Abstract
Background and objectives: Coronary artery calcification (CAC) is common in advanced chronic kidney disease (CKD), yet its onset and time course are uncertain. The study objective was to assess longitudinal relationships among CAC, kidney function, and traditional and putative cardiovascular disease (CVD) risk factors.
Design, setting, participants, & measurements: This is a prospective cohort analysis from the Spokane Heart Study, a long-term observational study of community-dwelling adults who were assessed every 2 yr for CAC (electron-beam computed tomography), CVD risk factors, and laboratory testing. Estimated GFR (eGFR) was determined by the reexpressed Modification of Diet in Renal Disease equation.
Results: CAC was present in 28% (245 of 883) at baseline. After 6 yr, new-onset CAC developed in 33% (122 of 371); severity increased from a median CAC score of 38 to 152 in those with baseline CAC. Neither eGFR (101 ± 34 versus 104 ± 31 ml/min per 1.73 m2, respectively) nor serum phosphorus (3.25 ± 0.49 versus 3.29 ± 0.48 mg/dl, respectively) differed by CAC presence or absence at baseline; however, multivariate models (generalized estimating equations for incidence and prevalence) revealed that independent predictors of CAC over time were greater baseline CAC scores, higher serum phosphorus levels, lower eGFR levels, and traditional CVD risk factors. Each 1-mg/dl increase in phosphorus imparted odds ratios for CAC of 1.61 (incidence) and 1.54 (prevalence), risks comparable to traditional CVD risk factors.
Conclusions: CAC becomes more frequent and severe over time. Higher levels of serum phosphorus and reduced kidney function independently predicted CAC.
When kidney function declines, death from cardiovascular disease (CVD) predominates over progression of chronic kidney disease (CKD) (1–3). Excess CVD risk even in early-stage CKD is not fully explained by traditional, Framingham-type CVD risk factors. In ESRD, coronary artery calcification (CAC) is a common finding associated with clinical CVD (4). In ESRD, the extent of CAC is related to serum phosphorus and the calcium-phosphorus product along with traditional CVD risk factors (4,5). Many years ago, a meticulous autopsy study of hemodialysis patients described a distinctive coronary artery pathology characterized by medial calcification (6). Vascular pathology was similar in nondialysis patients with CKD, but arterial calcification was less frequent and severe (6). Although CAC appears before ESRD, its onset and time course in relation to decreased kidney function are unknown.
Experimental models point to hyperphosphatemia, a hallmark of bone and mineral metabolism disorders in CKD, as a potential causal link to vascular calcification. Uremic rodents are protected from developing aortic calcification and/or atherosclerotic lesions by treatment with noncalcemic phosphate binders (7,8). In gene knockout models for phosphorus-regulating factors, fibroblast growth factor 23, or Klotho, nonuremic mice develop elevated serum phosphorus levels and vascular calcification (9,10). Observational studies of people with and without clinically apparent kidney disease also show that higher serum phosphorus levels within the “normal” range are associated with clinical CVD and CAC (11–14). The study objective was to assess longitudinal relationships among CAC, kidney function, and traditional and putative CVD risk factors, including serum phosphorus, among participants in the Spokane Heart Study, a long-term observational study of adults in the community.
Materials and Methods
Participants
The Spokane Heart Study recruited community-dwelling men and women who were ≥18 yr of age and were employed in the fields of law enforcement, fire fighting, health care, education, and business. All participants gave written informed consent for the study, which was reviewed and approved by the local institutional review boards for Washington State University and the Spokane community hospitals. Between April 1994 and November 2003, people who were believed to be in good health and have no known CVD or other illnesses associated with major comorbidities or reduced life expectancy were enrolled (15). Data were collected at 2-yr intervals for ≥6 yr. Those who attended at least two data collection sessions (n = 883) were identified for this study.
Measurements
A multifaceted battery of tests related to health status was administered to participants at each measurement occasion (16). CAC determined by electron-beam computed tomography was defined as the primary outcome. The CAC score was quantified as pixel density in a gated scan with 3-mm image slices. Pixel density was reported in Hounsfield units and summed to produce a total score for all coronary arteries (16).
At each assessment, blood was collected in the fasting state for standard clinical chemistries and hematology testing. Blood samples were analyzed at a central laboratory (Pathology Associates Medical Laboratory, Spokane, WA). Laboratory analyses were obtained within 6 mo of the measurement of CAC. Serum creatinine values were aligned with those from the Fourth National Health and Nutrition Examination Survey (NHANES; 1999 to 2000) that corresponded with the approximate midpoint of recruitment for the Spokane Heart Study. Creatinine measurements were assessed by age, gender, and racial characteristics. As a result, the measured serum creatinine level was reduced by 0.27 mg/dl, the average difference between observed (Spokane Heart Study) and expected (NHANES) values. The reexpressed Modification of Diet in Renal Disease (MDRD) formula was used to compute estimated GFR (eGFR) (17).
Diabetes was defined as fasting blood glucose concentration of ≥126 mg/dl documented on at least two study visits or taking prescribed medicines to treat hyperglycemia. Hypertension was defined as a systolic BP >140 mmHg or a diastolic BP >90 mmHg documented on at least two study visits or taking prescribed antihypertensive agents.
Study Design and Data Analyses
Among the total of 883 participants, 214 had two scans, 416 had three scans, and 253 had four scans. For statistical model-building purposes, total CAC score was dichotomized into a positive (>0 or ≥10) or negative (0 or <10) scan. Both models yielded similar inferences (data not shown). Accordingly, CAC score >0 was used as the cut point for a positive scan to balance distribution between scan categories. Among participants with positive scans, CAC scores were computed to evaluate progression.
Means ± SD were computed for continuous variables, and frequencies were determined for discrete measures. Independent t tests were used to detect differences between groups on continuous measures. χ2 statistics were used for categorical data. Multiple variable testing was performed with generalized estimating equations (GEE) to assess longitudinal relationships between CAC and variables of interest (incident and prevalent analyses). Prespecified covariates included both categorical (gender, status of diabetes and hypertension, and CAC presence at baseline for prevalent analysis) and time-dependent (age, lipids, eGFR, and serum phosphorus and calcium) variables. Nonzero CAC scores (log-transformed because of skewness), changes in eGFR, and serum phosphorus were assessed by random regression modeling with at least the baseline and one follow-up measurement. Data analyses were performed with SPSS 16 (SPSS, Inc., Chicago, IL).
Results
CAC was present in 28% (249 of 883) of participants at study entry. Participants with CAC more commonly were male, had hypertension, and had histories of smoking and family members with CVD (Table 1). They were older and had higher levels of total and LDL cholesterol. Levels of eGFR and serum phosphorus did not differ by CAC status. Serum calcium was slightly higher in the group with CAC at baseline.
Table 1.
Baseline characteristics of Spokane Heart Study participants by CAC status
| Characteristic | CAC(n = 249) | No CAC(n = 635) | P | Total(n = 883) |
|---|---|---|---|---|
| Male (n [%]) | 178 (72) | 316 (50) | <0.001 | 494 (56) |
| Hypertension (n [%]) | 105 (42) | 160 (25) | <0.001 | 265 (30) |
| Diabetes (n [%]) | 16 (6) | 21 (3) | 0.060 | 37 (4) |
| Smoking history (n [%]) | 138 (55) | 262 (41) | <0.001 | 400 (45) |
| Family history of CVD (n [%]) | 121 (51) | 222 (37) | <0.001 | 343 (41) |
| White race (n [%]) | 245 (98) | 611 (96) | 0.133 | 856 (97) |
| Age (yr; mean ± SD) | 54 ± 9 | 46 ± 9 | <0.001 | 48 ± 9 |
| Total cholesterol (mg/dl; mean ± SD) | 220 ± 38 | 209 ± 36 | <0.001 | 212 ± 37 |
| LDL cholesterol (mg/dl; mean ± SD) | 139 ± 34 | 128 ± 31 | <0.001 | 131 ± 32 |
| HDL cholesterol (mg/dl; mean ± SD) | 52 ± 15 | 53 ± 16 | 0.126 | 53 ± 16 |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 101 ± 34 | 104 ± 31 | 0.201 | 104 ± 32 |
| Phosphorus (mg/dl; mean ± SD) | 3.25 ± 0.49 | 3.29 ± 0.48 | 0.251 | 3.28 ± 0.48 |
| Calcium (mg/dl; mean ± SD) | 9.61 ± 0.39 | 9.56 ± 0.37 | 0.040 | 9.57 ± 0.37 |
| Body mass index (kg/m2; mean ± SD) | 27 ± 4 | 27 ± 4 | 0.114 | 27 ± 4 |
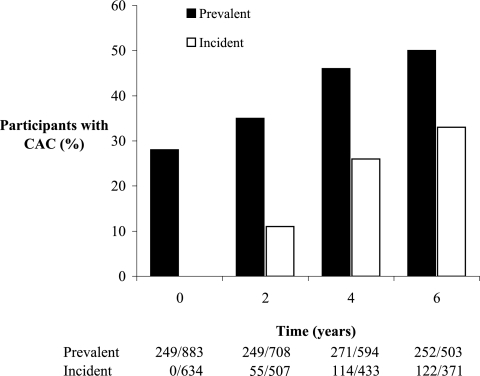
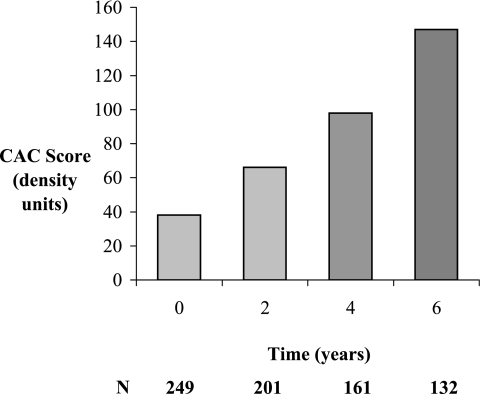
During 6 yr of follow-up, new-onset (incident) CAC developed in 33% (122 of 371), yielding an overall prevalence of 50% (252 of 503; P < 0.001 for both analyses; Figure 1). Among the subset with CAC at baseline, severity increased from a median score of 38 to 152 density units after 6 yr (P < 0.001; Figure 2). The eGFR declined similarly during 6 yr of follow-up in participants with and without baseline CAC (−11 ± 36 and −12 ± 30 ml/min per 1.73m2, respectively; P < 0.001 for change, P = 0.935 for interaction). Average values for serum phosphorus increased over time but did not differ by baseline CAC status (0.09 ± 0.51 and 0.11 ± 0.49 mg/dl, respectively; P < 0.001 for change, P = 0.562 for interaction).
Figure 1.
Prevalent and incident CAC among Spokane Heart Study participants. Prevalent and incident CAC increased progressively during 6 yr of follow-up (P < 0.001 for both analyses).
Figure 2.
Median scores for positive CAC scans among Spokane Heart Study participants with CAC at baseline. Median score increased from 38 to 152 after 6 yr (P < 0.001).
The GEE approach to multiple variable modeling for CAC prevalence revealed that independent predictors were greater baseline CAC, older age, male gender, higher serum levels of phosphorus, and lower levels of eGFR and HDL cholesterol (Table 2). Diabetes status just missed statistical significance. Hypertension status and serum levels of calcium and LDL cholesterol showed NS relationships with CAC. A second GEE multiple variable model that included the same covariates (except for baseline CAC) was performed to evaluate incident CAC. Independent predictors in this model were nearly identical to those in the first model (Table 3). Of particular interest, higher serum phosphorus, a putative CVD risk factor associated with loss of kidney function, significantly influenced development of CAC. In both multiple variable models, each 1-mg/dl increase in serum phosphorus predicted increased risk for CAC over time (prevalence odds ratio 1.54 [95% confidence interval 1.17 to 2.04; P = 0.002]; incidence odds ratio 1.61 [95% confidence interval 1.20 to 2.14; P = 0.001]), which represents an impact similar to traditional CVD risk factors. Multiple variable models for incidence and prevalence of CAC were also evaluated with inclusion of smoking status and family history of CVD, because these risk factors differed by baseline CAC status. These covariates did not consistently influence CAC or alter significant predictors in either model (data not shown).
Table 2.
Multiple variable model for risk of CAC over time among all Spokane Heart Study participants (prevalence)
| Variable | β | OR | 95% CI | χ2 | P |
|---|---|---|---|---|---|
| Baseline CAC present (yes/no) | 5.733 | 308.79 | 145.14 to 656.97 | 221.48 | <0.001 |
| Age (per decade) | 1.175 | 3.24 | 1.10 to 1.15 | 101.07 | <0.001 |
| Male (yes/no) | 1.010 | 2.75 | 1.80 to 4.18 | 22.13 | <0.001 |
| HDL cholesterol (per 1 mg/dl) | −0.020 | 0.98 | 0.97 to 0.99 | 11.84 | 0.001 |
| Phosphorus (per 1 mg/dl) | 0.435 | 1.54 | 1.17 to 2.04 | 9.40 | 0.002 |
| eGFR (per 10 ml/min per 1.73 m2) | −0.065 | 0.94 | 0.89 to 0.99 | 5.90 | 0.015 |
| Diabetes (yes/no) | 0.576 | 1.78 | 0.97 to 3.24 | 3.52 | 0.061 |
| LDL cholesterol (per 10 mg/dl) | 0.003 | 1.03 | 0.98 to 1.08 | 1.50 | 0.221 |
| Calcium (per 1 mg/dl) | 0.187 | 1.20 | 0.83 to 1.75 | 0.96 | 0.327 |
| Hypertension (yes/no) | 0.025 | 1.03 | 0.71 to 1.48 | 0.02 | 0.892 |
CI, confidence interval; OR, odds ratio.
Table 3.
Multiple variable model for risk of new-onset CAC over time among Spokane Heart Study participants without CAC at baseline (incidence)
| Variable | β | OR | 95% CI | χ2 | P |
|---|---|---|---|---|---|
| Age (per decade) | 1.213 | 3.36 | 2.64 to 4.29 | 95.51 | <0.001 |
| Male (yes/no) | 1.10 | 3.00 | 1.92 to 4.67 | 23.50 | <0.001 |
| HDL cholesterol (per 1 mg/dl) | −0.020 | 0.98 | 0.97 to 0.99 | 11.19 | 0.001 |
| Phosphorus (per 1 mg/dl) | 0.474 | 1.61 | 1.20 to 2.14 | 10.47 | 0.001 |
| eGFR (per 10 ml/min per 1.73 m2) | −0.065 | 0.94 | 0.88 to 0.99 | 4.87 | 0.027 |
| Diabetes (yes/no) | 0.54 | 1.72 | 0.92 to 3.21 | 2.92 | 0.087 |
| LDL cholesterol (per 10 mg/dl) | 0.028 | 1.03 | 0.98 to 1.08 | 1.32 | 0.250 |
| Hypertension (yes/no) | 0.065 | 1.07 | 0.73 to 1.56 | 0.11 | 0.737 |
| Calcium (per 1 mg/dl) | 0.029 | 1.03 | 0.71 to 1.48 | 0.02 | 0.879 |
Discussion
Among individuals without clinical CVD at entry into the Spokane Heart Study, CAC was a common finding. CAC occurrence and severity increased progressively during 6 yr of follow-up. Higher levels of serum phosphorus and lower levels of kidney function emerged as independent predictors of CAC over time, along with traditional CVD risk factors.
As in patients with advanced stages of CKD, the serum phosphorus level was a significant determinant of CAC in this relatively healthy population. Reduced kidney function, predominantly within the clinically normal range, was yet another predictor of CAC indicating that the effect of lower eGFR may also be explained by complications other than higher serum phosphorus. Across the CKD continuum, risk for CAC has been reported to be increased by older age, male gender, and usually diabetes (4,5,18). Diabetes fell short of statistical significance in this study, most likely owing to the small number of participants with diabetes. The strongest determinant of CAC in the prevalent analysis was having a positive scan at baseline, which is consistent with the progressive nature of vascular calcification.
Although calcification can be a component of typical atherosclerotic disease, in the setting of uremia, calcium chiefly deposits within the media and internal elastic lamina (6,19). Eventually, resultant injury responses to calcification contribute to advancing sclerosis in the coronary arteries and throughout the circulation. Experimental models in both uremic and nonuremic animals support hyperphosphatemia as central to the process of vascular calcification. Irrespective of kidney function, mice or rats with higher serum phosphorus develop prominent aortic calcification that can be corrected by phosphate-binding drugs or dietary restriction (7–10,20). A growing series of observations in humans are consistent with experimental models in that higher serum phosphorus has been reported to predict CAC and CVD events in people without clinically apparent kidney disease (11–13). Similar observations have recently been extended to a nondialysis CKD population among whom higher serum phosphate was independently associated with prevalent CAC as well as with calcification of the aorta and cardiac valves (14); however, these relationships are complex and most apparent in multivariate analyses that control for a series of potential confounders.
The body of evidence implicating phosphorus in CAC isvitally important. These data shed light on a nontraditional mechanism for CVD, which may help to explain unexpected responses to traditional therapies in patients with CKD. For example, atorvastatin and rosuvastatin failed to reduce risk for CVD events in hemodialysis patients with and without diabetes in Die Deutsche Diabetes Dialyse Studie (4D Study) and A study to evaluate the Use of Rosuvastatin in subjects On Regular hemodialysis: an Assessment of survival and cardiovascular events (AURORA), unusually “negative” statin studies (21,22). Moreover, adverse CVD events in CKD are often due to heart failure, which may be related to higher serum phosphorus, at least in part because of its effect to promote cardiac valvular and muscle calcification (7,15,23,24). Clinical trials of phosphate reduction have been limited by selection of only participants with ESRD and insufficient clinical outcomes data to draw firm conclusions (25,26). Observational human data combined with experimental studies make a case for testing effects of treatments that reduce phosphate on CVD outcomes in patients who are in earlier stages of CKD or perhaps even in those who do not have CKD but have CAC. On the basis of the concept that calcification is central to mechanisms of CVD, treatment strategies that minimize tissue deposition of calcium (e.g., reduced phosphate diet and/or noncalcemic phosphate binders) are of particular interest.
This study has worthy strengths, including long duration of prospective follow-up among a cohort based in the community with serial measures of CAC, eGFR, phosphorus, and CVD risk factors performed biennially. Conversely, limitations of the Spokane Heart Study must be considered. First, there is potential for misclassification, because even the reexpressed version of the MDRD equation is less reliable at higher eGFR levels and kidney function declines with age; however, repeated measures of eGFR along with the GEE approach to time-dependent analyses enhance reliability. Second, specialized tests of bone and mineral metabolism have not been performed. Phosphorus-regulating factors are likely pertinent to CAC mechanisms and for future analyses in this long-term study. Although a cross-sectional study of patients with CKD found that the effect of phosphorus on CAC was independent of parathyroid hormone or vitamin D, such observations should be validated prospectively in populations with and without CKD (15). Finally, influences of albuminuria and other emerging CVD risk factors on CAC remain to be investigated. Despite these limitations, counterbalancing strengths of the Spokane Heart Study allow it to be informative in a translational sense by corroborating observations made in experimental models on the one hand and supporting a foundation for specific hypotheses to be tested in clinical trials on the other hand.
Conclusions
CAC was common in apparently healthy adults in the community. Frequency and severity of CAC became greater over time. Higher levels of serum phosphorus, a putative CVD risk factor, and reduced kidney function at levels conventionally considered to be within the normal range independently predicted the occurrence of CAC in addition to traditional CVD risk factors.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for the Spokane Heart Study has been provided through the E.L. Wiegand Foundation and Washington State University with in-kind support from Providence Medical Research Center and Sacred Heart Medical Center.
This study was presented in abstract form at the annual meeting of American Society of Nephrology; November 6 through 9, 2008; Philadelphia, PA.
C. Harold Mielke, MD, and J. Paul Shields, MD, were instrumental in the original design and implementation of the Spokane Heart Study. Dennis Dyck, PhD, has provided succeeding leadership for the study and encouraged the authors to pursue analyses related to CKD. Most important, this work was made possible by the dedicated volunteer participants who readily committed to long-term participation in the Spokane Heart Study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Coronary Calcification in Chronic Kidney Disease: Morphology, Mechanisms and Mortality,” on pages 1883–1885.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCullough CE, Hsu C-Y: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RRUKPDS Group: Development and progression of nephropathy in type 2 diabetes: The United Kingdom prospective diabetes study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients: A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Kuzela DC, Huffer WE, Conger JD, Winter SD, Hammond WS: Soft tissue calcification in chronic dialysis patients. Am J Pathol 86: 403–424, 1977 [PMC free article] [PubMed] [Google Scholar]
- 7.Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov IG, Vanholder R, Lacour B, Drueke TB, Massy ZA: Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation 112: 2875–2882, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Neven E, Dams G, Postnov A, Chen B, De Clerck N, De Broe ME, D'Haese PC, Persy V: Adequate phosphate binding with lanthanum carbonate attenuates arterial calcification in chronic renal failure rats. Nephrol Dial Transplant 24: 1790–1799, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Razzaque MS, St.-Arnaud R, Taguchi T, Lanske B: FGF-23, vitamin D and calcification: The unholy triad. Nephrol Dial Transplant 20: 2032–2035, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto K, Ito M, Segawa H, Kuwahata M: Molecular targets of hyperphosphataemia in chronic renal failure. Nephrol Dial Transplant 18: iii79–iii80, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan GCholesterol And Recurrent Events (CARE) Trial Investigators: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Gaziano M, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mielke CH, Shields JP, Bromeling LD: Coronary artery calcium scores for men and women of a large asymptomatic population. Cardiovascular Disease Prevention 2: 194–198, 1999 [Google Scholar]
- 16.Mielke CH, Shields JP, Broemeling LD: Risk factors and coronary artery disease for asymptomatic women using electron beam computed tomography. J Cardiovasc Risk 8: 81–86, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function: Measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kramer H, Toto R, Peshock R, Cooper R, Victor R: Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J Am Soc Nephrol 16: 507–513, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Goodman WG: Vascular calcification in chronic renal failure. Lancet 358: 1115–1116, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD: Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18: 2116–2124, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz EGerman Diabetes and Dialysis Study Investigators: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad FAURORA Study Group: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Keith DS, Nichols G, Gullion CM, Betz Brown J, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Burke SK, Raggi PTREAT to Goal Working Group: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Suki WNDialysis Clinical Outcomes Revisited Investigators: Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients: Results of a randomized clinical trial. J Ren Nutr 18: 91–98, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.