Abstract
Background and Objectives: Peritoneal dialysis (PD) is a common maintenance renal replacement modality for children with ESRD frequently compromised by infectious peritonitis and catheter exit site and tunnel infections (ESI/TI). The effect of topical mupirocin (Mup) and sodium hypochlorite (NaOCl) solution was evaluated as part of routine daily exit site care on peritonitis and ESI/TI rates, causative microorganisms, and catheter survival rates.
Design, setting, participants, & measurements: Retrospective chart review of children on home continuous cycling PD between April 1, 2001 and June 30, 2007 was performed. Infection rates were examined based on exit site protocol used in two different periods: Mup alone, April 1, 2001 to November 17, 2004; and Mup and NaOCl (Mup+NaOCl), November 18, 2004 to June 30, 2007.
Results: Eighty-three patients (mean PD initiation age: 12.1 ± 5.8 yr) received home PD over 2009 patient months. Annualized rates (ARs) for peritonitis decreased from 1.2 in the Mup period to 0.26 in the Mup+NaOCl period (P < 0.0001). ARs for ESI/TI decreased from 1.36 in the Mup period to 0.33 in the Mup+NaOCl period (P < 0.0001). No infections with Mup-resistant organisms were observed when either Mup or Mup+NaOCl was used for prophylaxis. Gram-negative-organism associated peritonitis decreased from an AR of 0.31 in the Mup period to 0.07 in the Mup+NaOCl period (P < 0.001). Infection-related catheter removal rates decreased from 1 in 38.9 catheter-months in the Mup period to 1 in 94.2 in the Mup+NaOCl period (P = 0.01). Catheter survival rates were longer in the Mup+NaOCl period (Kaplan–Meier, P < 0.009).
Conclusions: The combination Mup+NaOCl in daily exit site care was very effective to reduce PD catheter-associated infections and prolong catheter survival in pediatric patients.
Peritoneal dialysis (PD) is a common modality for chronic renal replacement therapy in children with ESRD. In the United States, nearly half of incident and prevalent dialysis patients less than 19 yr old are treated with PD (1). However, the longevity and success of PD is frequently compromised by the infectious complications of peritonitis and PD catheter exit site and tunnel infections (ESI/TI). Peritonitis is a major cause of hospitalization, catheter loss, and transfer to hemodialysis. ESI/TI, which occurs in approximately one third of pediatric patients after 1 yr on PD, is a risk factor for the development of peritonitis and imposes a 2-fold risk of access revision and an almost 3-fold risk of hospitalization for access complications (2). Techniques to reduce or prevent ESI/TI and peritonitis are essential for the success of long-term chronic PD.
A major cause of ESI/TI and catheter-related peritonitis is gram-positive organisms, especially Staphylococcus aureus (3–7). Mupirocin (GlaxoSmithKline, Brentford, Middlesex, United Kingdom) is an antibiotic that binds to isoleucyl t-RNA synthetase, thereby inhibiting bacterial protein synthesis in gram-positive organisms (8). At the high concentrations achieved through topical application, mupirocin demonstrates bactericidal activity and has excellent activity against S. aureus with a minimal inhibitory concentration of <1 mg/L(9). Studies in adult PD patients show topical mupirocin application as part of routine exit site care reduces the rate of ESI/TI and peritonitis, particularly S. aureus-related infections (10–15). One pediatric PD study demonstrated no episodes of S. aureus-related infection after initiation of topical daily mupirocin with 3 yr of follow-up (16).
From April 2001 through October 2004, our pediatric dialysis center's routine exit site care protocol consisted of the application of topical mupirocin after daily cleansing with soap and water. In November 2004, sodium hypochlorite solution (ExSept Plus, Alcavis, Gaithersburg, MD) was added to the exit site care protocol. Sodium hypochlorite 0.114% (NaOCl) is an antiseptic solution that has broad-spectrum antimicrobial activity against gram-positive, gram-negative, atypical mycobacteria, and fungal organisms (Alcavis information sheet). Wadhwa and Reddy showed in adult patients that NaOCl is useful as a PD catheter exit site disinfectant to keep the resident microorganisms inhibited (17). In the current study, we assessed whether topical mupirocin alone and mupirocin in combination with NaOCl as part of routine daily exit site care were useful in pediatric dialysis patients.
Materials and Methods
We performed a retrospective chart review for all patients receiving maintenance home continuous cycling peritoneal dialysis (CCPD), who were followed in the Dialysis Unit at Texas Children's Hospital (Houston, TX) from April 1, 2001 through June 30, 2007. Patients were excluded if they received PD for less than a 3-mo period or if PD was initiated at another institution. The Baylor College of Medicine Institutional Review Board, with waiver of informed consent, approved the study protocol.
All PD catheters were placed by one of four pediatric surgeons at Texas Children's Hospital following International Society of Peritoneal Dialysis guidelines (18), which include downward-facing exit site, perioperative antibiotic prophylaxis, avoidance of sutures around the exit site, and immobilization of exit site to prevent trauma. The Dialysis Unit protocol for initial PD catheter care included immobilization of the PD catheter for 1 wk with subsequent dressing change and weekly saline flush starting at the end of the first week. The PD catheter was not used for at least 14 d.
The following patient demographic information was collected: age and date at PD initiation, gender, ethnicity, and cause of ESRD.
Two time periods based on exit-site care protocol practices were defined as follows:
(1) Mupirocin (Mup): April 1, 2001 through November 17, 2004. Daily exit site care was performed with soap and water. Mup was applied to the catheter exit site after the exit site was allowed to dry.
(2) Mup + NaOCl (Mup+NaOCl): November 18, 2004 through June 30, 2007. Daily exit site care was performed with soap and water, which was allowed to dry, followed by application of NaOCl spray for 1 min. After 1 min, the exit site was dried thoroughly with sterile gauze, followed by topical Mup application.
Each new exit site care protocol was prescribed for all prevalent patients at the beginning of, and new incident patients after the beginning of, each time period. As a result, some patients contributed data in both time periods.
Data for infection-related complications (ESI/TI, peritonitis), causative microorganisms, date/cause of catheter removal, and reason for termination of CCPD were obtained for further analysis.
Definitions (based on International Society of Peritoneal Dialysis Guidelines (18))
ESI.
Infection characterized by the presence of purulent drainage with or without erythema at the catheter exit site, with or without a positive culture.
TI.
Infection characterized by pain, swelling, and erythema over the subcutaneous catheter tunnel with or without a positive culture.
Peritonitis.
Infection characterized by cloudy effluent with white blood cell count >100/mm with >50% comprised of polymorphonuclear neutrophils. An effluent culture was always obtained, but positive culture was not required.
Statistical Analyses
Potential differences in PD-associated infection rates among time periods for all organisms and for type of organism were evaluated by χ2 analyses. For the purpose of this analysis, episodes of ESI and TI were combined. Differences in continuous variables between two time periods were assessed by t test analysis. Rates of catheter removal because of infection were compared in the Mup versus Mup+NaOCl using χ2 analyses. Differences in catheter survival between Mup versus Mup+NaOCl periods were assessed by Kaplan–Meier analysis. Catheter survival was censored for removal for noninfectious reasons, including termination of PD due to death, transfer to hemodialysis, transfer to another program, renal transplantation, catheter malfunction, or end of time period/end of study. Catheter survival between the Mup and Mup+NaOCl periods was examined in two ways. In the first analysis, catheter survival was assessed for all patients, irrespective of overlap from the Mup to the Mup+NaOCl period. For patients who overlapped from one time period to the next, catheter removal was censored for the end of the time period. When added to the next time period, the date of catheter placement was changed to correspond with the start of the Mup+NaOCl period, to represent the exposure time to the new exit site care protocol. To eliminate any potential bias, the second analysis excluded those patients who overlapped and included only patients who initiated dialysis in that particular period.
All analyses were performed using STATISTICA Software (StatSoft, Inc., Tulsa, OK). A P value <0.05 was considered significant for each analysis.
Results
Patient Demographics
Data were abstracted from charts of a cohort of 83 patients (44.6% male) comprising 2009 patient-months (pt-mo) of CCPD. Mean patient age at CCPD initiation was 12.1 ± 5.8 yr (age range 7 d to 22.6 yr). Fifty-six patients were treated in the Mup period, and 56 patients in the Mup+NaOCl period. Twenty-nine patients overlapped from the Mup period into the Mup+NaOCl period. Mean patient ages (±SD) for each time period were Mup = 11.2 ± 6 yr, Mup+NaOCl = 12.8 ± 5.4 yr (P = 0.14).
The causes of ESRD were similar to those reported for pediatric patients in the United States (1) (Table 1). Fourteen of 83 patients (16.9%) patients had a failed transplant and returned to dialysis (four with focal segmental glomerulosclerosis, two with renal dysplasia, two with other GN, two with obstructive uropathy, one with hereditary disease, and three with unknown etiology).
Table 1.
Cause of ESRD
| Cause of ESRD | Number of Cases | Percent of Patients |
|---|---|---|
| Renal dysplasia | 16 | 19.3 |
| Focal segmental glomerulosclerosis | 16 | 19.3 |
| Other GN | 16 | 19.3 |
| Hereditary diseases | 11 | 13.3 |
| Obstructive uropathy | 6 | 7.2 |
| Reflux nephropathy | 4 | 4.8 |
| Unknown | 5 | 6 |
| Other | 9 | 10.8 |
Infection Rates
ESI/TI.
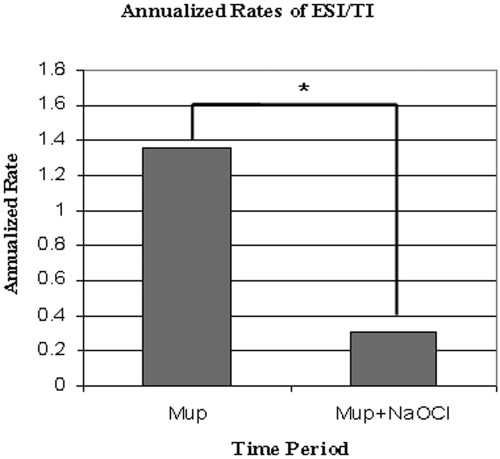
A comparison of the annualized rates (ARs) of ESI/TI in each of the two time periods is depicted in Figure 1. In the Mup period, 76 ESI/TI occurred in 668.8 pt-mo (AR 1.36). In the Mup+NaOCl period, ESI/TI rates decreased significantly to 39 infections in 1403.6 pt-mo (AR 0.33, P < 0.0001).
Figure 1.
AR (infection per patient-year) of ESI/TI. *Mup versus Mup+NaOCl, P < 0.0001.
Peritonitis.
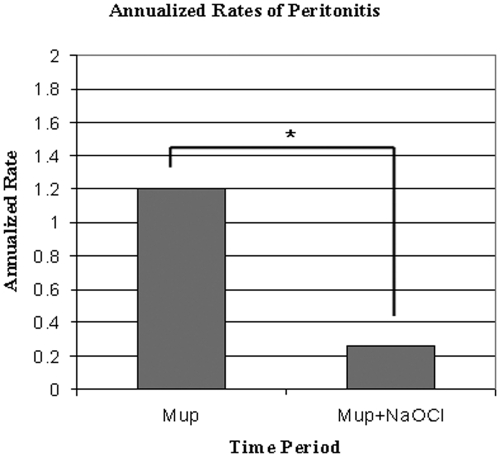
In the Mup period, 68 peritonitis episodes occurred in 668.8 pt-mo (AR 1.2). The peritonitis rate decreased significantly in the Mup+NaOCl period to 30 infections in 1403.6 pt-mo (AR 0.26, P < 0.0001) as shown in Figure 2.
Figure 2.
AR (infection per patient-year) of peritonitis. *Mup versus Mup+NaOCl, P < 0.0001.
Causative Organisms.
Of the 76 ESI/TI in the Mup period, 14 episodes were due to S. aureus (AR 0.25) and 27 episodes were due to coagulase-negative staphylococcus (AR 0.48). In the Mup+NaOCl period with 39 ESI/TI, three were due to S. aureus (AR 0.03) and three were due to coagulase-negative staphylococcus (AR 0.03), which significantly decreased when compared with the Mup period (P < 0.0001).
Seventeen episodes of peritonitis caused by gram-negative organisms occurred in 668.8 pt-mo (AR 0.31) in the Mup period as compared with eight episodes in 1403.6 pt-mo (AR 0.07) in the Mup+NaOCl period (P < 0.001) (Table 2). In particular, 8 of 17 gram-negative peritonitis episodes were caused by Pseudomonas aeruginosa in the Mup period, compared with none in the Mup+NaOCl period. The AR for peritonitis caused by gram-positive organisms decreased from 0.47 in the Mup period to 0.08 in the Mup+NaOCl period (P < 0.0001). The AR for culture-negative peritonitis also decreased significantly from Mup (AR 0.27) to the Mup+NaOCl period (AR 0.09, P < 0.003). No emergence of Mup-resistant organisms was observed.
Table 2.
| Organism | Mup | Mup+NaOCl |
|---|---|---|
| Gram-negative | 17 (0.31) | 8 (0.07) |
| 8 Pseudomonas | No Pseudomonas | |
| P < 0.001 | ||
| Gram-positive | 26 (0.47) | 9 (0.08) |
| P < 0.0001 | ||
| Fungus | 5 (0.09) | 3 (0.03) |
| Culture-negative | 15 (0.27) | 10 (0.09) |
| P < 0.003 | ||
| Multiple organism | 5 (0.09) | 0 (0) |
| 1 with fungus | ||
| Total episodes | 69 | 30 |
| P < 0.0001 | ||
| Total patient years/pt-mo | 55.7/668.8 | 117/1403.6 |
Annualized rates of infection as episodes per total patient-years shown in parentheses.
Statistical analysis by χ2 for *Mup versus Mup+NaOCl.
Catheter Survival
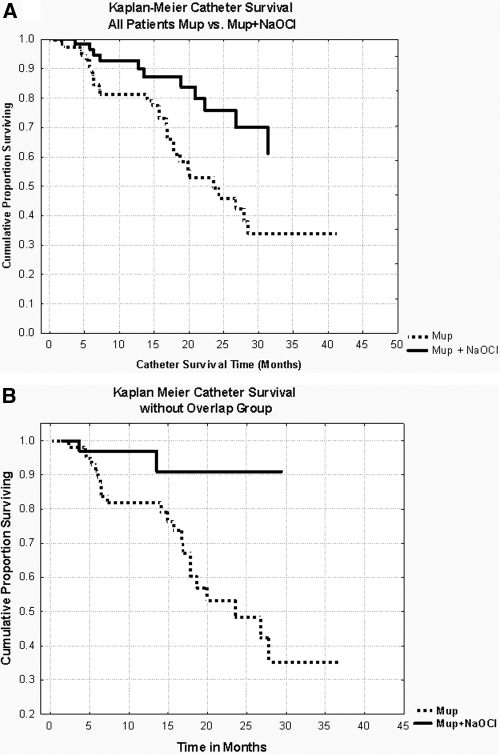
Catheter removal because of infection decreased from 1 in 38.9 catheter-months in the Mup period to 1 in 94.2 catheter-months in the Mup+NaOCl period (P = 0.01). Kaplan–Meier analysis revealed significantly longer catheter survival for all patients in the Mup+NaOCl era compared with the Mup era (P < 0.009) (Figure 3a). Catheter survival at 2 yr from dialysis initiation improved from 50% in the Mup period to 75% in the Mup+NaOCl period. Mup+NaOCl catheter survival remained significantly longer (P < 0.02), even after reducing any bias to survival by excluding the 29 patients who overlapped between the time periods (Figure 3b).
Figure 3.
Kaplan–Meier analysis of PD catheter survival in Mup versus Mup+NaOCl periods, censored for removal for noninfectious reasons. (A) All patients, P < 0.009. Catheter survival at 2 yr from dialysis initiation improved from 50% in Mup to 75% in Mup+NaOCl period. (B) Exclusion of patients who overlapped into the next time period, P < 0.02.
Discussion
The study presented here is the first to evaluate the effects of different PD catheter exit site care protocols (Mup versus Mup+NaOCl) on the rates of ESI/TI and peritonitis in pediatric dialysis patients. Before the use of topical Mup for PD exit site care, the AR of peritonitis at our institution was 1.7, with 76% of infections due to gram-positive organisms (unpublished data). Multiple studies in adults have been published regarding the efficacy of topical Mup for exit site care, reporting a 33% to 100% reduction in catheter-related infections due to S. aureus (10,12–15). Consistent with these findings, the rate of peritonitis due to gram-positive organisms in the Mup period decreased by 64% when compared with our center's peritonitis rate before the initiation of Mup. However, despite the initiation of Mup, the rates of ESI/TI (AR 1.36) and peritonitis (AR 1.2) were higher in our study than compared with reported data from other institutions that used Mup topical prophylaxis, which report AR between 0.16 to 0.77 for ESI/TI and AR between 0.09 to 0.48 for peritonitis (11–16). Given the continued high rate of ESI/TI and peritonitis, NaOCl was added to the exit site care protocol in November 2004 in attempts to further decrease the rates of catheter-related infections.
After NaOCl was added to our exit site care protocol, we observed a further 83% reduction in gram-positive peritonitis rates as well as a 77% reduction in gram-negative and a 67% reduction in culture-negative peritonitis rates when compared with the Mup period (94% reduction in gram-positive peritonitis rates when compared with time period before Mup initiation). Furthermore, addition of NaOCl to Mup led to a very significant decrease in ESI/TI rates (P < 0.0001). Finally, catheter removal from infectious causes also was reduced significantly, as evidenced by prolonged catheter survival in patients receiving Mup+NaOCl versus Mup in all patients (P < 0.009), an improvement that persisted even after excluding patients who overlapped between the groups (P < 0.02).
Although studies have reported the development of Mup-resistant S. aureus or other gram-positive organisms (19,20), we did not have any catheter-related infections due to Mup-resistant gram-positive organisms. However, consistent with findings and concerns regarding emergence of gram-negative infections (particularly infections caused by Pseudomonas spp) with the use of daily topical Mup (21,22), we experienced a high number of peritonitis episodes due to Pseudomonas in the Mup period. Notably, after addition of NaOCl to Mup, we observed no Pseudomonas-associated peritonitis episodes as well as a decrease in rates of fungal and polymicrobial peritonitis, suggesting NaOCl has effective antimicrobial activity against Pseudomonas and effective antifungal activity.
Limitations of our study include the retrospective design, the relatively small patient numbers compared with adult studies, and the number of patients who overlapped the two treatment periods. Although the patient population in our study was relatively small, it included 3.1% to 4% of the prevalent United States pediatric PD population per year who were patients at our pediatric dialysis center (1). An additional concern may be that patients treated in the Mup+NaOCl period had a slightly higher, although not statistically significant, mean age of 12.8 ± 5.4 yr (versus 11.2 ± 6 yr for Mup, P = 0.14), and previous studies have revealed that younger patients are more likely to develop peritonitis (3,23–26). The dramatic decrease in peritonitis rates during the Mup+NaOCl period appeared to be much more than accounted for by the small difference in mean age.
The results of our study lead us to conclude that the combination of daily Mup+NaOCl for PD catheter exit site care appears to be more effective for infection prophylaxis than Mup alone. Whether decreased infection rates resulted from the combination Mup+NaOCl or could have been achieved with NaOCl alone could not be determined by our study. Additional prospective studies are needed to confirm our findings and to further evaluate the utility of NaOCl as an effective cleansing and infection prophylaxis solution for daily PD exit site care.
Disclosures
None.
Acknowledgments
A portion of these data were presented in abstract form at the 28th Annual Dialysis Conference in Orlando, Florida on March 1, 2008.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Li S, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis 49[Suppl 2]: S1–S296, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Furth SL, Donaldson LA, Sullivan EK, Watkins SL: Peritoneal dialysis catheter infections and peritonitis in children: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 15: 179–182, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Honda M: The 1997 Report of the Japanese National Registry data on pediatric peritoneal dialysis patients. Perit Dial Int 19[Suppl 2]: S473–S478, 1999 [PubMed] [Google Scholar]
- 4.Yinnon AM, Gabay D, Raveh D, Schlesinger Y, Slotki I, Attias D, Rudensky B: Comparison of peritoneal fluid culture results from adults and children undergoing CAPD. Perit Dial Int 19: 51–55, 1999 [PubMed] [Google Scholar]
- 5.Lerner GR, Warady BA, Sullivan EK, Alexander SR: Chronic dialysis in children and adolescents. The 1996 annual report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 13: 404–417, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Piraino B: Staphylococcus aureus infections in dialysis patients: Focus on prevention. ASAIO J 46: S13–S17, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hoshii S, Wada N, Honda M: A survey of peritonitis and exit-site and/or tunnel infections in Japanese children on PD. Pediatr Nephrol 21: 828–834, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chain EB, Mellows G: Pseudomonic acid. Part 1. The structure of pseudomonic acid A, a novel antibiotic produced by Pseudomonas fluorescens. J Chem Soc [Perkin 1]: 294–309, 1977 [PubMed] [Google Scholar]
- 9.Hudson IR: The efficacy of intranasal mupirocin in the prevention of staphylococcal infections: A review of recent experience. J Hosp Infect 27: 81–98, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Bernardini J, Piraino B, Holley J, Johnston JR, Lutes R: A randomized trial of Staphylococcus aureus prophylaxis in peritoneal dialysis patients: Mupirocin calcium ointment 2% applied to the exit site versus cyclic oral rifampin. Am J Kidney Dis 27: 695–700, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Lim CT, Wong KS, Foo MW: The impact of topical mupirocin on peritoneal dialysis infection in Singapore General Hospital. Nephrol Dial Transplant 20: 2202–2206, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Mahajan S, Tiwari SC, Kalra V, Bhowmik DM, Agarwal SK, Dash SC, Kumar P: Effect of local mupirocin application on exit-site infection and peritonitis in an Indian peritoneal dialysis population. Perit Dial Int 25: 473–477, 2005 [PubMed] [Google Scholar]
- 13.Thodis E, Bhaskaran S, Pasadakis P, Bargman JM, Vas SI, Oreopoulos DG: Decrease in Staphylococcus aureus exit-site infections and peritonitis in CAPD patients by local application of mupirocin ointment at the catheter exit site. Perit Dial Int 18: 261–270, 1998 [PubMed] [Google Scholar]
- 14.Uttley L, Vardhan A, Mahajan S, Smart B, Hutchison A, Gokal R: Decrease in infections with the introduction of mupirocin cream at the peritoneal dialysis catheter exit site. J Nephrol 17: 242–245, 2004 [PubMed] [Google Scholar]
- 15.Casey M, Taylor J, Clinard P, Graham A, Mauck V, Spainhour L, Brown P, Burkart J: Application of mupirocin cream at the catheter exit site reduces exit-site infections and peritonitis in peritoneal dialysis patients. Perit Dial Int 20: 566–568, 2000 [PubMed] [Google Scholar]
- 16.Auron A, Simon S, Andrews W, Jones L, Johnson S, Musharaf G, Warady BA: Prevention of peritonitis in children receiving peritoneal dialysis. Pediatr Nephrol 22: 578–585, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Wadhwa NK, Reddy GH: Exit-site care in peritoneal dialysis. Contrib Nephrol 154: 117–124, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, Kuijper EJ, Li PK, Lye WC, Mujais S, Paterson DL, Fontan MP, Ramos A, Schaefer F, Uttley L; ISPD Ad Hoc Advisory Committee: Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 25: 107–131, 2005 [PubMed] [Google Scholar]
- 19.Annigeri R, Conly J, Vas S, Dedier H, Prakashan KP, Bargman JM, Jassal V, Oreopoulos D: Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int 21: 554–559, 2001 [PubMed] [Google Scholar]
- 20.Perez-Fontan M, Rosales M, Rodriguez-Carmona A, Falcon TG, Valdes F: Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 39: 337–341, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, Mir S, Ha IS, Fischbach M, Simkova E, Watson AR, Möller K, von Baum H, Warady BA: Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 72: 1374–1379, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, Piraino B: Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol 16: 539–545, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Warady BA, Hebert D, Sullivan EK, Alexander SR, Tejani A: Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 11: 49–64, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Honda M, Iitaka K, Kawaguchi H, Hoshii S, Akashi S, Kohsaka T, Tuzuki K, Yamaoka K, Yoshikawa N, Karashima S, Itoh Y, Hatae K: The Japanese National Registry data on pediatric CAPD patients: a ten-year experience. A report of the Study Group of Pediatric PD Conference. Perit Dial Int 16: 269–275, 1996 [PubMed] [Google Scholar]
- 25.Neu AM, Ho PL, McDonald RA, Warady BA: Chronic dialysis in children and adolescents. The 2001 NAPRTCS Annual Report. Pediatr Nephrol 17: 656–663, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Boehm M, Vecsei A, Aufricht C, Mueller T, Csaicsich D, Arbeiter K: Risk factors for peritonitis in pediatric peritoneal dialysis: a single-center study. Pediatr Nephrol 20: 1478–1483, 2005 [DOI] [PubMed] [Google Scholar]