Abstract
Background and objectives: Preemptive transplantation is ideal for patients with advanced chronic kidney disease (CKD). The practice has been to perform coronary angiography (CA) on all patients aged >50, all diabetics, and all patients with cardiac symptoms or disease with a view to revascularization before transplantation. Historically patients have delayed CA until established on renal replacement therapy due to concerns of precipitating the need for chronic dialysis. The objectives of this study were to establish the risk of contrast nephropathy in patients with advanced CKD who undergo screening CA, and to determine whether or not preemptive transplantation is achievable.
Design and setting: This retrospective analysis included 482 patients with stage IV/V CKD seen in West London predialysis clinics from 2004 to 2007. Seventy-six of 482 (15.8%) patients considered as potential transplant recipients met the authors' criteria for coronary angiography. Modification of Diet in Renal Disease (MDRD) GFR measurements were recorded for the 12 mo preceding and 12 mo following CA unless a defined endpoint was reached (transplantation, dialysis, or death).
Results: Mean MDRD GFR at CA was 12.51 ± 3.51 ml/min. The trend was not significantly different 6 mo pre- and postangiography. Cumulative dialysis-free survival was 89.1% 6 mo postangiography. Twenty-three of 76 (30.3%) patients had flow-limiting coronary artery disease. Twenty-five of 76 (32.9%) patients underwent transplantation with 22 of 25 (88.0%) transplants being performed preemptively.
Conclusions: The data suggest CA screening does not accelerate the decline in renal function for patients with advanced CKD, facilitating a safe preemptive transplant program.
It is well established that chronic kidney disease (CKD) is associated with premature atherosclerosis and results in an increased risk of cardiovascular morbidity and mortality (1). For patients with CKD, transplantation offers a greater survival advantage over other forms of renal replacement therapy (2). To minimize perioperative cardiac events, and reduce long-term cardiac morbidity, our practice has been to perform coronary angiography with a view to revascularization of significant coronary artery disease (CAD) before transplantation in all potential transplant recipients with one or more of the following criteria: age >50 yr, type 1 or type 2 diabetes, cardiac symptoms or disease irrespective of age, or an abnormal electrocardiogram other than left ventricular hypertrophy. Historically, there has been a tendency to delay coronary angiography in transplant recipients until they have been established on renal replacement therapy due to the concerns that contrast induced nephropathy (CIN) and cholesterol embolization syndrome may precipitate the need for chronic dialysis.
The reported incidence of CIN following coronary angiography ranges from 3.3% to 37% (3–5), with pre-existing renal disease being the greatest independent predictor. The severity of renal dysfunction measured by serum creatinine directly correlates with the incidence of CIN (6). The greatest concern for the patient is the need for dialysis treatment in the setting of CIN, which in one study has been reported to occur in 7.1% patients in the short term, and 0.9% in the long term (7). Acute dialysis requirement following percutaneous intervention is associated with a marked increase in hospital mortality (8). The renal dysfunction associated with CIN peaks at 3 to 5 d following the administration of contrast and improves in 1 to 3 wk (9). In those patients in whom renal insufficiency persists >3 wk, cholesterol embolization syndrome may be a contributory factor.
It is possible that the safety profile of coronary angiography has improved in the modern era, with the use of adequate hydration, N-Acetylcysteine, smaller volumes of nonionic contrasts, biplane imaging techniques, staged procedures in cases requiring intervention, and careful postangiography monitoring (10). If this is the case, potential transplant recipients should not be put off coronary angiography until established on renal replacement therapy, as this only heightens their cardiovascular burden, which may be halted or slowed down by successful kidney transplantation (11).
The purpose of this study was to establish the risk of contrast nephropathy to patients with advanced CKD (stages IV or V) who undergo elective and timely screening coronary angiography, and to determine whether or not preemptive transplantation is achievable.
Materials and Methods
Patient Population
This retrospective analysis included all predialysis patients seen in eight West London clinics between October 2004 and August 2007, n = 482. Seventy-six potential transplant recipients met the criteria for proceeding to coronary angiogram, which was performed at two hospital sites within the same institution. Patients on dialysis undergoing cardiac assessment before transplantation were excluded. All patients were seen by a consultant cardiologist before angiography and the risks of the procedure, including CIN, were explained. More than one cardiologist was involved, but practice was consistent according to local protocol and national guidelines.
Reno-protection
Reno-protection in the form of adequate hydration, 0.9% saline 500mls at least 2 h before and 2 h after the procedure, and oral N-Acetylcysteine 600mg twice daily at day −1, 0, and + 1 was administered in all cases. All potentially nephrotoxic drugs were withheld 24 h before the angiogram, and recommenced 48 h later, if not contraindicated. Seventy-four of 76 (97.4%) patients received Visipaque® contrast media, the remaining two of 76 (2.6%) received Omnipaque® contrast media.
Statistical Analyses
Simplified Modification of Diet in Renal Disease (MDRD) GFR results were generated from serum creatinine at time points pre- and postcoronary angiography and slopes were derived from mean values. Serum creatinine measurements were made on each visit to the clinic; frequency of visits varied between 2 to 12 wk depending on patient's symptoms and the rate of progression of his or her CKD. Values were recorded if within 28 d of 12, 9, 6, and 3 mo before and after the date of the angiogram; if out with 28 d, no value was entered for analysis. By nature of this retrospective analysis of real-life care for patients who were not in a trial protocol, there were missing data points as a result of nonattendance in clinic at appropriate time point. For −12, −9, −6, −3, 0, +3, +6, +9, +12 mo points data were absent in 17 of 76, ten of 76, five of 76, two of 76, 0 of 76, four of 68, five of 56, five of 40, and six of 38 patients at risk, respectively. No values were ascribed in these instances. Patients were also advised to have their serum creatinine checked once between 3 to 7 d after their angiogram; seven of 76 patients failed to attend for this investigation.
Dialysis- and transplant-free survival was analyzed using the Kaplan Meier analysis performed using SPSS Version 16.0. Dialysis-free survival is censored for transplantation or death. All numeric values are expressed as mean ± 1 SD unless stated otherwise. MDRD GFR pre- and postangiography and contrast volumes in the setting of biplane versus monoplane angiography were analyzed using the paired t test.
Definitions
A history of myocardial infarction (MI) was defined as present if the patient had electrocardiogram evidence of ST-elevation, MI previously, or if the patient had a documented acute presentation with cardiac symptoms, with associated troponin rise according to the reference range in the biochemistry laboratory at that time. A history of smoking was defined as present if the patient was a current smoker or if the patient was an exsmoker of less than 5 yr. Flow-limiting coronary artery disease was defined as >50% stenosis of one or more dominant coronary vessels. Any atheromatous lesion <50% not requiring intervention was defined as nonflow limiting. Endpoints included remaining predialysis, undergoing transplantation, commencing dialysis, and death.
Results
Demographics
The clinical and demographic characteristics of the patients who met the criteria for proceeding to coronary angiography are outlined in Table 1. The male:female ratio was approximately 3:2, with a mean age of 56.33 ± 9.59 yr. The population was predominantly Caucasian, although 17 of 76 (22.4%) were Asian, reflecting the diverse ethnicity of the population in West London. Thirty-three of 76 (43.4%) of the patients were diabetic, with diabetic nephropathy being the most common cause of CKD in this group of patients. Only two of 76 (2.6%) had a history of previous MI. Twenty-three of 76 (30.3%) patients had flow-limiting CAD, 29 of 79 (38.2%) patients had nonflow limiting CAD, and 24 of 76 (31.5%) patients had normal coronary arteries. All patients were followed up for 12 mo following angiography, unless they reached a defined endpoint resulting in a mean follow up of 8.71 ± 4.0 mo.
Table 1.
The clinical and demographic characteristics of the patients
| Demographics | n = 76 |
|---|---|
| Male gender | 49 of 76 (64.5%) |
| Female gender | 27 of 76 (35.5%) |
| Mean age (years) | 56.33 ± 9.59 |
| Caucasian | 52 of 76 (68.4%) |
| Asian | 17 of 76 (22.4%) |
| Other ethnicity | 7 of 76 (9.2%) |
| Diabetes | 33 of 76 (43.4%) |
| Diabetic nephropathy | 28 of 76 (36.8%) |
| Mean SBP (mmHg) | 142 ± 15 |
| Mean DBP (mmHg) | 77 ± 12 |
| Mean total cholesterol (mmol/l) | 3.92 ± 0.99 |
| History of smoking | 17 of 76 (22.4%) |
| History of previous MI | 2 of 76 (2.6%) |
| Flow-limiting coronary artery disease | 23 of 76 (30.3%) |
| Nonlimiting coronary artery disease | 53 of 76 (69.3%) |
| Mean follow up (months) | 8.71 ± 4.0 |
MI, myocardial infarction; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Treatment
Sixty-five of 76 (85.5%) patients received some form of lipid-lowering therapy; the majority of patients received statin monotherapy (80.3%). One of 76 (1.3%) patients was enrolled in the SHARP trial, and was blinded to treatment with either statin + ezetimibe, or statin alone. Thirty of 76 (39.5%) of patients had monoplane angiography, with a mean contrast dose of 69.1mls, and 46 of 76 (60.5%) patients had biplane angiography, with a mean contrast dose of 47.8mls (t test, P = 0.07). All 23 patients with flow-limiting disease underwent revascularization; 10 of 23 (43.5%) patients required coronary artery bypass grafting and 13 of 23 (56.5%) required percutaneous intervention. Eleven of 13 (84.6%) patients undergoing percutaneous revascularization had a staged procedure. The two patients that did not undergo staged procedures received a moderate contrast volume, between 60 to 80mls. Table 2 summarizes the treatment details.
Table 2.
The treatment details
| Treatment | Details | n = 76 |
|---|---|---|
| Lipid-lowering therapy | Statin | 61 of 76 (80.3%) |
| Fibrate | one of 76 (1.3%) | |
| Ezetimibe | two of 76 (2.6%) | |
| SHARP trial | one of 76 (1.3%) | |
| Total | 65 of 76 (85.5%) | |
| Angiography | Monoplane | 30 of 76 (39.5%) |
| Biplane | 46 of 76 (60.5%) | |
| Mean volume of contrast (ml) | 55.74 ± 50.18 | |
| Revascularization | Percutaneous (Unstaged) | 2 of 76 (2.6%) |
| Percutaneous (Staged) | 11 of 76 (%) | |
| Surgical (CABG) | 10 of 76 (13.2%) | |
| Total | 23 of 76 (30.3%) |
CABG, coronary artery by-pass graft.
eGFR Pre- and Postangiography
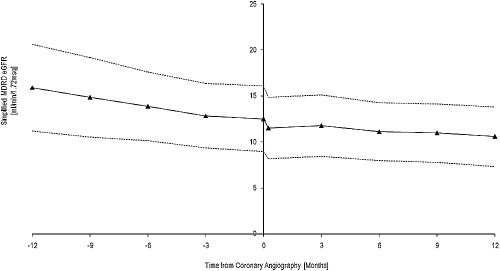
Mean MDRD GFR at angiography was 12.46 ± 3.41ml/min. Figure 1 demonstrates the trend in MDRD GFR, which was not significantly different 6 mo pre- and postangiography (slope change in MDRD GFR −0.21 ± 0.27 versus −0.21 ± 0.30 ml/min/mo [t test, P = 0.42]). It also demonstrates the temporary decline in MDRD eGFR following administration of contrast media. The use of statins did not significantly impact the degree of rise in serum creatinine at 1 wk postangiography (t test, P = 0.51).
Figure 1.
Modification of Diet in Renal Disease (MDRD) eGFR before and after coronary angiography.
Contrast Volumes
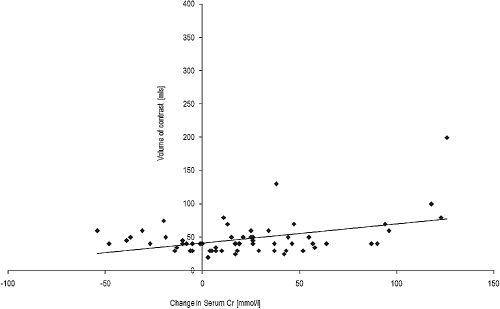
The mean contrast volume administered was 55.74 ± 50.18mls. Figure 2 demonstrates the relationship between contrast volume and difference in serum creatinine from baseline up to 1 wk after contrast. There was a significant relationship between increasing contrast volume and rise in creatinine at 1 wk (R = 0.423). Unconventionally, one patient received a large 400ml volume of contrast for an aortogram due to concern about a possible aortic dissection, and this patient has not been included in this subanalysis. This patient had a significant decline in renal function, but despite a period of anuria was managed without the need for dialysis. In a number of patients (n = 18) an improvement in serum creatinine was noted at 1 wk postcontrast, which may be explained by the reno-protection protocol, which included prehydration and withholding of potentially nephrotoxic drugs. This improvement was not sustained and the slope of GFR in these patients reverted to the precontrast slope in the proceeding months after angiography.
Figure 2.
Change in serum creatinine up to 1 wk after contrast administration.
Biplane angiography was used in 46 of 76 (60.5%) patients and was associated with a reduction in contrast volume, although not statistically significant (t test, P = 0.07). If CIN is defined as a rise in serum creatinine >10% above baseline within 1 wk of contrast administration, only six of 17 (35.3%) patients had contrast nephropathy with the smallest dose of contrast (20 to 39ml), compared with four of four (100%) patients with the highest doses of contrast (>100mls). Overall, 18 of 69 patients had CIN, of which four of 18 (22.2%) patients with a mean MDRD GFR of 12.05 ± 1.75 ml/min started dialysis during the 12 mo follow up; patients without CIN had a similar outcome with eight of 51 (15.7%) patients with a mean MDRD GFR of 10.34 ± 2.07ml/min started on dialysis. Those patients who remained dialysis independent had a better baseline MDRD GFR 13.35 ± 3.39 ml/min in the group who got CIN, and 14.03 ± 3.85 ml/min in the group that did not get CIN.
Dialysis-Free Survival
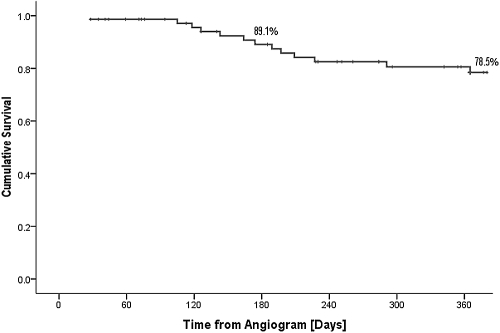
Thirteen of 76 (17.1%) patients started dialysis during the follow-up period. No patients required dialysis treatment immediately postcontrast administration, and only one patient commenced dialysis within 3 mo of undergoing coronary angiography. Figure 3 demonstrates cumulative dialysis-free survival, which was 89.1% at 6 mo postcoronary angiography. Forty of 76 (53.3%) patients remained dialysis independent and one of 76 (1.3%) patients died from trauma awaiting transplantation. More diabetic patients started dialysis within the period of study, but cumulative dialysis-free survival following angiography to 12 mo was not statistically different in diabetics versus nondiabetics (Logrank P = 0.09).
Figure 3.
Kaplan-Meier analysis demonstrating cumulative dialysis free survival.
Time to Transplantation
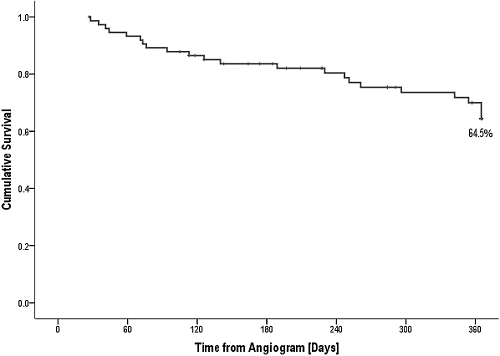
Of the 76 patients that had coronary angiography, 25 of 76 (32.9%) patients underwent transplantation, with 22 of 25 (88.0%) transplants being performed before the need for dialysis. Of these patients receiving preemptive transplantation, 18 of 22 received live donor transplants, two of 22 received a deceased donor kidney transplant, and two of 22 received a simultaneous pancreas kidney transplant. Nine of 22 patients were transplanted within 3 mo of coronary angiography. The renal function for these preemptively transplanted patients before they exited the study as a result of transplantation was not declining more or less abruptly than the rest of the cohort. Figure 4 demonstrates cumulative patient survival without transplantation, which was 64.5% at 12 mo.
Figure 4.
Kaplan-Meier analysis demonstrating time to transplantation.
Discussion
Our data suggest that coronary angiography did not accelerate decline in renal function for patients with advanced CKD. A transient decline in GFR was demonstrated in the first week following coronary angiography but this was entirely reversible. This paper is of relevance to all clinicians involved with patients requiring cardiac catheterization in the setting of advanced CKD. If the procedure is performed appropriately with small volumes of contrast, biplane angiography using N-Acetylcysteine, and adequate hydration around the time of the procedure, then the risk of contrast exposure can be minimized in this population.
Significant flow-limiting CAD was identified in just under a third of patients, and just over a third of patients had nonflow-limiting disease. Only 31.5% of patients (24 of 76) had normal coronary angiograms. Listing for transplantation was facilitated, 25 of 76 (32.9%) of screened patients underwent transplantation within 12 mo of coronary angiography; the large majority were transplanted preemptively. Our overall rates for preemptive transplantation are currently above the national average, 17% versus 14% (12). Of the 76 patients screened, the first patient to commence dialysis did so at 3 mo postcoronary angiography; this patient did not have a potential live donor. Nine of 22 (40.9%) preemptive transplants were performed within 3 mo of coronary angiography, which highlights how it is possible to complete workup of the donor and recipient within 3 mo.
We report a cumulative dialysis-free survival of 89.1% at 6 mo postcoronary angiography. This may be attributed in part to the identification of strategies that minimize contrast nephropathy. As this was censored for transplantation, it is not possible to know if the 22 patients preemptively transplanted would have required dialysis before the end of the follow-up period. We carried out staged procedures to reduce the volume of contrast administered in a single sitting particularly for those with complex and widespread lesions, an approach used by many operators in cardiac catheter laboratories (13). The use of continuous veno-venous hemofiltration, initiated before exposure to contrast and continued for 18 to 24 h after the procedure, has been reported to result in a significant reduction in the degree of renal injury in a cohort of patients known to be at considerable risk (mean baseline creatinine 265.2 μmol/L) (14). This approach obviously has significant implications in terms of resources, and was not a strategy we utilized.
The mechanisms responsible for worsening of renal function were not formally identified in this study design. CIN results from a combination of pathophysiologic processes. Hypertonic contrast media causes the production of oxygen-free radicals leading to apoptosis in renal tubular cells (15). Aside from direct tubular injury, contrast media also has effects on the metabolism of certain vasoconstrictors including adenosine and endothelin, causing disturbances to renal hemodynamics (16). We have demonstrated that the dose-dependent association of volume of contrast administered with CIN and contrast volumes can to some extent be controlled. Recent reports have suggested that the use of biplane angiography is associated with a significant reduction in contrast volumes (17). In this study, biplane angiography was used in a proportion of patients and was associated with a reduction in contrast volume, although this did not reach statistical significance.
Cholesterol embolization syndrome occurs when disruption to a vascular plaque releases cholesterol crystals into the bloodstream, resulting in localized inflammation and fibrosis, most commonly seen in the skin, digits, kidneys, and eyes and often persisting for weeks. The initial event may be either due to spontaneous plaque rupture, or as a secondary phenomenon following the administration of thrombolytics or following vascular endothelial trauma (18). One prospective study evaluating patients undergoing coronary angiography found that no patients had dermatological manifestations of cholesterol embolization syndrome, but a persistently elevated creatinine at 3 wk was reported to occur in 2% of patients studied, which was attributed to cholesterol embolization to the kidneys (19).
Many of the recent studies investigating the effect of individual preventive measures against contrast nephropathy have included data from patients with less advanced CKD, whereas this study has looked at utilizing a combination of preventive measures in patients with advanced CKD; the average serum creatinine on the day of angiogram was 371.81 ± 88.79 mmol/L in this study. In one study that used hemofiltration around the time of contrast exposure, patients had a mean creatinine of 265.2 ± 88.4mmol/L (14). In one study that assessed how low volumes of contrast affected renal function after exposure, patients had a mean creatinine of 184.4mmol/L (17). In a recent study into the type of contrast media used during angiography and its association with contrast nephropathy, the mean serum creatinine was 255.2 ± 132 mmol/L (20), and finally in a randomized control trial using N-Acetylcysteine to reduce CIN, the mean serum creatinine was 199.76 ± 47.52 mmol/L (4).
Recognition and management of cardiovascular risk factors should begin earlier than the pretransplant screening stages and should remain an integral part of long-term care. Sixty-five of 76 (85.5%) patients in this study were already prescribed lipid-lowering therapy. There has been some interest in the literature concerning the potential protective benefits of high-dose short-term statin use. Statins appear to have pleiotropic effects, including antioxidant properties (15), and oxidative stress has been suggested as one of the possible mechanisms responsible for CIN. Unfortunately, no significant difference in renal function 48 h postcontrast was demonstrated in a randomized control study comparing patients treated with a total of 160mg simvastatin doses over 36 h versus no simvastatin (21) In this study, the use of standard-dose statins did not significantly impact the degree of rise in serum creatinine at 1 wk postangiography (t test, P = 0.51).
International guidelines recommend aggressive intervention in patients with renal disease and acute coronary syndromes (22). In spite of this, there remains a reluctance to undertake angiography and angioplasty in this population because of a lack of evidence base for managing CAD in patients with CKD and without symptoms. There is little evidence within the general population without renal failure that intervention on asymptomatic nondiabetic patients results in improved survival (23). This significantly impacts the cardiac assessment of potential renal transplant recipients and how to manage coronary artery lesions that are identified. A recent prospective study in a Scottish CKD population challenged the current practice of coronary angiographic screening (24). The authors reported no direct evidence of patient benefit from cardiac screening, and in fact suggested that it may serve as a barrier to being placed on a waiting list.
We know significant numbers of our potential transplant patients have premature CAD, which continues to burden them after transplantation, particularly in the immediate postoperative period. Our cardiac assessment and intervention protocol during the pretransplant period may be considered aggressive, but our aim is to have fully optimized our patients' cardiac status in preparation for transplantation. We advocate coronary angiography to assess cardiac status once the donor and recipient pair are assessed suitable by all other noncardiac criteria. We hypothesize that coronary angiography and revascularization before transplantation for high-risk patients is essential for reducing short- to medium-term cardiac morbidity and mortality and are examining this hypothesis prospectively.
Limitations
This study was retrospective and not controlled, but represents a unique and large cohort of predialysis patients screened in a consistent fashion. Data on dialysis-free survival is uncontroversial; however, by nature of its design, there were a minor number of missing data for serum creatinine in patients at risk at each time point. These have been clearly outlined in the Results section and must be taken into account. Data were not systematically missing for any one individual. It is acknowledged that 38 of 76 patients received either a transplant or dialysis treatment within the 12 mo follow-up period after screening and, as a result, could not contribute data points postcoronary angiography. While censoring for these patients lessens the numbers at risk for analysis after angiography, there is no reason to suspect that these patients were more likely to suffer accelerated decline in renal function, and bias as a result seems unlikely. Pragmatically, by virtue of their coronary angiography, 22 of 38 of these patients had preemptive transplantation, the most ideal treatment for their already advanced renal disease.
As a result of this study we have been able to draw some useful conclusions that may influence potential transplant-recipient screening practice within transplant units. Coronary angiographic screening did not accelerate the decline in renal function for patients with advanced CKD, which has enabled the facilitation of a safe and prompt preemptive transplantation program.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Herzog C: How to manage the renal patient with coronary heart disease: The agony and the ecstasy of opinion-based medicine. J Am Soc Nephrol 14: 2556–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Fung JW, Szeto CC, Chan WW, Kum LC, Chan AK, Wong JT, Wu EB, Yip GW, Chan JY, Cheuk YU, Woo KS, Sanderson JE: Effect of N-acetylcysteine for prevention of contrast nephropathy in patients with moderate to severe renal insufficiency: A randomised trial. Am J Kidney Dis 43: 801–808, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Garovic VD: Contrast nephropathy after coronary angiography. Mayo Clin Proc 79: 211–219, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern EF, Hill JA, Winniford M, Cohen MB, VanFossen DB: Nephrotoxicity of ionic and non-ionic contrast media in 1196 patients: A randomised trial. Kidney Int 47: 254–261, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Pichard AD, Satler LF, Leon MB: The prognostic implications of further renal function deterioration within 48 hours of interval coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 36: 1542–1548, 2000 [DOI] [PubMed] [Google Scholar]
- 8.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW: Acute renal failure after coronary intervention: Incidence, risk factors and relationship to mortality. Am J Med 103: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Berns AS: Nephrotoxicity of contrast media. Kidney Int 36: 730–740, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Pucelikova T, Dangas G, Mehran R: Contrast-induced nephropathy. Catheter Cardiovasc Interv 71: 62–72, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Meier-Kriesche H, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplantation 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Statistics and Audit Directorate UK Transplant. Transplant Activity in the UK 2006–2007:18 [Google Scholar]
- 13.Hirshfield J: Invasive cardiovascular procedures — Optimizing patient safety. New En Journ Med 349:1311–1312, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Marenzi G, Marana I, Lauri G, Assanelli E, Grazi M, Campodonico J, Trabattoni D, Fabbiocchi F, Montorsi P, Bartorelli L: The Prevention of radio contrast-agent–Induced nephropathy by hemofiltration. N Engl J Med 349:1333–1340, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Katholi RE, Wood WT, Taylor GJ, Dietrick CL, Womack KA, Katholi CR, McCann WP: Oxygen free radicals and contrast nephropathy. Am J Kidney Dis 32: 64–71, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Russo D, Minutolo R, Cianciaruso B, Memoli B, Conte G, De Nicola L: Early effects of contrast media on renal hemodynamics and tubular function in chronic renal failure. J Am Soc Nephrol 6: 1451–1458, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Kane G, Doyle B, Lerman A, Barness G, Best P, Rihal C: Ultra-low contrast volumes reduce rates of contrast induced nephropathy in patients with chronic kidney disease undergoing coronary angiography. Journ Am Coll of Cardiol 51: 89–90, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Bashore TM, Gehrig T: Cholesterol emboli after invasive cardiac procedures. J Am Coll Cardiol 42: 217–218, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Saklayen MG, Gupta S, Suryaprasad A, Azmeh W: Incidence of atheroembolic renal failure after coronary angiography. A prospective study. Angiology 48: 609–613, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Tadros GM, Malik JA, Manske CL, Kasiske BL, Dickinson SE, Herzog CA, Wilson RF, Das G, Panetta CJ: Iso-osmolar radiocontrast Iodixanol in patients with chronic kidney disease. J Invasive Cardiol 4: 499e1–499e8, 2005 [PubMed] [Google Scholar]
- 21.Jo S, Koo B, Park J, Kang H, Cho Y, Kim Y, Youn TJ, Chung WY, Chae IH, Choi DJ, Sohn DW, Oh BH, Park YB, Choi YS, Kim HS: Prevention of radio contrast medium-induced nephropathy using short term high dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial – A randomized control study. Am Heart J 155: 499e1–499e8, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Kristensen SD, Widimsky P, McGregor K, Sechtem U, Tendera M, Hellemans I, Gomez JL, Silber S, Funck-Brentano C, Kristensen SD, Andreotti F, Benzer W, Bertrand M, Betriu A, De Caterina R, DeSutter J, Falk V, Ortiz AF, Gitt A, Hasin Y, Huber K, Kornowski R, Lopez-Sendon J, Morais J, Nordrehaug JE, Silber S, Steg PG, Thygesen K, Tubaro M, Turpie AG, Verheugt F, Windecker S; ESC Committee for Practice Guidelines (CPG): Guidelines for the diagnosis, treatment of non-ST-segment elevation acute coronary syndromes: The task force for the diagnosis, treatment of non-ST-segment elevation acute coronary syndromes of the European society of cardiology. Eur Heart J: 28:1598–1660, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Boden WE, O'Rouke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini J, Weintraub WS.for the COURAGE Trial Research Group: Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356: 1503–1516, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Patel RK, Mark PB, Johnston N, McGeoch R, Lindsay M, Kingsmore DB, Dargie HJ, Jardine AG: Prognostic value of cardiovascular screening in potential renal transplant recipients: A single-centre prospective observational study. Am J Transplant 8: 1673–1683, 2008 [DOI] [PubMed] [Google Scholar]