antibody to rat PECAM/CD31 (M20) was purchased from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA).
Antibody purification and isotyping
Clarified hybridoma culture supernatants were passed over a bed of GammaBind Plus (GE Healthcare, Piscataway, NJ) and after washing with 10 bed volumes of PBS, the antibody was eluted with glycine (0.1M, pH 3.0) into tubes containing 25 µL HEPES (1.0M, pH 7.0) . Peak fractions were collected and dialyzed against PBS at 4 °C overnight with stirring. Sodium azide was added and the antibody was stored at 4 °C. Isotyping was done with the Clonotyping System-HRP from Southern Biotechnology following standard protocols.
P ELISA
Rat lung P was diluted to a concentration of 1.0 mg/mL in water and sonicated on ice. The sonicated P was diluted to a final concentration of 0.1 mg/mL in water and pipetted into the wells of a 96-well plate at 50µL/well. The plates were placed, uncovered, in a 37 °C oven and allowed to dry overnight. Prior to use, the plates were blocked with 5% FBS in PBS for one hour. The block was removed and primary antibody (ie diluted serum from tail bleeds or 50 µL of medium from fusion plates) were added and incubated for 2 hours at room temperature. The plates were then washed three times with PBS and horseradish peroxidase-conjugated goat anti-mouse Ig secondary antibody (KPL, Gaithersburg, MD), diluted in block, was added and incubated for one hour at room temperature. The secondary antibody was removed by washing three times with PBS and 75 µL of the substrate o-phenylene diamine (Sigma-Aldrich, St. Louis, MO) was added to the wells. The reaction was stopped after 5 minutes by adding an equal volume of 2N H2SO4 and the plates were read at 490 nm.
Western blotting
Samples (5 µg) of rat organ homogenates and P were boiled in 1× reducing sample buffer and proteins were resolved by SDS-PAGE then transferred to nitrocellulose. The equality of the protein loads was verified by staining the nitrocellulose blot with Ponceau S and/or by silver staining a duplicate PAGE gel. Blots were used only if the variation between samples was less than 10%. Blots were blocked for 30 minutes at room temperature in 2% powdered milk dissolved in PBS/0.1% Tween 20 followed by a 2 hour incubation with the primary antibody (hybridoma culture medium diluted 1:10 in PBS/0.1% Tween 20, or purified antibody diluted to 2 µg/mL in block). The blots were then washed and incubated in horseradish peroxidase-conjugated goat anti-mouse Ig (GE Healthcare, Piscataway, NJ) for one hour at room temperature. After additional washes, signals were visualized by saturating the blots with SuperSignal West Pico (Pierce) and exposing them to X-ray film.
Tissue preparation and immunostaining
To prepare tissues for paraffin embedding, rats were anesthestized and the vasculature was perfused with Ringer’s followed by 10% neutral buffered formalin (NBF). The tissues were removed, cut into 0.5 cm slices and post-fixed for 6 hours in NBF prior to processing and embedding in paraffin. Sections (5 µm) were mounted on Colorfrost/Plus slides (Fisher Scientific). Tissue for cryosections were also fixed by perfusion with NBF, then perfused with 30% sucrose in NBF. The tissues were removed, cut into 0.5 cm slices, post fixed for 6 hours in 30% sucrose in NBF, then placed in 30% sucrose in PBS overnight at 4 °C. The tissues were embedded in TBS (Triangle Biomedical Sciences, Durham, NC) and 5 µm sections were cut on a Microm HM505E cryomicrotome and mounted on Colorfrost/Plus slides.
Prior to immunostaining, deparaffinized, rehydrated sections were subjected to antigen retrieval in 10 mM citrate buffer (pH 6.0) in a vegetable steamer, then blocked in 5% FBS in PBS/0.1% Tween 20 at room temperature for 30 minutes. Sodium azide (0.05%) was added to the block in order to inactivate endogenous tissue peroxidases. The sections were incubated with primary antibody for 2 hr at room temperature, washed 3 × 5 min in PBS/0.1% Tween 20 then incubated with biotinylated secondary antibody for 1 hr at room temperature followed by horseradish peroxidase-conjugated streptavidin (KPL, Gaithersburg, MD). To prevent cross reactivity with endogenous rat immunoglobulins in the tissues, a rat-serum-adsorbed, biotinylated goat anti-mouse- Ig (KPL, Gaithersburg, MD) was used to detect mouse monoclonal antibodies. After washing 3 × 5 min, the color reaction was developed with DAB (BioGenex, San Ramon, CA) and the sections were counterstained with hematoxylin prior to dehydration and mounting in Permount (Fisher Scientific). Digital images were acquired using a Nikon Eclipse E800 light microscope equipped with a Nikon DXM1200 digital camera.
Immunofluorescence staining of heart sections was done with paraffin-embedded tissue subjected to antigen retrieval as above. Cryosections, fixed for 5 minutes in NBF at room temperature, were used for lung and tumor immunofluorescence analyses. Heart, lung and tumor sections were washed in water, quenched (1 mM CuSO4, 50 mM ammonium acetate in water; 20 minutes), washed again in water then blocked and incubated with a mixture of the appropriate primary antibodies for 2 hr at room temperature. The slides were washed 3 × 5 min in PBS/0.1% Tween 20, then incubated for 1 hr with Alexafluor-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) in the dark at room temperature. After a final washing sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and confocal images were acquired with a Nikon 800 microscope fitted with a Perkin Elmer Wallac UltraView confocal head and a triple line laser (Perkin Elmer Wallac, Gaithersburg, MD).
1D gel and LC-MS/MS analyses
Lung P was incubated with β-octylglucoside (1.0% in PBS) at 37 °C for 1 hour in the presence of a protease inhibitor cocktail. After centrifugation proteins in the resulting supernatant were resolved by SDS-PAGE. Proteins were visualized by silver staining and the gel was aligned with a Western blot of the same sample probed with mAb G278. The appropriate gel slice was excised and proteins were digested in-gel with trypsin to yield a peptide mixture that was extracted from the gel and separated by a C18 reversed-phase microcolumn using a linear acetonitrile gradient over 60 minutes delivered by an Agilent 1100 HPLC system. The eluted peptides were directly introduced into a 3D ion trap mass spectometer (LCQ DecaXP, Thermo Fisher Scientific, Inc., Waltham, MA, USA). SEQUEST algorithms were used to designate acquired MS/MS spectra to peptide sequences from available public rat databases downloaded from UniProt and NCBI RefSeq (non-redundant) protein databases (April, 2006). Searches were performed as tryptic peptides only with a precursor mass tolerance of 2.0 Da. Accepted peptide identifications were based on a minimum ΔCn score of 0.1 and minimum cross correlation scores of 1.8 (z = 1), 2.5 (z = 2) and 3.5 (z = 3). Protein identification results were extracted from Sequest outfiles and filtered and grouped with DTASelect software using the above criteria.
cDNA cloning and recombinant expression
Total RNA was extracted from rat lungs with an RNeasy Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. Reverse transcription (RT) was performed with 5 µg of total lung RNA, 400 pmol of a podxl reverse primer 5’-TCAGAGGTGTGTATCTTCCTC-3’, and 200 units of Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Five microliters of the RT reaction was used in a PCR reaction with 100 pmol of the reverse primer and 100 pmol of forward primer 5’- CACCATGCGCCCCACTCTGGCG-3’ modified on the 5’ end to facilitate cloning into the Gateway Vector system (Invitrogen, Carlsbad, CA). The resulting PCR product was resolved on a 0.8% agarose gel, and the single band of approximately 1500 kb was excised, purified using a Perfectprep Gel Cleanup Kit (Eppendorf North America) then cloned into the pENTR/D-TOPO vector. The insert was sequenced and compared to the NCBI database, then cloned into pDEST17 using Gateway’s LR reaction. Purified recombinant podxl was analyzed by mass spectrometry to verify the identity of the protein.
In vivo imaging and biodistribution
Purified mAb G278 and an isotype matched control antibody were radioiolabeled with 125I using Iodobeads (Pierce, Rockford, IL). For in vivo imaging, anesthetized rats were injected via the tail vein with radiolabeled G278 and images were captured at the time points indicated. For the biodistribution assays, 10 µg of labeled G278 or the isotype matched control IgG were inoculated via the tail vein or the left ventricle and 1 hour later blood samples were collected prior to transcardial perfusion with 200 ml of PBS followed by organ dissection and measurement of bound radiolableld antibody. Organs were also collected from animals 24 hr after tail vein injection. Results are expressed as percent of injected dose per gram of tissue (%ID/g).
Results
ELISA and Western blot analysis of proteins expressed in lung EC membranes
A panel of monoclonal antibodies was generated by immunizing mice with lung endothelial cell plasma membranes (P) following standard protocols. When the fusion was tested by lung P ELISA, a number of parent hybridomas were selected based on strong immunoreactivity to antigens expressed on rat lung EC. One of these parents, G278 (IgG1, kappa), was selected for further study based on the ELISA results and Western blotting. In the P ELISA assay G278 produced a very strong OD of 2.6. By comparison, the OD from a monoclonal antibody to aminopeptidase P, a protein abundantly expressed in rat lung EC [4, 13], was 1.2 in the same assay. When tested by Western blotting, no specific signal was observed in the lane containing total lung homogenate (Figure 1A, lane H), however, because of the antigen enrichment afforded by subfractionation of the luminal plasma membrane of lung EC, a strong signal at approximately 130 kDa was readily detectable on blots of lung P (Figure 1A, lane P).
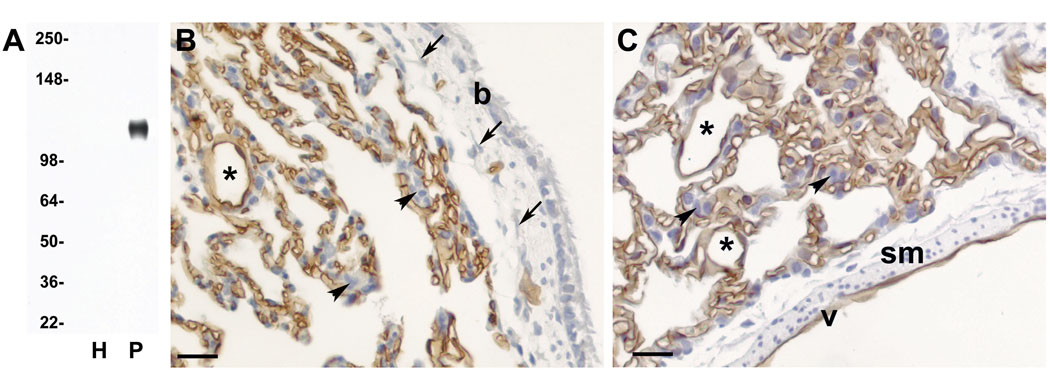
Figure 1.
A: Western blot analysis of homogenate (H) and luminal plasma membranes (P) isolated from normal rat lungs. Five micrograms of protein were loaded in each lane and blots were probed with mAb G278. Molecular weight markers are on the left. B & C: Immunohistochemical analysis of paraffin-embedded sections from normal rat lung. Panel B shows a section through the wall of a bronchiole and adjoining lung parenchyma and Panel C shows a longitudinal section through a large vein and the adjoining parenchyma. All endothelial cells in all capillaries, small veins (*) and on the lumen of the large vein (v) are stained with G278, while fibroblasts (arrows), smooth muscle cells (sm), and epithelial cells in the alveolae (arrowheads) and bronchiole (b) are unstained. Although not shown in these images, mAb G278 also stained the endothelium of all arteries and arterioles
In order to verify that the signals seen on the Western blots originated from an antigen expressed on the endothelium, sections of paraffin-embedded lung were immunostained with mAb G278. A strong signal was observed on all EC while no signal was seen on epithelial cells, fibroblasts or smooth muscle cells (Figures 1B & C) or on lymphocytes, nerves or the pleural mesothelium (not shown). Immunostaining was observed in all capillaries as well as all veins and veinules (Figures 1B & C) arteries and arterioles (not shown), of all sizes.
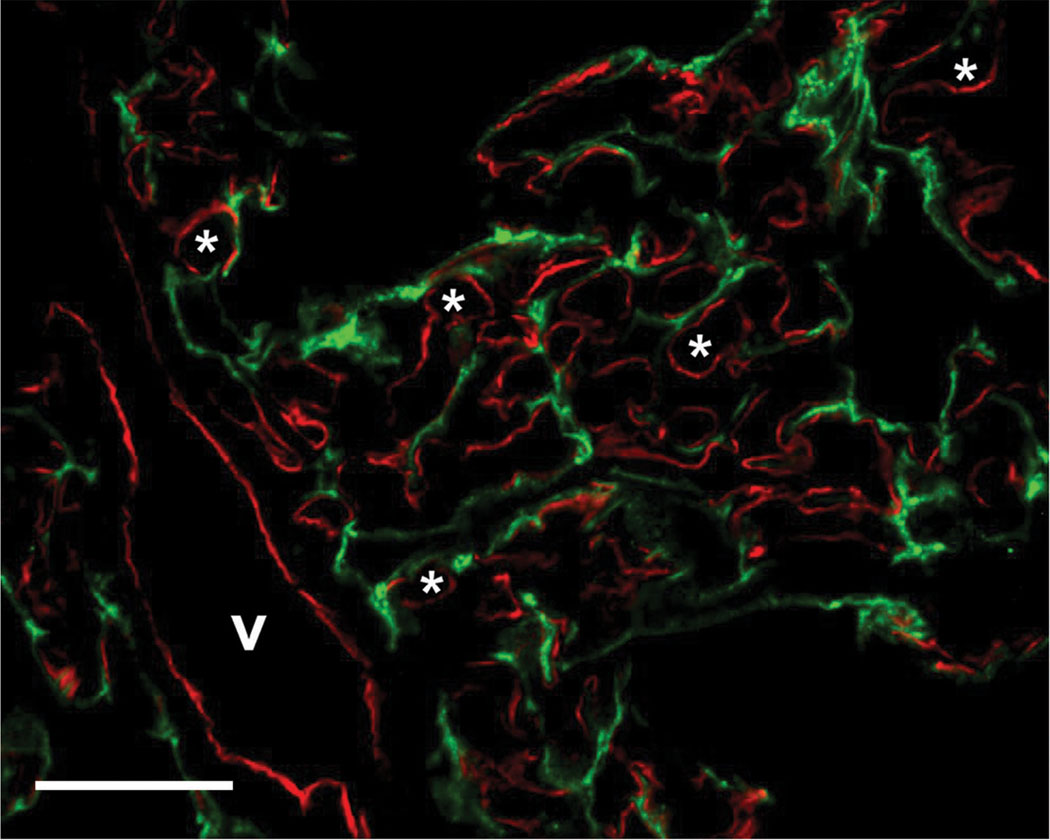
In addition to a dense vascular network, the lung also has an extensive lymphatic network. To determine if lymphatic EC were also immunostained, lung cryosections were analyzed by dual immunofluorescence with G278 and anti-podoplanin, an antibody that specifically labels lymph vessels. Figure 2 shows that there is no co-localization of the G278 signal (in red) with the podoplanin signal (in green), indicating that G278 specifically labels the blood vascular endothelium.
Figure 2.
Dual immunofluorescence analysis of normal rat lung cryosections. Sections were immunostained with mAb G278 (red channel) and a goat polyclonal antibody to the lymphatic marker podoplanin (green channel). There is no colocalization of the two signals indicating that mAb G278 does not immunostain lymphatic endothelial cells. v: small vein; *: capillary lumen. Bar = 20 µm
mAb G278 recognizes podocalyxin (podxl)
G278 does not effectively immunoprecipitate it’s cognate antigen isolated from P, therefore in order to identify the protein target we used a gel-based liquid chromatography tandem mass spectrometry approach (G-LC-MS/MS), in which proteins in P are first resolved by SDS-PAGE, then the appropriate band is excised, in-gel trypsin digested and the resulting peptides are analyzed by MS/MS. The antigen recognized by G278 is solubilized by SDS, Triton X-100, NP40, CHAPS and β-octylglucoside (not shown). The latter detergent was selected for the G-LC-MS/MS approach because, while it effectively solubilizes the target antigen, the majority of the proteins in P remain in the silica pellet following centrifugation thus minimizing the presence of irrelevant proteins in the sample. G-LC-MS/MS analysis of the protein band corresponding to the Western blot signal identified the antigen as podxl. Eight unique peptides were identified covering 14.4% of the rat podxl amino acid sequence (Figure 3A & B). A typical MS/MS spectrum is shown in Figure 3C. In order to confirm the identity of the protein target for mAb G278, rat podxl was cloned and recombinantly expressed. Western blot analysis of the recombinant protein confirmed that mAb G278 specifically recognizes rat podxl (not shown) and moreover demonstrated that the epitope to which the antibody binds is resident in the protein core and not dependent on post-translational modifications.
Figure 3.
G-LC-MS/MS analysis of the protein recognized by mAb G278. Proteins extracted by selective detergent solubilization were resolved by SDS-PAGE and the gel slice containing the target antigen was analyzed. A: Unique proteolytic peptides of rat podocalyxin (Column 2) identified by G-LC-MS/MS. The primary amino acid positions are listed in column 1; the charge status of each identified peptide is listed in column 3. B: Peptides (highlighted in blue) covering 14.4% of the rat podocalyxin (NCBI: gi6492252) amino acid sequence which were identified in the excised gel band containing the target antigen. C: a typical MS/MS spectrum, FLELLCHSAK.
Western blotting and proteomics analysis of podxl expression in rat heart, kidney, liver and brain
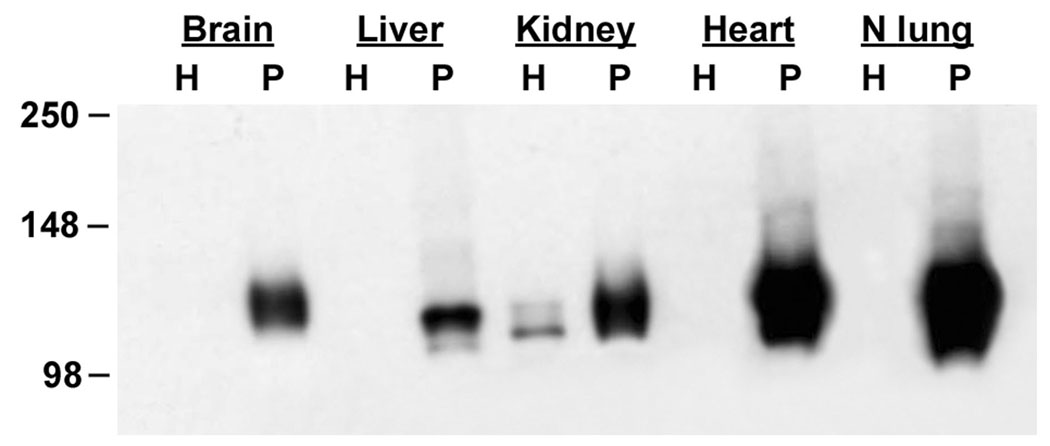
Expression of podxl in other vascular beds was examined by Western blot analysis of homogenates and P isolated from rat brain, heart, liver, and kidney. As before, no signal was observed in H prepared from lung, or in H from brain, heart, or liver (Figure 4). A weak doublet was observed in the homogenate from kidney (Figure 4, lane 5) which is likely due to the presence of podxl in the podocytes of the glomeruli and in the thin segment of the loop of Henle (see below). Podxl expression was observed in P isolated from each of the organs with the strongest signals being in lung and heart P, followed by kidney, brain, and liver P. Podxl in P from heart and lung migrates as a doublet with a strong signal seen at approximately 130 kDa and a weaker signal at approximately 120 kDa (seen to better advantage in Figure 10C). In brain and kidney P only the 130 kDa band is observed while in liver P two bands of approximately 125 kDa and 115 kDa are seen. These differences are probably due to natural variations between organs since it is known that the molecular weight of rat podxl varies depending on the cell type [14]. The presence of podxl in the EC membranes from each of these organs was also confirmed by proteomics analyses. In each case we were able to detect at least one unique podxl peptide (not shown).
Figure 4.
Western blot analysis of podxl expression in rat organs. Homogenates (H, 5 ug) and P (5 ug) prepared from rat brain, liver, kidney, heart, and normal lung were analyzed by Western blotting with mAb G278. Podocalyxin is seen in P from all organs but is not detected in organ homogenates with the exception of kidney.
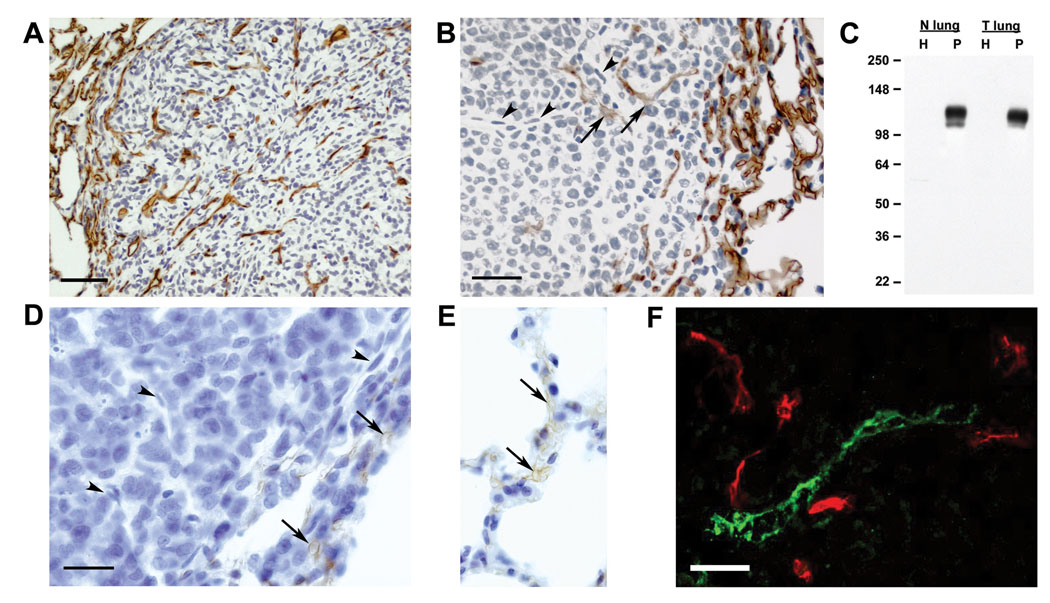
Figure 10.
Analysis of podocalyxin expression in a rat metastatic mammary tumor and sarcoma models. Panel A: Metastatic lung tumors of the rat sarcoma cell line FRcJ-4 were immunostained with G278. Signals are observed in the adjacent normal lung tissue and throughout the tumor mass itself. Panel B: Metastatic lung tumors of the rat mammary carcinoma cell line 13762 were immunostained with G278. Podxl expression is observed in the vessels adjacent to the tumor and in the corona of the tumor itself. Deeper in the tumor nodule podocalyxin expression by endothelial cells is diminished (arrows) or lost (arrowheads). Panel C: Homogenates (H) and P isolated from normal rat lungs (N lung) and tumor-bearing lungs (T lung) analyzed by Western blots probed with mAb G278. Molecular weight markers are shown on the left. Panel D & E: Anti-PECAM/CD31 immunostaining of tumor and normal vessels. PECAM/CD31 expression is seen in vessels in the corona of a 13762 tumor and adjacent normal lung (Panels D & E, arrows), but is absent in endothelial cells found deeper in the tumor mass (Panel D, arrowheads). Panel F: Dual immunofluorescence of a section cut through the corona of a 13762 mammary carcinoma nodule. There is no colocalization of the G278 signal (red channel) and anti-podoplanin (green channel) showing that mAb G278 does not immunostain the tumor lymphatic endothelium. Bar in Panels A & B = 50 µm. Bar in Panels D & E = 20 µm
Immunohistochemical analysis of podxl expression in other rat organs
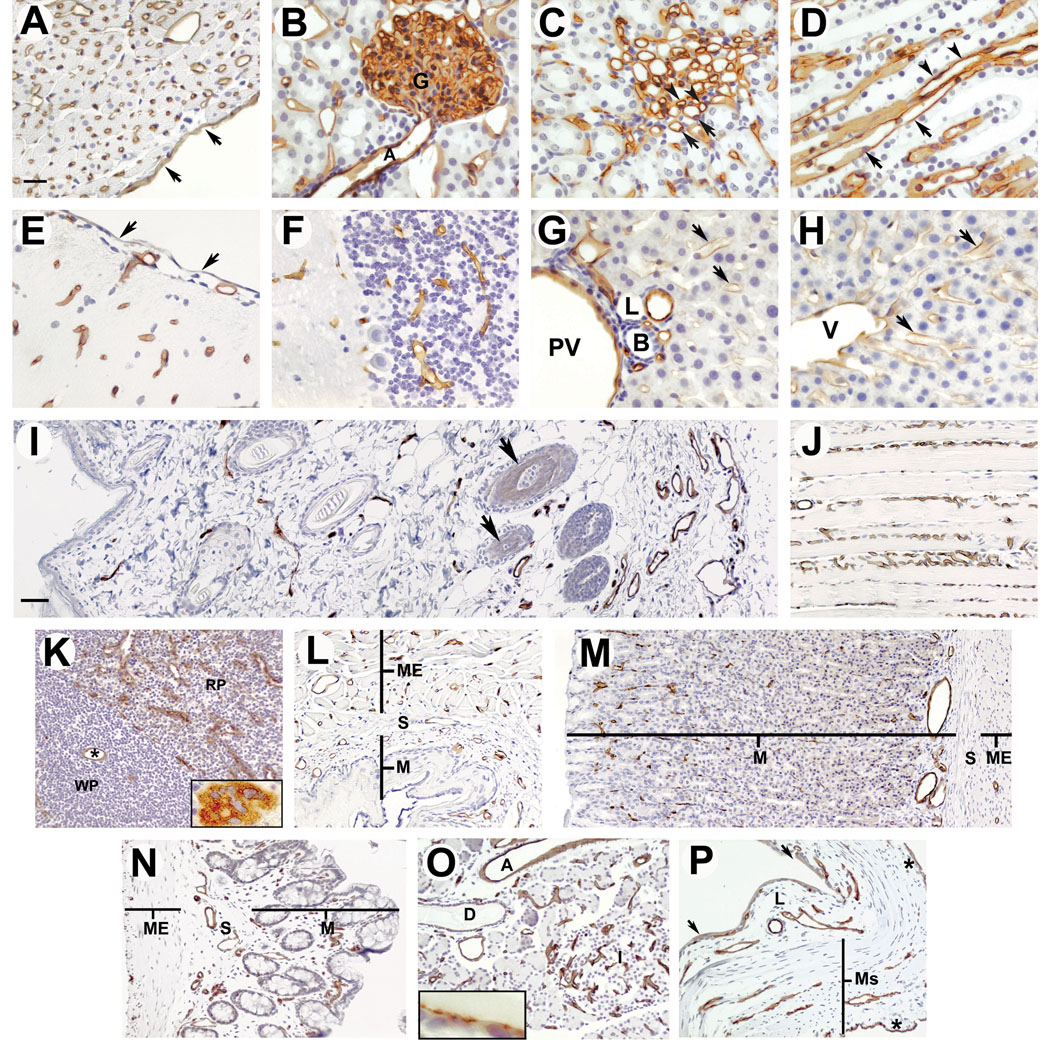
The distribution of podxl in rat organs was examined by immunohistochemistry. G278 performed equally well on fresh frozen, fixed frozen and paraffin embedded tissues, but immunostaining of paraffin embedded sections is shown here because of the quality of the histological architechture. Analysis of the heart, kidney, liver and brain show that the signals seen by Western blotting in Figure 4 were from podxl expressed by the EC. In these organs, as in lung tissue, all capillaries, arteries, arterioles, veins and veinules of all sizes were stained. In the heart, G278 stained all blood vessels as well as the endocardium but showed no immunoreactivity with the cardiomyocytes (Figure 5A) or the epicardium (not shown). G278 also stained all blood vessels in the cortex, glomeruli, medulla and papilla of the kidney (Figures 5B, C &D, respectively). No extra-endothelial stain was seen in the kidney with the exception of the podocytes in the glomeruli and the thin segment of the Loop of Henle. Vessels in all parts of the brain examined also expressed podxl, but no staining was seen in the nervous tissue itself nor the pia mater (Figures 5E & F). In the portal area of the liver, portal veins, hepatic arteries and other small vessels stained with G278 but no immunoreactivity was seen in the bile collecting ducts, lymphatics or heptaocytes (Figure 5G). Hepatic venules and the endothelium lining the liver sinusoids also expressed podxl (Figure 5H) but the mesothelial covering was negative (not shown).
Figure 5.
Immunohistochemical analysis of podocalyxin expression in paraffin-embedded sections of normal rat organs. Blood vessels in all organs examined were clearly stained with mAb G278. Panel A: endothelial cell staining in heart; the endocardium is indicated by arrows. B: kidney cortex showing staining of peritubular vessels, capillaries and podocytes in the glomerulus (G) and a glomerular arteriole (A); C & D: kidney medulla and kidney papilla, respectively; vessels are indicated by arrowheads, thin segments of the Loop of Henle are indicated by arrows; E: vessel staining in cerebrum; pia mater is indicated by arrows; F podocalyxin expression of vessels in the cerebellum; G: portal area of liver showing staining of the portal vein (PV) and endothelia lining the sinusoids (arrows). B: bile collecting duct; L: lymphatic vessel; H: liver showing podocalyxin expression in a hepatic veinule (V) and in the endothelia lining surrounding sinusoids (arrows); I: panoramic view of skin; vessels throughout the thickness of the skin are immunostained with G278; the stain seen on hair follicles (arrows) is similar to that seen on control slides and is non-specific; Ep: epidermis, Sb: sebaceous gland; J longitudinal section of skeletal muscle showing stained capillaries in the endomysium: K: spleen showing staining of central artery (*) in the white pulp (WP) and surrounding vessels in the red pulp (RP); inset shows a megakaryocyte stained with G278; L, M & N: panoramic views of esophagus, stomach and large intestine, respectively, showing vessel staining in the mucosa and lamina propria of the mucosal layer (M), in the submucosa (S) and in the muscularis externa (ME); O: Pancreas, showing staining of all vessels throughout the organ, and on the surface of the epithelium lining interlobular ducts (inset); I, pancreatic islet; D, interlobular duct; A, interlobular arteriole; P: urinary bladder showing staining of vessels in the lamina propria (L) and muscularis (Ms), and staining of the mesothelium (asterisks), light staining was also seen on some portions of the transitional epithelium (arrows). Bar in panel A = 20 µm; panels B through H are at the same magnification as Panel A. Bar in panel I = 50 µm; panels J through P are at the same magnification as Panel I.
When the immunohistochemical analysis was extended to include skin, skeletal muscle, spleen, esophagus, stomach, colon, pancreas, and bladder, we found that G278 also stained the endothelia in all of the blood vessels of these tissues as well. In the epidermis, dermis and hypodermis of the skin, EC were the only cell type that stained with G278 (Figure 5I). Likewise, in skeletal muscle only the endothelium was stained (Figure 5J). Podxl expression was also seen in the central and pulp arteries of the spleen (Figure 5K) as well as in the trabecular arteries and veins (not shown). Megakaryocytes, which are known to express podxl [14] were also stained (Figure 5K, inset) and only a few cells in the mesothelium on the surface of the spleen were immunopositive (not shown). In the esophagus and stomach and large intestine (Figures 5L, 5M and 5N respectively), blood vessels in the muscularis mucosa and lamina propria of the mucosal layer, as well as those in the submucosa, and the muscularis externa were immunopositive. Blood vessels throughout the pancreas also expressed podxl (Figure 5O), including capillaries in the islets, as well as intralobular and interlobular vessels of all sizes. Staining was sometimes observed on the cells lining the interlobular ducts (Figure 5O, inset), but not on the epithelium of the intralobular ducts (not shown). In the bladder (Figure 5P) endothelial immunostaining was seen in blood vessels of the muscularis and the lamina propria. Staining was sometimes seen on the transitional epithelium lining the bladder, and a strong podxl signal was observed on the mesothelium.
podxl is not expressed in caveolae
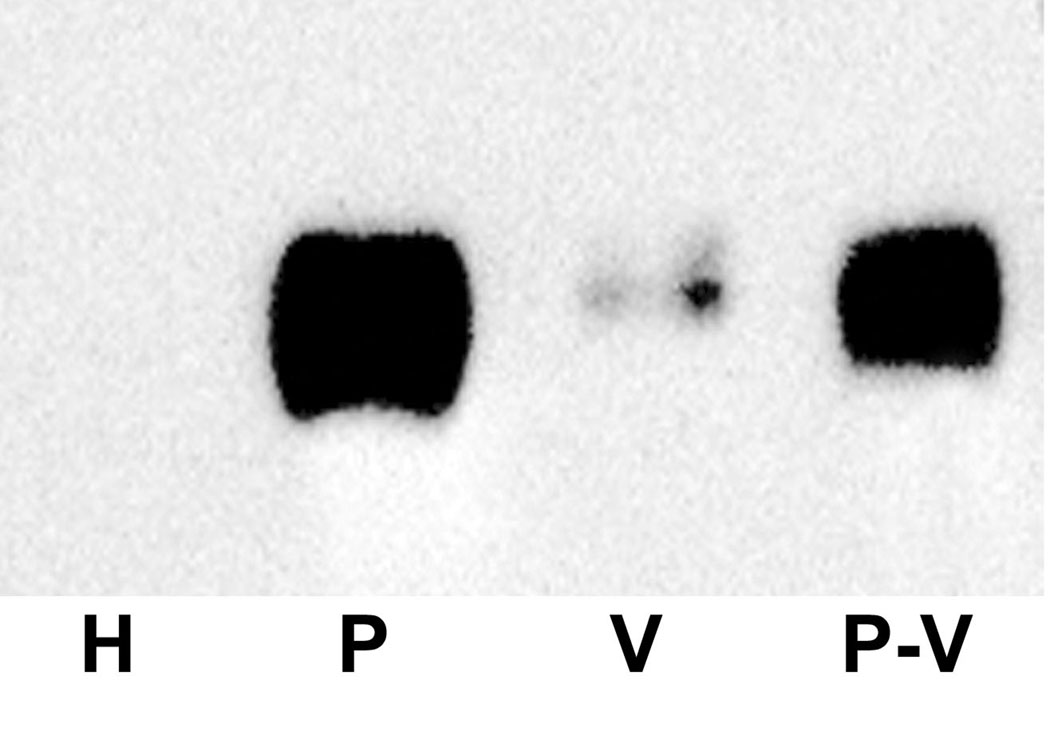
Continuous EC are heavily invested with flask-shaped invaginations called caveolae which mediate the uptake and transport of blood-borne molecules across the endothelium [13, 15, 16]. Caveolae (V) were isolated by further subfractionation of lung P and analyzed by Western blotting with G278. As shown in Figure 6A, strong podxl signals were seen in P and in P depleted of caveolae (P-V), but very little podxl could be detected in V. When tissues were stained by dual immunofluorescence for caveolin-1 (the major protein in caveolae) and for podxl (Figure 7A) there was little or no colocalization of the signals when exact cross sections of capillaries (Figure 7B) or the endocardium (Figure 7C) were examined.
Figure 6.
Podocalyxin protein is not enriched in caveolae. Panel A: Western blot of homogenate from normal lung (H, 5 µg), normal lung P (P, 5 µg), caveolae (V, 2 µg), and the caveolae-depleted subfraction of P (P-V, 5µg) were probed with mAb G278. Strong signals are seen in NP and in P-V, but very little is seen in V indicating that podxl is expressed primarily outside of the caveolae.
Figure 7.
Dual immunofluorescence analysis of rat heart sections immunostained with mAb G278 (red) and a goat polyclonal antibody to the caveolar marker caveolin-1 (green). When seen in exact cross section there is little or no colocalization of the two signals in capillaries (square in Panel A, and Panel B) nor in the endocardium (rectangle in Panel A, and Panel C) indicating that podxl is primarily located outside of the caveolae. Scale bar in Panel A = 20 µm.
In vivo targeting
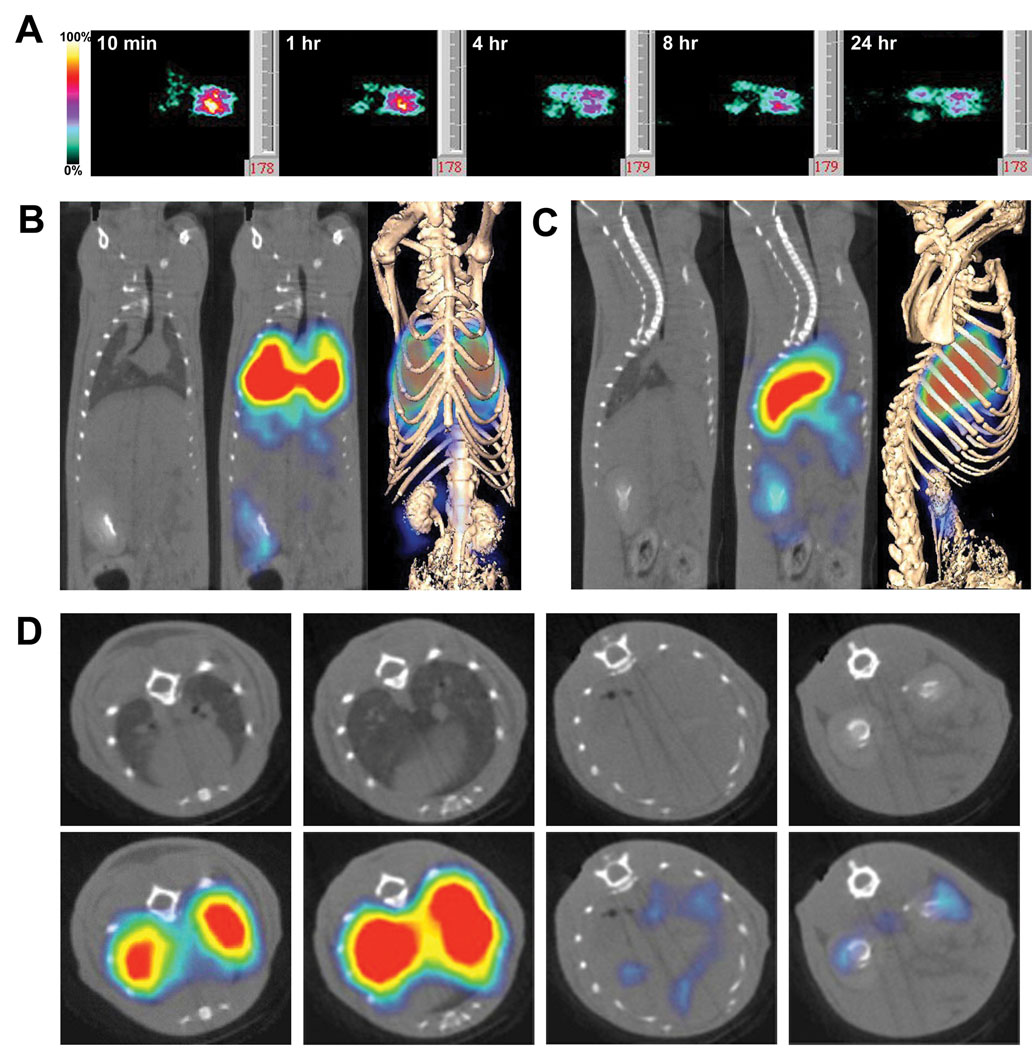
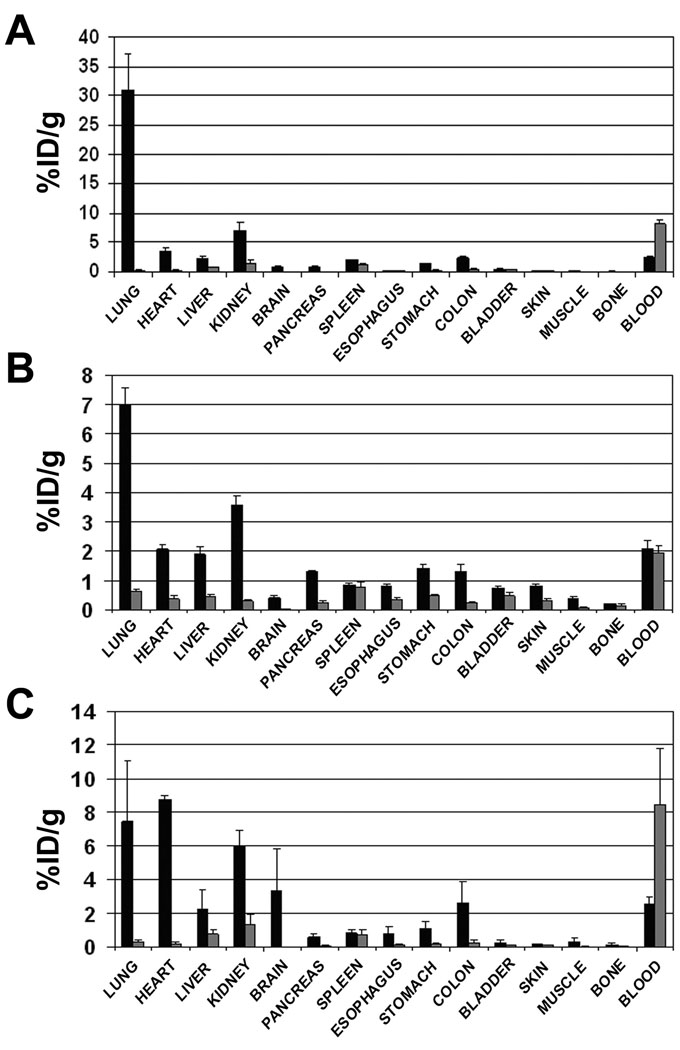
Western blot analysis and immunohistochemistry showed that podxl is expressed in every vascular bed examined, but the accessibility of the antigen to binding by G278 via the blood stream could not be determined by these analyses. Planar gamma-scintigraphy was used on rats following tail vein injection of 125I-G278 in order to assess podxl targeting in various organs. Images were acquired at 10 minutes, one, four, eight and 24hr post-injection. By 10 minutes a clear, strong lung image was evident, and some accumulation could also be observed in, kidney and liver (Figure 8A). At one hour intensities of the lung and liver images had decreased slightly and continued to decline throughout the timecourse while at the same time the signal increased somewhat in kidney. Because podxl is not expressed in caveolae (Figure 6 and Figure 7) 125I-G278, once bound to the endothelium, likely would not be internalized but instead would remain on the luminal surface and be subjected to deiodination which is known to occur in blood [17]. The redistribution of the signal from lung to kidney is likely due to the processes involved in eliminating free 125I. Consistent with this hypothesis is the appearance of a signal in thyroid seen at later time points (not shown). Clearer pictures of organ targeting were obtained with high-resolution pinhole single photon-emission computed tomography (SPECT) imaging combined with X-ray computed tomography (CT). Figures 8B, C & D show that 1 hr after tail vein injection there is a very strong signal in lung and much weaker signals in the heart, liver and kidneys. When antibody binding was quantified in a biodistribution assay the results were consistent with the SPECT images. The majority of 125I-G278, slightly more than 30% ID/g, was measured in lung 1 hr after tail vein injection, while heart, liver, kidney and other organs bound substantially less (Figure 9A). Under the same conditions little or no accumulation of a radiolabeled isotype-matched control antibody was detected in these tissues. At the 24 hr time point there was roughly a four-fold decrease in 125I-G278 found in the lung (Figure 9B). There was also a decrease in radioactivity in the heart, liver and kidney as well, but the magnitude was not as great as that seen in the lung. In these experiments the measured radioactivity in the various organs was inconsistent with the amounts of podxl seen in Western blots of lung, heart, kidney, liver and brain (Figure 4). This may be due to the fact that when reagents are inoculated via the tail vein, the first capillary bed that will be encountered is in the lungs where a large portion of the injected molecule could be removed by this highly vascularized organ. In order to allow G278 to pass once through the vasculature of other organs in the body prior to entering the lung, the radiolabeled G278 or an isotype-matched control was injected via the left ventricle and radioactivity was measured at the 1 hr time point as before. As seen in Figure 9C, bound 125I-G278 in most organs was similar to that seen in animals injected by tail vein and there was a slight decrease (15%) in kidney. However bound 125 I-G278 increased 4.6-fold in brain and 2.5-fold in heart indicating that the podxl expressed in these vascular beds is accessible and will bind G278. The majority of the isotype-matched control antibody remained in circulation and there was little or no accumulation of this antibody in the other tissues.
Figure 8.
In vivo imaging of organ immunotargeting. Rats were injected via the tail vein with 125I-G278 (10 µg at 10 µCi/ µg). Panel A: Planar γ-scintigraphic images were acquired at 10 min and at 1, 4, 8, and 24 hr. At 10 min the antibody has accumulated primarily in the lungs, but signals can also be seen in the areas of the liver and kidneys. Panels B, C & D: SPECT/CT imaging of G278 targeting showing coronal (B), saggital (C) and axial slices (D). The far right images in Panels B & C show the fusion of volumetric SPECT texture with the CT isosurface. Axial slices in D were taken at the level of the heart and lungs (far left), the lungs (second from left), the liver (third from left) and the kidneys (far right). Because of the high signal in lungs, the threshold is too low to see bound radiolabel in the heart.
Figure 9.
Biodistribution analysis of G278. 125I-G278 (black bars) or a radiolabeled isotypematched control antibody (gray bars) was injected into rats via the tail vein (Panels A & B) or via the left ventricle (Panel C). Tissues and blood samples were harvested at 1 hr (Panels A & C) or 24 hr (Panel B), weighed and gamma radioactivity was measured. Results are expressed as percent injected dose per gram of tissue (%ID/g).
podxl expression can be modulated in the tumor microenvironment
Tumor blood vessels differ from their normal counterparts in a number of parameters including size, shape and permeablilty. Using a subtractive proteomics approach, we have previously identified alterations in the protein profiles of endothelia isolated from normal rat lungs and from tumor-bearing lungs [5]. We wished to determine if podxl expression was affected by the tumor microenvironment using the same 13762 rat mammary carcinoma model as well as the FRcJ-4 rat sarcoma model [18]. When sections of lungs bearing FRcJ-4 nodules were immunostained with G278, podxl expression was seen on vessels in the normal portions of the lung and on the endothelium throughout the tumor mass itself (Figure 10A). Likewise examination of 13762 tumor lungs showed podxl expression on vessels in the normal lung tissue and in the outer margins of the tumor nodule. However, in the deeper portions of the tumor podxl expression was diminished or absent (Fig 10B). Microscopic examination of these deeper vessel-like structures showed that they were composed of cells which were similar to and continuous with podxl-positive EC (Figure 10B, arrowheads). Western blot analysis of P isolated from normal and 13762 tumor-bearing lungs showed that podxl expression was comparable in the two samples (Fig 10C). These data indicate that podxl expression in the normal areas of tumor-bearing lungs is not affected by the presence of the tumors and that the tumor microenvironment can influence podxl expression but varies from tumor to tumor. Loss of an EC marker in the tumor microenvironment was not limited to podocalyxin. Figure 10 shows that PECAM/CD31 was detectable in blood vessels at the perimeter of the tumor (Panel D) and in the adjacent normal lung tissue (Panel E) but was absent from vessel-like structures within the tumor mass (Panel D). We also used dual immunofluorescence to evaluate the ability of mAb G278 to distinguish blood vascular EC from lymphatic EC within the tumor. Figure 10F shows a section through the outer margin of a tumor nodule which there is no colocalization of mAb G278 (red) with anti-podoplanin (green) indicating that tumor lymphatics, like those in normal tissue (see Figure 2), are not recognized by mAb G278.
Discussion
Podocalyxin, along with CD34 and endoglycan, belong to the CD34 family of sialomucins which are similar in structure and genomic organization (for a review see [19]). In the present study we examined the distribution of podxl on rat endothelia in normal and tumor tissues using a monoclonal antibody, mAb G278, developed in our laboratory. G278 binds to the protein core of this heavily sialylated and O-glycosylated member of the sialomucin protein family and, as such, it is likely able to detect all forms of rat podxl irrespective of the nature and extent of post-translational modification. This feature was exploited in the present study to determine the expression levels of podxl in different vascular beds. Western blot analysis of luminal plasma membranes islolated from rat lung, heart, brain, liver and kidney showed that podxl is most highly expressed on the EC in lung and heart. The epitope recognized by G278 is exposed on the luminal EC surface which allowed us to image various organs and to determine the biodistribution of radiolabled antibody delivered into the blood stream. When inoculated via the tail vein the antibody was delivered to the “first-pass” organ, namely the lungs, and bound primarily in that vascular bed. However, delivery via the left ventricle allowed the antibody to reach other organs prior to entering the lungs which altered the biodistribution profile of 125I-G278. Using both multi-organ Western blot analysis and biodistribution we have shown that the podxl protein is most highly expressed in rat lung and heart.
Podxl was first identified as the major sialoprotein of glomerular podocytes [20] and was subsequently shown to be expressed on rat endothelia by indirect imunofluorsecence [20–22]. We have expanded on the latter studies and have examined podxl expression in a number of rat organs by immunohistochemistry. G278 is capable of immunostaining fresh frozen and fixed frozen tissues as well as formalin fixed paraffin-embedded (FFPE) tissues. FFPE sections offer the advantage of preserving the histological architecture of the organ allowing the investigator to better appreciate the relationship of stained cells with surrounding cells and stroma. Indeed, using dual immunofluorescence we were able to resolve the difference in location of podxl on the luminal EC plasma membrane and caveolin-1 on the cytoplasmic face of the membrane. Western blotting of caveolae isolated from rat lung P also demonstrated that little or no podxl is found in these organelles. These results are consistent with the immuno-electron microscopy of Horvat, et al [21] which showed no vesicle staining in lung EC with a rabbit polyclonal antibody.
Lin et al. [23] demonstrated that podxl is universally expressed by EC in the dog. These authors, however, also reported podxl expression in a number of extra-vascular locations (heart muscle fibers, skeletal muscle, neuronal cells in the cerebrum, etc) which were not observed in G278- stained rat tissues. These differences may reflect variations in podxl distribution in the two species. The only extra-endothelial staining that we observed in rat tissues was in cells known to express podxl, namely the podocytes [20, 22, 24], the thin segment of the Loop of Henle and mesothelium [23], and megakaryocytes [14]. In addition, we found occasional staining on the epithelial cells lining the interlobular ducts of the pancreas and on some transitional epithelial cells in the bladder. The presence of podxl in pancreatic ductal epithelial cells is of interest in light of the report by Ney, et al [25] that identified podxl as useful marker to differentiate pancreatic ductal adenocarcinomas from other tumor types. They found that 44% of the ductal tumors expressed podxl. Our observation that podxl is expressed on some epithelial cells in the interlobular but not the intralobular ducts raises the question as to whether this protein might serve as a suitable marker to further define the origins of subsets of pancreatic ductal adenocarcinomas. Lin, et al [23] did not report on podxl expression in lymph vessels of the dog, but in the present study we found that in the rat mAb G278 did not cross react with lymphatic EC. Our results show that G278 can clearly discriminate between blood and lymphatic EC in both normal and tumor tissues.
MAb G278 works exceptionally well to immunostain rat blood vessels of all types and all sizes and consistently outperformed antibodies to the more commonly used pan-endothelial markers CD34 (Testa, et al, submitted) and PECAM/CD31 (Figure 10D and E). Commercially available anti-PECAM pAbs which cross-react with rat PECAM are produced by immunization with the human protein, which shares only a 60% identity with the rat homolog. Poor immunostaining with these antibodies may be the result of less than optimal reactivity with rat PECAM. In addition, brief (5 min) formalin fixation of cryosections eliminated reactivity of the commercial anti-PECAM antibodies with rat EC but did not affect immunostaining of human EC (not shown). Formalin fixation did not eliminate rat EC immunostaining by mAb G278. We have also compared mAb G278 and anti-PECAM pAbs with respect to immunoisolation of rat endothelial cells and found that mAb G278 was far superior in this function as well.
In this study we have demonstrated that podxl is expressed on the normal vasculature of adult rat organs as well as in the tumor microenvironment where angiogenic processes are ongoing. This latter observation is consistent with previous reports of podxl expression in embryoinc and extra-embryonic vessels during development [26, 27]. Interestingly, we have observed that rat EC will lose podxl expression with continued in vitro cultivation [3]. The only other situation in which we have seen an absence of podxl is in the apparent vasculature in the interior of metastatic mammary tumor nodules growing in lung. The inner vessels in these tumors also lacked PECAM/CD31 expression. Heukamp, et al [28] also observed that podxl and another EC marker, CD34, were absent from the microvasculature of dysplastic hepatic nodules. Modulation of podxl and other EC proteins in the tumor vasculature is not surprising. Studies from our laboratory [5] and others [29–32], using a variety of techniques, have compared normal and tumor EC in an effort to identify tumor vascular markers. These reports have demonstrated a profound effect on the protein profiles of EC within the tumor microenvironment. Although podxl was absent in the deeper portions of 13762 tumors, the loss was not detectable by Western blot analysis of P from normal and tumor lung. This may be due to a slight upregulation of podxl expression in the tumor margin and/or the adjacent normal tissue which would not have been evident by immunohistochemistry. Also of note was the fact that the molecular weights of podxl in tumor and normal lung P were the same. The molecular weight of rat podxl can vary depending in the cellular source (see for example [14]), and in human tumor cells the molecular weight of podxl can be substantially larger than that of normal tissue [33]. The observed differences in molecular weights are likely due to alterations in the extensive postranslational modifications that constitute the majority of the protein’s mass. In the present study we observed no difference in the molecular weights of the podxl bands in P from normal or tumor lung. Tumor EC, although influenced by the tumor microenvironment, are not transformed cells and retain many features of normal EC. Thus modification of podxl in tumor and normal EC would be similar resulting in proteins of the same molecular weights. The mechanism(s) which underly the loss of podxl expression is unknown and although its implications have not been elucidated we have observed that in 13762 tumors, where podxl expression is lost, the core of the nodule is almost always necrotic while in the FRcJ-4 tumors necrotic centers were not often seen. It has been suggested that the charge repulsion effects supported by this strongly anionic molecule may help to maintain open vascular lumens and glomerular filtration slits [14, 34], however the cause-and-effect relationship between podxl expression and tumor vessel patency remains to be determined.
Podxl has been assigned both adhesive and anti-adhessive functions. In podxl knockout mice the podocytes form tight and adherens junctions rather that the normal foot processes and slit diaphragms and the pups die within 24 hours of birth with anuria [35]. Studies have also shown that podocalyxin acts as a ligand for L-selectin on high endothelial venules [36] and enhances cell adhesion to platelets [37]. Conversely, several transfection studies have suggested that podxl acts to disrupt the formation of cell-cell junctions [38, 39] and leads to increased tumor cell migration and invasion in vitro [40]. Podxl expression has also been associated with more aggressive forms of prostate [41] and breast cancer [38] and is a commonly expressed marker of blasts in acute leukemia [42].
MAb G278 is a robust antibody that reliably binds to podocalyxin in a variety of biochemical, immunological and in vivo applications. We believe G278 to be an invaluable tool not only as a means of immunostaining rat blood vessels but as reagent with which to elucidate the functions of podocalyxin.
Acknowledgements
This work was supported by grants (to JES) from the National Cancer Institute (PO1CA104898, RO1CA83989 and R24CA095893) and National Heart Lung and Blood Institute (RO1-HL074063, RO1-HL052766 and RO1-HL058216). We thank Kally Pascua, Alexina Wempren, Melinda Schnitzer and Michelle Pacheco for their excellent technical assistance.
References
- 1.Schnitzer JE. Update on the cellular and molecular basis of capillary permeability. Trends in Cardiovasc Med. 1993;3:124–130. doi: 10.1016/1050-1738(93)90012-U. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nature biotechnology. 2004;22:985–992. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: A pathway to overcome cell barriers to drug and gene delivery. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 6.Valadon P, Garnett JD, Testa JE, Bauerle M, Oh P, Schnitzer JE. Screening phage display libraries for organ-specific vascular immunotargeting in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:407–412. doi: 10.1073/pnas.0506938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh P, Schnitzer JE. Cell biology: A laboratory handbook. Academic Press; 1998. Immunoisolation and subfractionation of plasma membranes to purify caveolae separately from glycosyl-phosphatidylinositol-anchored protein microdomains.; in Celis J; pp. 34–46. [Google Scholar]
- 8.Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Science. Vol. 269. New York, NY: 1995. Separation of caveolae from associated microdomains of gpi-anchored proteins; pp. 1435–1439. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol. 2003;285:H1720–H1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 10.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including vamp, nsf, snap, annexins, and gtpases. The Journal of biological chemistry. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 11.Schnitzer JE, Oh P. Aquaporin-1 in plasma membrane and caveolae provides mercurysensitive water channels across lung endothelium. Am J Physiol. 1996;270:H416–H422. doi: 10.1152/ajpheart.1996.270.1.H416. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 13.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nature biotechnology. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen A, Solin ML, Reivinen J, Juvonen E, Vaisanen R, Holthofer H. Podocalyxin in rat platelets and megakaryocytes. The American journal of pathology. 1999;154:813–822. doi: 10.1016/S0002-9440(10)65328-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by gtp-driven fission from the plasma membrane of endothelium. The Journal of cell biology. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnitzer JE, Oh P, McIntosh DP. Science. Vol. 274. New York, NY: 1996. Role of gtp hydrolysis in fission of caveolae directly from plasma membranes; pp. 239–242. [DOI] [PubMed] [Google Scholar]
- 17.Russell J, O'Donoghue JA, Finn R, Koziorowski J, Ruan S, Humm JL, Ling CC. Iodination of annexin v for imaging apoptosis. J Nucl Med. 2002;43:671–677. [PubMed] [Google Scholar]
- 18.Kraemer M, Tournaire R, Dejong V, Montreau N, Briane D, Derbin C, Binetruy B. Rat embryo fibroblasts transformed by c-jun display highly metastatic and angiogenic activities in vivo and deregulate gene expression of both angiogenic and antiangiogenic factors. Cell Growth Differ. 1999;10:193–200. [PubMed] [Google Scholar]
- 19.Furness SG, McNagny K. Beyond mere markers: Functions for cd34 family of sialomucins in hematopoiesis. Immunologic research. 2006;34:13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- 20.Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. The Journal of cell biology. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D. Endothelial cell membranes contain podocalyxin--the major sialoprotein of visceral glomerular epithelial cells. The Journal of cell biology. 1986;102:484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miettinen A, Dekan G, Farquhar MG. Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. The American journal of pathology. 1990;137:929–944. [PMC free article] [PubMed] [Google Scholar]
- 23.Lin WL, Pang VF, Liu CH, Chen JY, Shen KF, Lin YY, Yu CY, Hsu YH, Jou TS. Pleomorphic extra-renal manifestation of the glomerular podocyte marker podocalyxin in tissues of normal beagle dogs. Histochemistry and cell biology. 2007;127:399–414. doi: 10.1007/s00418-006-0252-8. [DOI] [PubMed] [Google Scholar]
- 24.Dekan G, Miettinen A, Schnabel E, Farquhar MG. Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection. Localization of antigens and bound iggs by immunoelectron microscopy. The American journal of pathology. 1990;137:913–927. [PMC free article] [PubMed] [Google Scholar]
- 25.Ney JT, Zhou H, Sipos B, Buttner R, Chen X, Kloppel G, Gutgemann I. Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Human pathology. 2007;38:359–364. doi: 10.1016/j.humpath.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Doyonnas R, Nielsen JS, Chelliah S, Drew E, Hara T, Miyajima A, McNagny KM. Podocalyxin is a cd34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood. 2005;105:4170–4178. doi: 10.1182/blood-2004-10-4077. [DOI] [PubMed] [Google Scholar]
- 27.McNagny KM, Pettersson I, Rossi F, Flamme I, Shevchenko A, Mann M, Graf T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. The Journal of cell biology. 1997;138:1395–1407. doi: 10.1083/jcb.138.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heukamp LC, Fischer HP, Schirmacher P, Chen X, Breuhahn K, Nicolay C, Buttner R, Gutgemann I. Podocalyxin-like protein 1 expression in primary hepatic tumours and tumour-like lesions. Histopathology. 2006;49:242–247. doi: 10.1111/j.1365-2559.2006.02489.x. [DOI] [PubMed] [Google Scholar]
- 29.Herbert JM, Stekel D, Sanderson S, Heath VL, Bicknell R. A novel method of differential gene expression analysis using multiple cdna libraries applied to the identification of tumour endothelial genes. BMC genomics. 2008;9:153. doi: 10.1186/1471-2164-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer research. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 31.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain a of fibronectin is a vascular marker of solid tumors and metastases. Cancer research. 2007;67:10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 32.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Science. Vol. 289. New York, NY: 2000. Genes expressed in human tumor endothelium; pp. 1197–1202. [DOI] [PubMed] [Google Scholar]
- 33.Schopperle WM, Kershaw DB, DeWolf WC. Human embryonal carcinoma tumor antigen, gp200/gctm-2, is podocalyxin. Biochemical and biophysical research communications. 2003;300:285–290. doi: 10.1016/s0006-291x(02)02844-9. [DOI] [PubMed] [Google Scholar]
- 34.Dekan G, Gabel C, Farquhar MG. Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:5398–5402. doi: 10.1073/pnas.88.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM. Anuria, omphalocele, and perinatal lethality in mice lacking the cd34-related protein podocalyxin. The Journal of experimental medicine. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for l-selectin: Parallels to cd34. The Journal of experimental medicine. 1998;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larrucea S, Butta N, Rodriguez RB, Alonso-Martin S, Arias-Salgado EG, Ayuso MS, Parrilla R. Podocalyxin enhances the adherence of cells to platelets. Cell Mol Life Sci. 2007 doi: 10.1007/s00018-007-7374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Somasiri A, Nielsen JS, Makretsov N, McCoy ML, Prentice L, Gilks CB, Chia SK, Gelmon KA, Kershaw DB, Huntsman DG, McNagny KM, Roskelley CD. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer research. 2004;64:5068–5073. doi: 10.1158/0008-5472.CAN-04-0240. [DOI] [PubMed] [Google Scholar]
- 39.Takeda T, Go WY, Orlando RA, Farquhar MG. Expression of podocalyxin inhibits cellcell adhesion and modifies junctional properties in madin-darby canine kidney cells. Molecular biology of the cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sizemore S, Cicek M, Sizemore N, Ng KP, Casey G. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer research. 2007;67:6183–6191. doi: 10.1158/0008-5472.CAN-06-3575. [DOI] [PubMed] [Google Scholar]
- 41.Casey G, Neville PJ, Liu X, Plummer SJ, Cicek MS, Krumroy LM, Curran AP, McGreevy MR, Catalona WJ, Klein EA, Witte JS. Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Human molecular genetics. 2006;15:735–741. doi: 10.1093/hmg/ddi487. [DOI] [PubMed] [Google Scholar]
- 42.Kelley TW, Huntsman D, McNagny KM, Roskelley CD, Hsi ED. Podocalyxin: A marker of blasts in acute leukemia. American journal of clinical pathology. 2005;124:134–142. doi: 10.1309/7BHLAHHU0N4MHT7Q. [DOI] [PubMed] [Google Scholar]