Abstract
Background and objectives: Stability of parathyroid hormone (PTH) at −80°C for long storage periods has never been studied. This can be of importance for the conclusions of studies where blood banks have been constituted. The study's aim was to evaluate stability of PTH when stored as serum or plasma EDTA samples at −80°C.
Design, setting, participants & measurements: Samples were collected from 16 chronic hemodialysis patients using EDTA and gel-separator tubes. Plasma and serum were aliquoted; one aliquot was assayed with Elecsys and Liaison methods to determine the “baseline” values and another aliquot after 1, 3, 6, and 12 mo. The factors “method,” “tubes,” “subjects,” and “time” were included in a mixed linear model to evaluate their effects on measured PTH values. The prediction interval methodology was used to assess where a future result could be obtained with a defined probability.
Results: With the Liaison method, the maximum storage times with either dry or EDTA tubes were estimated to be 9 and 2 mo, respectively. With the Elecsys method, samples could be stored at least 2 yr with acceptable level of degradation.
Conclusion: PTH stability at −80°C is not infinite. Maximum storage time and acceptance limits (30%) were defined, showing that with one method, samples should be stored for not more than 2 mo, whereas the other could be stored for up to 2 yr. With any PTH assay, the maximum storage time should be evaluated to ascertain that samples will keep their initial reactive profile after prolonged storage periods.
Parathyroid hormone (parathormone [PTH]) determination is routinely performed in laboratories for the diagnosis and management of renal osteodystrophy (ROD). Worldwide, nephrologists rely on the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (1) to initiate or maintain appropriate therapeutic treatments. Recently, some papers have illustrated the need for a standardization of the pre-analytical (2,3) and analytical (4,5) phases. However, to our knowledge, the stability of PTH at −80°C for a long storage period (1 yr or more) has never been studied. To know if there is, or is not, a significant degradation of PTH when samples are stored for a long period, such as in studies where a serum or plasma EDTA bank has been constituted (6–8), might thus be of importance for the conclusions of these studies. The aim of our study was to evaluate the stability of PTH when stored for a long period as serum or plasma EDTA samples at −80°C, and to see if there was an influence of the method (Roche Elecsys or DiaSorin Liaison) on the results obtained.
Materials and Methods
Samples were collected in 16 hemodialyzed patients at 08:00h, immediately before commencing renal dialysis. All the samples were drawn into 5-ml EDTA (EDTA) and gel separator with clot activator tubes (Dry) purchased from Terumo (Haasrode, Belgium). In all cases, the tubes were filled completely and brought to the laboratory within 30 min. They were centrifuged at + 4°C, and plasma and serum were immediately aliquoted in cap-closed tubes and stored at −80°C. An aliquot was directly assayed on Liaison (DiaSorin, Saluggia, Italy) and Elecsys (Roche Diagnostics, Mannheim, Germany) to determine the “baseline” values for serum and EDTA plasma.
For each patient, one aliquot of serum and plasma EDTA was thawed after respectively 1, 3, 6 and 12 mo, vortexed, centrifuged, and assayed on the two automates. We did not observe any sign of lyophilisation in the samples.
The Roche Elecsys and Diasorin Liaison PTH are two “second generation” or “intact” PTH automated chemiluminescent methods. It means that they measure the (1-84) PTH, but also the non-(1-84) PTH (generally called [7-84] PTH), which is known to accumulate in the blood of patients suffering from chronic kidney diseases. These methods use a pair of antibodies, polyclonal from goat origin for Liaison and murine monoclonal for Elecsys. In our hands, the coefficients of variation obtained with the Roche Elecsys PTH and the DiaSorin Liaison are <10% (9).
Data Treatment
All the results were reported as recovery values, that is the ratios of the analyte concentration at each storage time to the analyte concentration at time 0. The factors “method” (Elecsys or Liaison), “tubes” (Dry or EDTA), “subjects” (n = 16), and “time” (in months) were included in a linear mixed model to evaluate their effects on PTH concentration. Statistical significance of those factors was evaluated using α = 0.05. Bootstrap prediction intervals for the linear mixed model were also computed when relevant (10). JMP v7 (SAS Institute, Cary, NC) and R v2.8.1 (CRAN, http://cran.r-project.org) were used for model fitting and evaluation and to compute prediction intervals.
Results
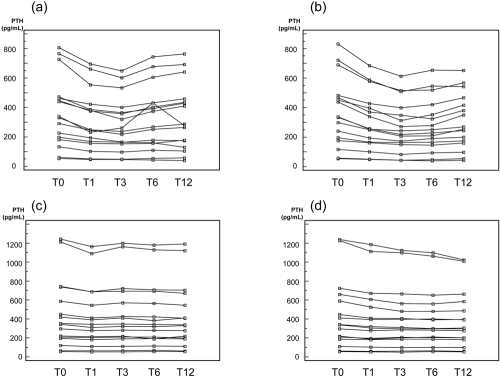
The absolute values obtained for Dry and EDTA samples at baseline and after 1, 3, 6, and 12 mo of storage at −80°C on Liaison and Elecsys are presented in Table 1 and Figure 1.
Table 1.
Absolute values obtained for Dry and EDTA samples at baseline and after 1, 3, 6, and 12 mo of storage at −80°C on Liaison and Elecsys
| Sample | Liaison |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry |
EDTA |
|||||||||
| Baseline | 1 mo | 3 mo | 6 mo | 12 mo | Baseline | 1 mo | 3 mo | 6 mo | 12 mo | |
| 1 | 226 | 194 | 165 | 160 | 130 | 240 | 194 | 172 | 191 | 199 |
| 2 | 807 | 695 | 648 | 743 | 764 | 831 | 685 | 613 | 653 | 652 |
| 3 | 766 | 661 | 602 | 677 | 691 | 690 | 578 | 516 | 519 | 568 |
| 4 | 331 | 248 | 238 | 267 | 287 | 334 | 256 | 217 | 229 | 245 |
| 5 | 133 | 103 | 97 | 109 | 105 | 116 | 100 | 82 | 93 | 97 |
| 6 | 728 | 553 | 534 | 605 | 642 | 723 | 587 | 509 | 546 | 543 |
| 7 | 473 | 387 | 364 | 391 | 430 | 468 | 370 | 349 | 324 | 380 |
| 8 | 448 | 375 | 358 | 403 | 435 | 438 | 340 | 272 | 279 | 351 |
| 9 | 291 | 246 | 218 | 251 | 264 | 299 | 249 | 206 | 208 | 253 |
| 10 | 440 | 381 | 320 | 373 | 409 | 451 | 397 | 313 | 352 | 416 |
| 11 | 339 | 231 | 261 | 292 | 277 | 337 | 254 | 243 | 249 | 269 |
| 12 | 198 | 168 | 163 | 178 | 174 | 195 | 165 | 163 | 166 | 174 |
| 13 | 461 | 423 | 400 | 431 | 458 | 483 | 427 | 399 | 421 | 467 |
| 14 | 182 | 157 | 152 | 157 | 177 | 176 | 162 | 149 | 145 | 160 |
| 15 | 53 | 46 | 46 | 44 | 39 | 53 | 48 | 43 | 40 | 43 |
| 16 | 61 | 51 | 49 | 54 | 58 | 57 | 51 | 44 | 47 | 54 |
| Sample | Elecsys |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry |
EDTA |
|||||||||
| Baseline | 1 mo | 3 mo | 6 mo | 12 mo | Baseline | 1 mo | 3 mo | 6 mo | 12 mo | |
| 1 | 220 | 213 | 217 | 189 | 217 | 218 | 188 | 202 | 209 | 202 |
| 2 | 1244 | 1162 | 1200 | 1179 | 1190 | 1239 | 1185 | 1122 | 1099 | 1023 |
| 3 | 738 | 687 | 693 | 694 | 671 | 659 | 606 | 562 | 558 | 585 |
| 4 | 343 | 309 | 322 | 319 | 330 | 337 | 304 | 296 | 295 | 297 |
| 5 | 119 | 109 | 111 | 114 | 111 | 110 | 102 | 98 | 100 | 99 |
| 6 | 1212 | 1090 | 1163 | 1130 | 1120 | 1226 | 1113 | 1098 | 1061 | 1009 |
| 7 | 744 | 688 | 720 | 707 | 703 | 723 | 672 | 665 | 652 | 662 |
| 8 | 586 | 545 | 569 | 564 | 546 | 590 | 524 | 480 | 482 | 487 |
| 9 | 352 | 344 | 346 | 341 | 338 | 340 | 319 | 311 | 299 | 305 |
| 10 | 449 | 412 | 426 | 423 | 408 | 446 | 409 | 406 | 391 | 395 |
| 11 | 297 | 276 | 282 | 280 | 277 | 296 | 277 | 285 | 283 | 277 |
| 12 | 209 | 203 | 209 | 209 | 199 | 209 | 196 | 207 | 205 | 200 |
| 13 | 420 | 392 | 411 | 384 | 411 | 412 | 395 | 397 | 399 | 391 |
| 14 | 196 | 182 | 190 | 193 | 191 | 188 | 182 | 187 | 187 | 181 |
| 15 | 63 | 63 | 64 | 69 | 61 | 61 | 60 | 61 | 64 | 59 |
| 16 | 56 | 54 | 54 | 57 | 56 | 55 | 53 | 50 | 55 | 54 |
Figure 1.
Plot of the absolute PTH values obtained for (A) [Liaison; Dry], (B) [Liaison; EDTA], (C) [Elecsys; Dry], and (D) [Elecsys; EDTA] at baseline (T0) and after 1, 3, 6, and 12 mo of storage at −80°C.
The concentration of PTH was modeled as a linear mixed model of factors “methods,” “tubes,” “subjects,” and “time” using the following model:
where Y is the measured PTH concentration (pg/ml), γ0,j is the random intercept, γ4,j is the random slope for time and the βi's (i = 1 to 3) are the coefficients of the factors “methods,” “tubes,” and “subjects, respectively. ε ∝ N(0;σε2 is the residual error assumed normally distributed, γ0,j ∝ N(0;σγ12 and γ4,j ∝ N(0;σγ24 are also assumed normally distributed and account for the variability of all of the j = 1 to 16 subjects.
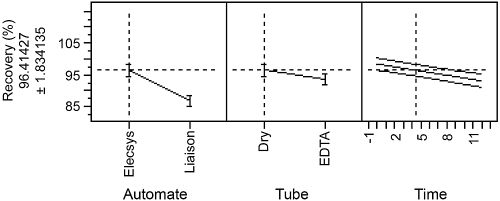
The model adjusted R2 equaled 0.9, suggesting a good adequacy of the model fitted: 90% of the observed variability of the PTH concentration is explained by the retained factors. Statistical evaluation of the coefficient of the factors showed that the type of method used to analyze the samples is highly significant (p-value <0.0001), as shown in Figure 2. Similarly, the type of tube used to collect the sample is also significant (p-value = 0.041; Figure 2). The time of storage showed a statistically (p-value = 0.002) decreasing slope of −2.06, as indicated in Figure 2.
Figure 2.
Effect of the factors “method,” “tube,” and “time” on PTH concentration.
Due to the significant effect of the factors “method” and “tube,” separate analysis for each pair of [“method”; “tube”] was performed to further assess the stability of PTH concentration over time using a linear mixed model. For each pair of [“method”; “tube”], time of storage has a significant effect over the concentration of PTH (p-value <0.05). The slope of the degradation was subject-dependent, and about 60% of the predicted slopes for each subject were found negative. This finding asserts a non-negligible degradation of PTH over time at −80°C, although not systematic for all biologic sources of samples.
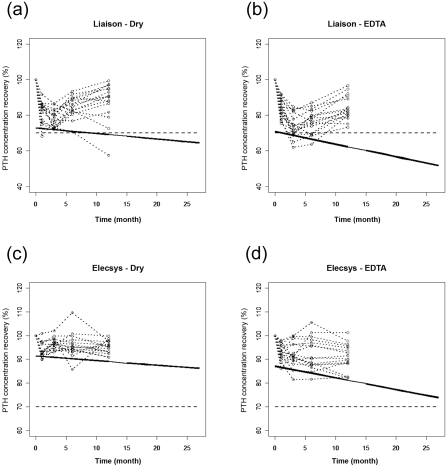
Although the average degradation slope is statistically significant, and knowing that it is variable from one patient to another, this degradation may have a practical implication. The question is what impact these slopes will have on the future results that will be obtained when thawing a single sample of any subject and analyzing it after 6, 12, or even 24 mo of storage. This question can be effectively answered through a graphical representation and using prediction interval methodology (10,11). Prediction intervals allow us to predict where a future result can be obtained with a defined probability. Figures 3A through D show the recovery of PTH concentration of each subject obtained during the storage of 16 patients' samples for each pair [“method”; “tube”]. Added to these figures are drawn their 95% lower prediction interval (bold continuous line). Thus, these prediction limits show where a single result of any possible future patient can be obtained at each storage time with 95% probability. It is thus evident from these figures that stable storage of these samples at −80°C is not infinite. There is indeed a maximum storage time that should be evaluated. Furthermore, this is a requirement for laboratories aiming at accreditation such as the International Organization for Standardization ISO 17025 (12) or ISO 15189 (13). One effective way to determine this storage time is to add to the graph acceptance limits as shown in Figures 3A through D. These acceptance limits represent the maximum degradation that is acceptable. The maximum storage time is defined when this acceptance limit crosses the lower prediction interval. Those acceptance limits could be, for example, the limits chosen for assessing the validity of the method, or two or three times the intermediate precision SD of the method. The acceptance limits shown in Figures 3A through D are settled by allowing a degradation of maximum −30%, i.e., a minimum recovery of 70% (dashed line) (14). The maximum storage times when using the Liaison method with either Dry tubes or EDTA ones depicted in Figures 3A and B are the shortest and are estimated at 9 and 2 mo, respectively. So, samples collected with EDTA tubes should not be stored more than 2 mo using this type of automate, as the degradation will exceed the maximum acceptable level of degradation of 30%. By opposite when using the Elecsys method, the maximum storage times are of at least 27 mo whatever the type of tube used, as shown in Figures 3C and D. When using the Elecsys automate, samples could be stored up to 2 yr, still with acceptable stability of the PTH. Indeed, the degradation will not exceed the maximum acceptable level of −30%. Furthermore, the statistical difference between the two methods has shown here the practical implication of such a lack of comparability on the stability of PTH.
Figure 3.
Prediction of the linear degradation of PTH concentration over time for each pair of [“method”;”tube”]: (A) [Liaison; Dry]. (B) [Liaison; EDTA]. (C) [Elecsys; Dry]. (D) [Elecsys; EDTA]. The circles and the dotted lines show the evolution of the PTH recovery with respect to time point “0 mo.” The bold continuous lines are the lower 95% prediction limits and the dashed lines are the lower acceptance limits settled at 70% of recovery.
Discussion
We presented here the first study on the storage of PTH at −80°C for a long period. We introduced an original method that allowed us to take into account different parameters, such as the method used to determine PTH, the kind of tube used for sampling, the length of storage, and the patient variability. We were thus able to build a model that explained 90% of the variability of PTH concentration. We also established a maximum storage time for the storage of the peptide using an original methodology. This maximum storage time is an important parameter that should be taken into account in studies where samples are kept for a long period at −80°C before processing. Indeed, we have shown that there was a systematic degradation of PTH and, moreover, that this degradation was patient-dependent. In other words, we will observe degradation in some patient samples, but not in others; it means that we will be obliged to be restrictive and use the lowest maximum storage time for the limit of storage. In this way, a correct interpretation of PTH results obtained in stored samples is possible in clinical studies.
Our results showed that PTH was more stable in serum than in EDTA plasma when samples were kept for a long period at −80°C. This can be surprising, as previous studies have shown that PTH was more stable in EDTA plasma when samples were kept at room temperature (15). However, if we agree with the fact that the degradation of PTH is more important at room temperature in Dry tubes, we have already shown that PTH was more stable in serum when samples were stored at −20°C (2).
Another striking point is the impact of the assay method used on the maximum storage time. Indeed, we observed a more important degradation with Liaison than with Elecsys. With the Liaison, the results decreased from 1 mo to 3 mo, and then seemed to rise at 6 mo and 12 mo. We have no clear explanation for such a phenomenon, but we know that it is not due to analytical problems, as the two leveled internal quality controls that were run before and after each series of samples were all comprised in the laboratory acceptation range. The coefficients of variation calculated on these quality controls were <7% and <3% for the level 1 (mean: 64 pg/ml) and level 2 (mean: 552 pg/ml), respectively. One explanation for the discrepancies observed between the two methods could be linked to the C-terminal fragments of PTH. Indeed, it is now well known that these fragments accumulate in the serum of hemodialyzed patients (16) and that they are more stable than PTH itself (17). The Liaison is one of the methods that cross-reacts at the lesser extent with PTH (7-84), the most representative fragment of the C-terminal fragments. This is particularly true, when compared with the Elecsys (5), which could let us think that the decrease observed with the Liaison, and not the Elecsys, could be due to a degradation of the PTH molecule itself.
Conclusion
Storage of PTH at −80°C is not infinite, but method and patient dependent. An evaluation of the maximum storage time should be performed to ascertain that the samples will present the same reactive profile after storage for a long period. Only in such a way will consistent decisions be made with the results obtained from the analysis of the stored samples.
Disclosures
None.
Acknowledgments
The authors are very grateful to the anonymous reviewers for providing important comments that led to significant improvements of this article. A research grant from the Belgium National Fund for Scientific Research (FRS-FNRS) to E. Rozet is gratefully acknowledged.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 2.Cavalier E, Delanaye P, Carlisi A, Krzesinski JM, Chapelle JP: Stability of intact parathyroid hormone in samples from hemodialysis patients. Kidney Int 72: 370–372, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Holmes DT, Levin A, Forer B, Rosenberg F: Preanalytical influences on DPC IMMULITE 2000 intact PTH assays of plasma and serum from dialysis patients. Clin Chem 51: 915–917, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Joly D, Drueke TB, Alberti C, Houillier P, Lawson-Body E, Martin KJ, Massart C, Moe SM, Monge M, Souberbielle JC: Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: A cross-sectional study. Am J Kidney Dis 51: 987–995, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, Massart C, Monge M, Myara J, Parent X, Plouvier E, Houillier P: Inter-method variability in PTH measurement: Implication for the care of CKD patients. Kidney Int 70: 345–350, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Melamed ML, Eustace JA, Plantinga LC, Jaar BG, Fink NE, Parekh RS, Coresh J, Yang Z, Cantor T, Powe NR: Third-generation parathyroid hormone assays and all-cause mortality in incident dialysis patients: The CHOICE study. Nephrol Dial Transplant 23: 1650–1658, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Lehmann G, Stein G, Huller M, Schemer R, Ramakrishnan K, Goodman WG: Specific measurement of PTH (1–84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int 68: 1206–1214, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Reichel H, Esser A, Roth HJ, Schmidt-Gayk H: Influence of PTH assay methodology on differential diagnosis of renal bone disease. Nephrol Dial Transplant 18: 759–768, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cavalier E, Delanaye P, Carlisi A, Krzesinski JM, Chapelle JP: Analytical validation of the new version of the Liaison N-Tact PTH assay. Clin Chem Lab Med 45: 105–107, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Lahiri P, Li H: Parametric bootstrap approximation to the distribution of EBLUP and related prediction intervals in linear mixed models. The Annals of Statistics 36: 1221–1245, 2008 [Google Scholar]
- 11.De Gryze S, Langhans I, Vandebroek M: Using the correct intervals for prediction: A tutorial on tolerance intervals for ordinary least-squares regression. Chemom Intell Lab Syst 87: 147–154, 2007 [Google Scholar]
- 12.International Organization for Standardization (ISO): ISO/CEI 17025: General requirements for the competence of testing and calibration laboratories, Geneva, 2005 [Google Scholar]
- 13.International Organization for Standardization (ISO): ISO/CEI 15189: Medical laboratories – Particular requirements for quality and competence, Geneva, 2007 [Google Scholar]
- 14.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R: Quantitative bioanalytical methods validation and implementation: Best practices for chromatographic and ligand binding assays. Pharm Res 24: 1962–1973, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Glendenning P, Laffer LL, Weber HK, Musk AA, Vasikaran SD: Parathyroid hormone is more stable in EDTA plasma than in serum. Clin Chem 48: 766–767, 2002 [PubMed] [Google Scholar]
- 16.Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, D'Amour P: A non-(1–84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44: 805–809, 1998 [PubMed] [Google Scholar]
- 17.D'Amour P, Brossard JH: Carboxyl-terminal parathyroid hormone fragments: Role in parathyroid hormone physiopathology. Curr Opin Nephrol Hypertens 14: 330–336, 2005 [DOI] [PubMed] [Google Scholar]