Abstract
Background and objectives: Indoxyl sulfate and p-cresyl sulfate are important representatives of the protein-bound uremic retention solutes. Serum levels of p-cresyl sulfate and indoxyl sulfate are linked to cardiovascular outcomes and chronic kidney disease progression, respectively. They share important features such as the albumin-binding site, low dialytic clearance, and both originate from protein fermentation. Whether serum concentrations are related is, however, not known.
Design, setting, participants, & measurements: In an observational study in 75 maintenance hemodialysis patients, we studied agreement between indoxyl sulfate and p-cresyl sulfate serum concentrations, dialytic reduction rates, and dialytic clearances. Concentrations were determined by HPLC. Dialytic clearances were determined from total spent dialysate collections. In vitro spiking experiments were performed to explore protein binding characteristics.
Results: Indoxyl sulfate and p-cresyl sulfate total serum concentrations were not related (r = 0.02, P = 0.9), whereas free serum concentrations were only moderately related (r = 0.53, P < 0.001). Indoxyl sulfate and p-cresyl sulfate share the same albumin binding site, for which they are competitive binding inhibitors. Intriguingly, indoxyl sulfate and p-cresyl sulfate reduction rates (r = 0.91, P < 0.001) and dialytic clearances (r = 0.97, P < 0.001) correlated tightly.
Conclusions: Indoxyl sulfate and p-cresyl sulfate serum concentrations are not associated, suggesting different metabolic pathways. Indoxyl sulfate and p-cresyl sulfate are both valid markers to monitor behavior of protein-bound solutes during dialysis. Finally, they are competitive binding inhibitors for the same albumin binding site.
Protein-bound uremic retention solutes are implicated in the uremic syndrome and might be a missing link to explain the persistently high mortality rates in chronic kidney disease (CKD) (1–3). Efforts are mounting to reduce serum concentrations, either by reducing intestinal uptake (4) or by improving blood clearances (5,6).
Indoxyl sulfate and p-cresyl sulfate are perhaps the most widely used marker molecules to study the behavior of the protein-bound uremic retention solutes during hemodialysis, hemodiafiltration, peritoneal dialysis, and experimental adsorption-based blood purification devices (5–10). In addition, both indoxyl sulfate and p-cresyl sulfate are thought to contribute directly to uremic syndrome (1).
Indoxyl sulfate is considered a key player in the progression of CKD (11–13). A large multicenter trial is ongoing to study whether reduction of indoxyl sulfate serum concentrations will slow down CKD progression (Evaluating Prevention of Progression In Chronic Kidney Disease [EPPIC-1/2; www.clinicaltrials.gov NCT00500682/NCT00501046]). Furthermore, in vitro and animal data suggest that indoxyl sulfate contributes to CKD-associated bone mineral disease (14,15).
p-Cresyl sulfate, indirectly quantified as p-cresol, is associated with overall mortality and cardiovascular disease in hemodialysis (HD) patients (16,17). Preliminary findings suggested that p-cresyl sulfate contributes directly to endothelial dysfunction (18). Moreover, p-cresyl sulfate activates leukocyte free radical production (19), linking p-cresyl sulfate to inflammation.
Although frequently approached as individual uremic retention solutes, they share common ground. First, p-cresyl sulfate and indoxyl sulfate both originate from bacterial protein fermentation in the large intestine. Colonic microbiota degrade tryptophan to indole. Further hydroxylation results in 3-hydroxy-indole, the majority of which is sulfonated to produce indoxyl sulfate (20,21). In parallel, fermentation of tyrosine results in p-cresol. Recently, we reported on sulfate conjugation of p-cresol in CKD (8,22). Second, most p-cresyl sulfate and indoxyl sulfate circulates noncovalently bound to albumin, presumably at Sudlow site II. Ligands of this albumin binding site are aromatic and are either neutral or bear a negative charge located peripherally on the molecule (23,24). Finally, blood clearances of p-cresyl sulfate and indoxyl sulfate by dialysis are limited (9). Meyer et al. (25) elegantly elaborated a mathematical model describing the behavior of protein bound solutes during hemodialysis. In this model, clearances are directly associated with free fractions.
Although indoxyl sulfate and p-cresyl sulfate are related with respect to colonic protein fermentation, albumin binding, and theoretically, dialytic clearances, it is unclear to what extent blood concentrations are related. Tight agreement would alleviate the need to measure both. Furthermore, close agreement would provide alternative explanations for observed associations with clinical endpoints.
The aims of this study were to investigate (1) whether serum concentrations of indoxyl sulfate and p-cresyl sulfate are related, (2) to what extent their dialytic clearances are related, and (3) whether indoxyl sulfate and p-cresyl sulfate albumin binding is related in an unselected cohort of HD patients.
Materials and Methods
Study Population
Patients, treated with maintenance HD for at least 3 mo at the nephrology department of the University Hospital Gasthuisberg, Leuven, Belgium, were enrolled in this study. Eligible patients were 18 yr or older and able to give written informed consent. The study was performed according to the World Medical Association Declaration of Helsinki and approved by the local ethics committee. All patients provided written informed consent before enrollment.
Sample Collection
Blood was sampled at the start and at the end of a midweek dialysis. The slow flow/stop pump technique was used. Total spent dialysate was collected during the midweek dialysis session in 300-L polystyrene vessels. To calculate dialytic solute removals, spent dialysate collections were weighed, vigorously stirred, and sampled.
HPLC
p-Cresyl sulfate and indoxyl sulfate were quantified as described previously (26), using an HPLC (HPLC Alliance 2695; Waters, Zellik, Belgium), coupled to a Waters 2475 fluorescence detector. Free p-cresyl sulfate and indoxyl sulfate concentrations were measured in serum ultrafiltered at 37°C using 30,000-D molecular cut-off filters (Centifree UF devices; Amicon, Beverly, MA). Ultrafiltrates (600 μl) were concentrated using a vacuum concentrator (Christ rotary vacuum concentrator 2-18 and cool trap 2-50; Qlab, Vilvoorde, Belgium) at 30°C overnight. Dried ultrafiltrates were dissolved in 200 μl PBS by sonification for 30 min. Limits of quantification of total serum concentrations were 3.2 μM for indoxyl sulfate and 1.8 μM for p-cresyl sulfate. Limits of quantification of free serum concentrations were 1.1 and 0.6 μM, respectively. Recovery, tested in HD patients, was 102% for indoxyl sulfate and 105% for p-cresyl sulfate. Total, within-run, between-run, and between-day imprecision for indoxyl sulfate and p-cresyl sulfate were <6%. Intraindividual variation of indoxyl sulfate and p-cresyl sulfate serum concentrations was found to be limited. Over a 1-mo period, we observed modest variability in indoxyl sulfate (median, 7.2; interquartile range, 5.6 to 16.0 coefficient of variation [CV%]) and p-cresyl sulfate (median, 10.2; IQR, 7.6 to 14.0 CV%) serum concentrations (n = 10, 2-wk sampling interval).
Spiking Experiments
Serum from 20 HD patients was collected at the start of the HD session. Total and free indoxyl sulfate and p-cresyl sulfate serum concentrations were determined on aliquots before spiking and after spiking with indoxyl sulfate at a final concentration of 100 μM or p-cresyl sulfate at a final concentration of 166 μM. Concentrations were chosen to represent mean total serum concentrations in HD patients of indoxyl sulfate and p-cresyl sulfate, respectively. Samples were incubated in a 37°C water bath for 30 min.
Calculations
According to the law of mass action, binding can be expressed as
where Ct, Cb, and Cf represent the total, the bound, and free concentration of a given molecule.
The free fraction f is defined by the ratio between the unbound (free) concentration and the total concentration of a given ligand. Assuming a single binding site with a binding constant KA, this relation can be expressed as (24)
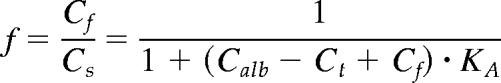 |
Dialytic solute removals (DSRs) of solute x were calculated as
where Vd is the spent dialysate volume and Cd,x is the dialytic solute concentration. Given that dialysate relative density is near 1, dialysate weight is taken as spent dialysate volume. Dialytic solute clearances (Kd,x) were calculated by
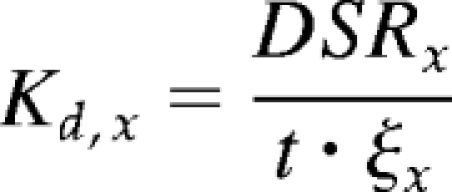 |
where t is the actual dialysis time (min) and ξx is the logarithmic mean serum concentration
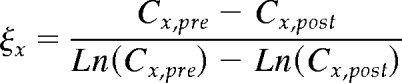 |
Statistics
Continuous variables are expressed as mean ± SD for normally distributed variables or median (interquartile range) otherwise. Normality was tested according to Shapiro-Wilk. Correlations were tested according to Spearman. A two-sided P < 0.05 was considered statistically significant.
Results
Study Population
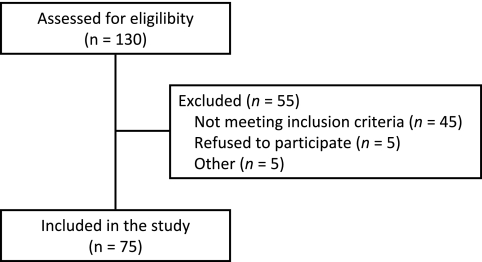
Between February and June 2006, 75 maintenance HD patients (Figure 1), followed at the nephrology department of the University Hospital Gasthuisberg, Leuven, Belgium, provided informed consent and were included in the study. Table 1 represents the demographic and baseline characteristics of the study population.
Figure 1.
Flow chart showing patient screening and inclusion.
Table 1.
Demographics, dialysis treatment, and biochemical variables
| Variable | |
|---|---|
| Age (yr) (median, minimum − maximum) | 75 (30–87) |
| Sex (man/woman) [n (%)] | 40/35 (54/46) |
| Vintage (mo) | 20 (9–31.5) |
| Qb,effective (ml/min) | 304.0 (40.1) |
| Qd (ml/min) | 505.6 (22.7) |
| Unipuncture/bipuncture [n (%)] | 8/67 (11–89) |
| Residual renal function (yes versus no) (%) | 39/36 (52/48) |
| Urea (mg/dl) | 119.3 (26.9) |
| Creatinine (mg/dl) | 6.9 (2.1) |
| Albumin (mg/L) | 39.5 (3.5) |
| Total p-cresyl sulfate (μM) | 183.6 (114.4–305.3) |
| Free p-cresyl sulfate (μM) | 10.3 (5.6–19.9) |
| Free fraction p-cresyl sulfate | 0.054 (0.042–0.070) |
| Total indoxyl sulfate (μM) | 104.7 (67.2–134.6) |
| Free indoxyl sulfate (μM) | 9.1 (5.4–12.5) |
| Free fraction indoxyl sulfate | 0.081 (0.068–0.106) |
Data are expressed as mean (SD) or median (25–75 percentile) as appropriate. To convert creatinine in mg/dl to μM, multiply with 88.4.
Qb,effective, effective blood flow; Qd, dialysate flow.
Serum Concentrations of Indoxyl Sulfate and p-Cresyl Sulfate
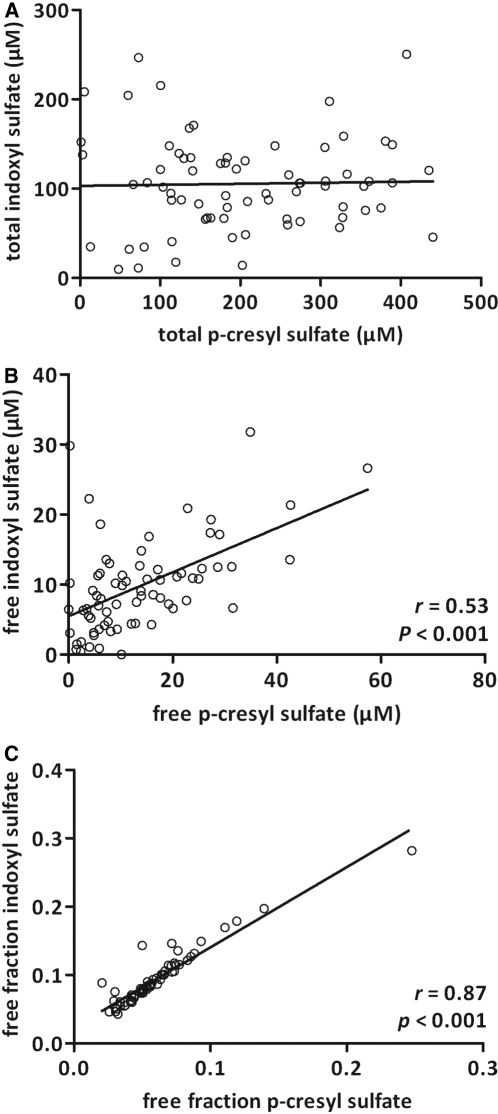
Associations between indoxyl sulfate and p-cresyl sulfate serum concentrations were analyzed using Spearman rank correlation analysis (Table 2). Total serum concentrations of indoxyl sulfate and p-cresyl sulfate were not correlated (P = 0.9). Free serum concentrations of indoxyl sulfate and p-cresyl sulfate showed a moderate direct association (r = 0.53, P < 0.001), whereas indoxyl sulfate and p-cresyl sulfate free fractions (f) were tightly correlated (r = 0.87, P < 0.001; Figure 2). On average, free fractions of indoxyl sulfate were 1.53 ± 0.20-fold higher than free fractions of p-cresyl sulfate.
Table 2.
Spearman rank correlations between uremic retention solutes
| Urea | Creatinine | IS, Total | IS, Free | IS, f | pCS, Total | pCS, Free | pCS, f | |
|---|---|---|---|---|---|---|---|---|
| Urea | 1.00 | — | — | — | — | — | — | — |
| Creatinine | r = 0.37, P = 0.002 | 1.00 | — | — | — | — | — | — |
| Indoxyl sulfate, total | r = 0.30, P = 0.01 | r = 0.47, P < 0.001 | 1.00 | — | — | — | — | — |
| Indoxyl sulfate, free | r = 0.38, P = 0.001 | r = 0.37, P = 0.001 | r = 0.81, P < 0.001 | 1.00 | — | — | — | — |
| Indoxyl sulfate, f | r = 0.33, P = 0.005 | r = 0.13, P = 0.3 | r = 0.3, P = 0.02 | r = 0.73, P < 0.001 | 1.00 | — | ||
| p-cresyl sulfate, total | r = 0.21, P = 0.08 | r = 0.21, P = 0.08 | r = 0.02, P = 0.9 | r = 0.30, P = 0.01 | r = 0.56, P = < 0.001 | 1.00 | — | — |
| p-cresyl sulfate, free | r = 0.28, P = 0.02 | r = 0.21, P = 0.08 | r = 0.15, P = 0.20 | r = 0.54, P < 0.001 | r = 0.76, P < 0.001 | r = 0.93, P < 0.001 | 1.00 | — |
| p-cresyl sulfate, f | r = 0.38, P = 0.001 | r = 0.27, P = 0.03 | r = 0.44, P < 0.001 | r = 0.54, P < 0.001 | r = 0.92, P < 0.001 | r = 0.62, P < 0.001 | r = 0.84, P < 0.001 | 1.00 |
IS, indoxyl sulfate; pCS, p-cresyl sulfate; f, free fraction.
Figure 2.
Agreement between (A) total serum concentrations, (B) free serum concentrations, and (C) free fractions of p-cresyl sulfate and indoxyl sulfate in a cohort of 75 hemodialysis patients. Spearman correlation coefficients are reported.
Dialytic Kinetics of Indoxyl Sulfate and p-Cresyl Sulfate
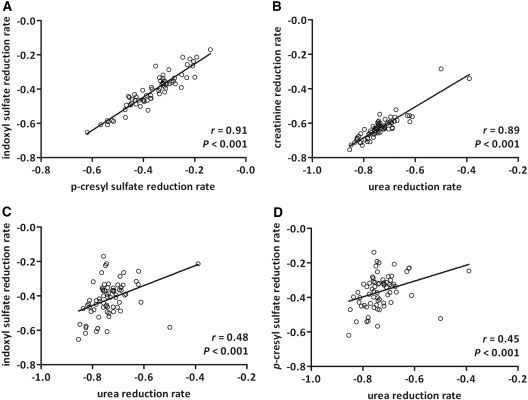
To study whether indoxyl sulfate and p-cresyl sulfate behave similar during dialysis, we first compared reduction rates (RRs) during hemodialysis. Indoxyl sulfate and p-cresyl sulfate RR correlated tightly (r = 0.91, P < 0.001). As expected, we also observed a good correlation between urea and creatinine RR (r = 0.89, P < 0.001). However, urea RRs were only moderately correlated with indoxyl sulfate RRs (r = 0.48, P < 0.001) and with p-cresyl sulfate RRs (r = 0.45, P < 0.001; Figure 3).
Figure 3.
Agreement between reduction rates of (A) p-cresyl sulfate and indoxyl sulfate, (B) urea and creatinine, (C) urea and indoxyl sulfate, and (D) urea and p-cresyl sulfate in a cohort of 75 hemodialysis patients. Spearman correlation coefficients are reported.
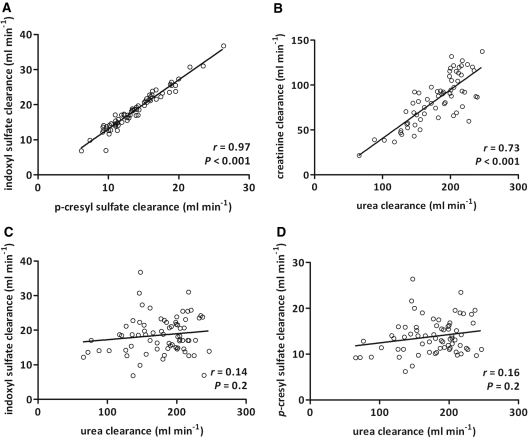
Second, we compared dialytic clearances of indoxyl sulfate, p-cresyl sulfate, urea, and creatinine. Indoxyl sulfate and p-cresyl sulfate clearances correlated tightly (r = 0.97, P < 0.001). On average, dialytic clearances of indoxyl sulfate were 33 ± 11% higher than dialytic clearances of p-cresyl sulfate. As expected, we also observed a good correlation between urea and creatinine dialytic clearances (r = 0.73, P < 0.001). However, dialytic clearances of indoxyl sulfate and p-cresyl sulfate were not significantly correlated with dialytic urea clearances (P = 0.2 for both; Figure 4).
Figure 4.
Agreement between dialytic clearances of (A) p-cresyl sulfate and indoxyl sulfate, (B) urea and creatinine, (C) urea and indoxyl sulfate, and (D) urea and p-cresyl sulfate in a cohort of 75 hemodialysis patients. Spearman correlation coefficients are reported.
Albumin Binding of Indoxyl Sulfate and p-Cresyl Sulfate
To determine whether indoxyl sulfate and p-cresyl sulfate compete for their albumin binding site, we performed in vitro spiking experiments on serum from 20 HD patients. Addition of p-cresyl sulfate significantly increased free serum concentrations and free fractions of both p-cresyl sulfate and indoxyl sulfate (Table 3). Also, addition of indoxyl sulfate significantly increased free serum concentrations and free fractions of both p-cresyl sulfate and indoxyl sulfate (P < 0.001 for all; Table 2).
Table 3.
Albumin binding of indoxyl sulfate and p-cresyl sulfate
| Variable | Baseline Concentration | Spiking p-Cresyl Sulfate |
Spiking Indoxyl Sulfate |
||
|---|---|---|---|---|---|
| Concentration | Change (%) | Concentration | Change (%) | ||
| p-Cresyl sulfate | |||||
| Total (μM) | 189.0 (130.6) | 354.3 (123.4) | 88.5 (54.1)a | 186.0 (125.0) | −4.9 (4.2)a |
| Free (μM) | 11.6 (13.4) | 30.1 (24.4) | 164.2 (109.4)a | 14.6 (17.2) | 24.7 (15.3)a |
| Free fraction (%) | 6.7 (4.0) | 8.6 (4.9) | 37.1 (20.3)a | 8.6 (6.2)a | 27.3 (15.9)a |
| Indoxyl sulfate | |||||
| Total (μM) | 114.3 (76.3) | 110.7 (73.7) | −3.1 (1.6)a | 210.4 (66.4) | 89.4 (125.4)a |
| Free (μM) | 12.8 (13.8) | 16.8 (17.2) | 28.5 (12.2)a | 28.3 (18.4) | 134.4 (144.0)a |
| Free fraction (%) | 9.3 (4.7) | 12.1 (6.1) | 34.3 (13.0)a | 11.4 (6.2) | 20.9 (7.5)a |
Data are expressed as median (interquartile range).
P < 0.001 versus baseline.
Changes in the free fraction of p-cresyl sulfate and indoxyl sulfate of individual patients correlated well (r = 0.85, P < 0.001) after spiking with indoxyl sulfate and after spiking with p-cresyl sulfate (r = 0.79, P < 0.001), despite opposite changes of total serum concentrations (Table 3). The observed small reductions in total p-cresyl sulfate and indoxyl sulfate total serum concentrations when spiked with indoxyl sulfate and p-cresyl sulfate, respectively, can be attributed to dilution.
Discussion
The main finding of this study in 75 maintenance HD patients is that, despite similar protein binding and blood clearances, total indoxyl sulfate and p-cresyl sulfate serum concentrations are not related.
Serum concentrations of indoxyl sulfate and p-cresyl sulfate are the result of generation from bacterial protein fermentation in the large intestine, metabolism including “detoxification” by sulfate conjugation and elimination. Residual renal function contributes little to total solute removal of protein-bound uremic retention solutes in HD patients (10). In this study, indoxyl sulfate and p-cresyl sulfate dialytic clearances are tightly correlated and were almost equal within individual patients. These findings indicate that the generation of indole and p-cresol is unrelated, despite the fact that they both originate from bacterial protein fermentation. Intervention studies also suggest that different metabolic pathways are involved. Oral intake of Lebenin (a preparation of antibiotic-resistant lactic acid bacteria) reduced serum concentrations of indoxyl sulfate but not of p-cresyl sulfate in HD patients (27). Vice versa, we recently observed that intake of the prebiotic oligofructose-enriched inulin significantly reduced p-cresol generation and p-cresyl sulfate serum concentrations, whereas indoxyl sulfate serum concentrations were not systematically reduced, nor was the indole generation rate (clinicaltrials.gov NCT00695513) (28). This allows for targeted therapies specifically reducing intestinal generation and adsorption of either p-cresol or indole.
Intriguingly, indoxyl sulfate and p-cresyl sulfate free fractions are tightly correlated (r = 0.87, P < 0.001). In the absence of a direct association of total serum concentrations, this strongly suggests interrelated protein binding. Albumin bears specific binding sites for anionic, neutral, and cationic ligands (24). Using competitive binding experiments with selective probes, indoxyl sulfate was shown to bind to Sudlow site II (29,30). We recently showed that p-cresyl sulfate binds to Sudlow site II as well (26). Although it is generally accepted that competition by uremic retention solutes is responsible for impaired adsorptive capacity of serum proteins including albumin in renal insufficiency, reported by Breyer and Radcliff more than half a century ago (31), little is known about competition between different uremic retention solutes for specific albumin binding sites. In this study, we showed that changes in total indoxyl sulfate concentrations affect free p-cresyl sulfate concentrations and vice versa through competitive binding inhibition.
Meyer et al. (25) modeled that dialytic clearances of protein-bound uremic retention solutes are directly proportional to free fractions. According to their model, one would expect good agreement in blood clearances. Indeed, we observed tight correlations between indoxyl sulfate and p-cresyl sulfate dialytic clearances (r = 0.97, P < 0.001) and RRs (r = 0.91, P < 0.001). In contrast, we observed no significant correlations between either p-cresyl sulfate or indoxyl sulfate dialytic clearances and urea dialytic clearances.
Our findings have several clinical implications. Total indoxyl sulfate and p-cresyl sulfate serum concentrations are not associated and free concentrations are only modestly associated. As a consequence, observed associations between total and, to a lesser extent, free solute concentrations of either one and clinical endpoints cannot be attributed to known physiologic effects of the other. In other words, p-cresyl sulfate and indoxyl sulfate are not interchangeable risk markers.
Intriguingly, indoxyl sulfate and p-cresyl sulfate free fractions are tightly correlated and, secondary to this, dialytic clearances of indoxyl sulfate and p-cresyl sulfate are also closely related. The implication is that either indoxyl sulfate or p-cresyl sulfate can be used as marker molecule to study the dialytic behavior of the Sudlow site II protein-bound uremic retention solutes during dialysis.
Finally, because we observed competitive albumin binding, therapies targeted at reducing indoxyl sulfate serum concentrations, e.g., AST-120 (Kremezin®; Kureha Chemical Industry, Tokyo, Japan) (4), are expected to reduce free p-cresyl sulfate serum concentrations, even if total p-cresyl sulfate concentrations are not affected.
In conclusion, indoxyl sulfate and p-cresyl sulfate are interchangeable marker molecules to monitor behavior of protein-bound uremic retention solutes during dialysis and are competitive binding inhibitors for the same albumin binding site. The absence of an association between total serum concentrations refutes the thesis that p-cresyl sulfate and indoxyl sulfate are interchangeable risk markers.
Disclosures
None.
Acknowledgments
We thank M. Dekens, E. Vanhalewyck, and A. Van Esch for excellent technical assistance and T. W. Meyer for critical appraisal of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu Cy: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305 2004 [DOI] [PubMed] [Google Scholar]
- 3.Depner T, Himmelfarb J: Uremic retention solutes: The free and the bound. J Am Soc Nephrol 18: 675–676, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N: A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis 47: 565–577, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Meyer TW, Peattie JW, Miller JD, Dinh DC, Recht NS, Walther JL, Hostetter TH: Increasing the clearance of protein-bound solutes by addition of a sorbent to the dialysate. J Am Soc Nephrol 18: 868–874, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Meijers BKI, Weber V, Bammens B, Dehaen W, Verbeke K, Falkenhagen D, Evenepoel P: Removal of the uremic retention solute p-cresol using fractionated plasma separation and adsorption. Artif Organs 32: 214–219, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Luo FJ, Patel KP, Marquez IO, Plummer NS, Hostetter TH, Meyer TW: Effect of increasing dialyzer mass transfer area coefficient and dialysate flow on clearance of protein-bound solutes: A pilot crossover trial. Am J Kidney Dis 43: 1042–1049, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of p-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y: Removal of the protein-bound solute p-cresol by convective transport: A randomized crossover study. Am J Kidney Dis 44: 278–285, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y: Superior dialytic clearance of [beta]2-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int 70: 794–799, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki T, Ise M, Hirata M, Endo K, Ito Y, Seo H, Niwa T: Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int 52: S211–S214, 1997 [PubMed] [Google Scholar]
- 12.Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S: The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int 52: S23–S28, 1997 [PubMed] [Google Scholar]
- 13.Niwa T: Organic acids and the uremic syndrome: protein metabolite hypothesis in the progression of chronic renal failure. Semin Nephrol 3: 167–182, 1996 [PubMed] [Google Scholar]
- 14.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T: Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant 23: 1892–1901, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Nii-Kono T, Iwasaki Y, Uchida M, Fujieda A, Hosokawa A, Motojima M, Yamato H, Kurokawa K, Fukagawa M: Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int 71: 738–743, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Meijers BKI, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1173–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 2009, in press [DOI] [PubMed] [Google Scholar]
- 19.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R: P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 22: 592–596, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Stanfel LA, Gulyassy PF, Jarrard EA: Determination of indoxyl sulfate in plasma of patients with renal failure by use of ion-pairing liquid chromatography. Clin Chem 32: 938–942, 1986 [PubMed] [Google Scholar]
- 21.Niwa T, Takeda N, Tatematsu A, Maeda K: Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem 34: 2264–2267, 1988 [PubMed] [Google Scholar]
- 22.de Loor H, Bammens B, Evenepoel P, De Preter V, Verbeke K: Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem 51: 1535–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Sudlow G, Birkett D, Wade D: The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol 11: 824–832, 1975 [PubMed] [Google Scholar]
- 24.Meijers BKI, Bammens B, Verbeke K, Evenepoel P: A review of albumin binding in CKD. Am J Kidney Dis 51: 839–850, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Meyer TW, Leeper EC, Bartlett DW, Depner TA, Lit YZ, Robertson CR, Hostetter TH: Increasing dialysate flow and dialyzer mass transfer area coefficient to increase the clearance of protein-bound solutes. J Am Soc Nephrol 15: 1927–1935, 2004 [DOI] [PubMed] [Google Scholar]
- 26.de Loor H, Meijers BK, Meyer TW, Bammens B, Verbeke K, Dehaen W, Evenepoel P: Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A 1216: 4684–4688, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y: Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 2009, in press [DOI] [PubMed] [Google Scholar]
- 29.Sakai T, Yamasaki K, Sako T, Kragh-Hansen U, Suenaga A, Otagiri M: Interaction mechanism between indoxyl sulfate, a typical uremic toxin bound to site II, and ligands bound to site I of human serum albumin. Pharm Res 18: 520–524, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Sakai T, Takadate A, Otagiri M: Characterisation of binding site of uremic toxins on human serum albumin. Biol Pharm Bull 18: 1755–1761, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Breyer B, Radcliff F: The adsorptive capacity of serum proteins in renal insufficiency. Austral J Exp Biol 32: 411–420, 1954 [DOI] [PubMed] [Google Scholar]