Abstract
Background and objectives: A secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) trial suggested that sevelamer reduced hospitalizations relative to calcium-based phosphate binders. However, whether changed medical costs associated with reduced hospitalizations or other medical services offset the higher cost of sevelamer is unclear. This DCOR secondary analysis aimed to (1) evaluate Medicare total, inpatient, outpatient, skilled nursing facility, and other costs in sevelamer-treated versus calcium-treated patients; (2) examine Medicare costs in specific categories to determine cost drivers; and (3) estimate and incorporate sevelamer and calcium binder costs.
Design, setting, participants, & measurements: DCOR trial participants were linked to the Centers for Medicare & Medicaid Services ESRD database. Medicare costs for 1895 dosed Medicare-primary-payer participants were evaluated. Phosphate binder costs were incorporated. Costs were indexed to 2001 (study base year). Sensitivity analyses were performed with randomized participants, two follow-up periods, and 2004 as index year.
Results: Inflation-adjusted Medicare per member per month (PMPM) costs were lower for sevelamer-treated than for calcium-treated participants by a mean differential of $199 PMPM (mean, $5236 versus $5435; median, $4653 versus $4933), mainly because of lower inpatient costs for the sevelamer group (mean, $1461 versus $1644; median, $909 versus $1144). However, after phosphate binder costs were incorporated, costs trended lower for calcium-treated than for sevelamer-treated patients (differential −$81, 95% confidence interval −$321 to $157 PMPM, using average wholesale price; −$25, −$256 to $213 PMPM, using wholesale acquisition cost).
Conclusions: Sevelamer reduced inpatient Medicare costs compared with calcium binders. However, when binder costs were added, overall PMPM costs favored calcium-treated over sevelamer-treated participants.
Phosphate-binding therapy is considered integral to the management of hyperphosphatemia in hemodialysis patients. Current National Kidney Foundation Kidney Disease Outcomes Quality Initiative clinical practice guidelines for bone metabolism and disease in chronic kidney disease indicate that calcium-based and non–calcium-, non–aluminum-, non–magnesium-containing phosphate binders (such as sevelamer) are effective and can be used as primary therapy (1). However, concerns about soft-tissue calcification with use of calcium-based binders and a small observational study suggesting that sevelamer may lead to reduced hospitalizations relative to calcium-based binders led to a large-scale randomized trial (Dialysis Clinical Outcomes Revisited [DCOR]) comparing sevelamer with calcium-based binders in Medicare-covered hemodialysis patients (2–4). Results showed no mortality difference in the overall population but significantly reduced hospitalization rates (10%) and days (12%) with sevelamer treatment (5,6). However, whether changed medical costs associated with reduced hospitalizations or other medical services offset the higher cost of sevelamer compared with calcium binders is unclear.
A recent economic study concluded that hospitalization risk would have to be reduced by 30% to offset the additional costs of sevelamer, assuming no difference in mortality (7). However, many cost inputs were estimated because actual costs were unavailable. This study reports methods and secondary economic analysis of the DCOR trial in which Medicare costs for study participants could be directly evaluated. The objectives were to (1) evaluate Medicare total, inpatient, outpatient, skilled nursing facility, and other costs for sevelamer versus calcium-treated patients; (2) examine Medicare costs in specific health care categories to determine cost drivers; and (3) estimate and incorporate sevelamer and calcium binder costs.
Materials and Methods
Perspective
This study is framed from the perspective of the U.S. Medicare system.
Study Design, Population, Data Sources, and Patient Characteristics
The DCOR trial was a multicenter, randomized, open-label, parallel-design trial. Participants (n = 2103) were enrolled from March 2001 through January 2002 and randomly assigned to sevelamer (Renagel; Genzyme, Cambridge, MA; n = 1053) or calcium-based phosphate binders (calcium acetate [PhosLo; Braintree Laboratories, Braintree, MA] or calcium carbonate [TUMS; GlaxoSmithKline, Philadelphia, PA]; n = 1050). Included participants were adults receiving hemodialysis therapy with Medicare as the primary payer. The trial was completed at the end of 2004. Case report form data from 2101 of 2103 participants were linked to the Centers for Medicare & Medicaid Services ESRD database; of 2101 participants, 1947 (92.7%) met the Medicare-as-primary-payer criterion regarding dialysis claims (at least $675 in dialysis costs in a month for at least 90% of the follow-up months), so health care service information and costs could be collected. Of 1947 participants randomized, 1895 (97.3%) received a study medication. Detailed methodologic information about the DCOR clinical trial and secondary analysis data sources, patient characteristics, and methods for mortality, morbidity, and hospitalization is reported elsewhere (4–6).
Follow-Up
Dosed participants were followed from the initial prescription date to the earliest date of death, kidney transplant, modality change to peritoneal dialysis, early site closure, or early termination plus 90 d. In separate sensitivity analyses, we also evaluated randomized participants followed from randomization date to these events and through the end of the study (intent-to-treat).
Outcome
The outcome was medical costs (Medicare health care costs plus phosphate binder costs). Medicare costs were defined by Medicare allowable amounts on submitted claims during the follow-up period. Per member per month (PMPM) costs were calculated for total, inpatient, outpatient, skilled nursing facility, and other medical claims. Each category of cost includes institutional and physician costs.
An actuarial model was used to further categorize health care costs for a better understanding of which costs drove differences between groups. To classify costs, institutional revenue codes, diagnosis-related groupings, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes were used. Medicare allowable amounts were used to calculate costs in each category.
Phosphate binder costs were estimated because Medicare did not cover most outpatient oral medications before Medicare Part D went into effect in January 2006. A mean dose per day was calculated for each participant based on doses at study start and study end. Medi-Span pricing data (average wholesale price [AWP] and wholesale acquisition cost [WAC]) were obtained for Renagel 800 mg, Phoslo 667 mg, TUMS 500 mg, and TUMS 1000 mg (Table 1) (8). AWP is a wholesaler-to-pharmacy list price that drug-price databases publish for use in reimbursement; WAC represents the manufacturer's list invoice price for a drug product to wholesalers. Neither AWP nor WAC includes discounts or rebates, nor do they represent the actual price that patients pay for a prescription. During the study time frame, AWP and WAC were used by state Medicaid programs and Medicare to reimburse pharmacies or hospitals for medications (AWP minus x% or WAC plus x% plus a dispensing fee). Thus, AWP and WAC represent a reasonable range of values for analyses of expected Medicare-related costs. The mean monthly cost for each participant was calculated based on the mean daily dose and AWP and WAC for each product. The sevelamer WAC was not available for 2001. We estimated 2001 WAC based on the percentage change in sevelamer AWP from 2001 to 2003 and sevelamer WAC in 2003.
Table 1.
Price of sevelamer hydrochloride and calcium-containing phosphate binders: 2001 to 2009a
| Medication |
Price Measure ($) | Year (as of July 1 of Each Year) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brand Name | Generic Name | Strength | Manufacturer | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| Renagel | Sevelamer | 800 mg | Genzyme | AWP | 1.16 | 1.23 | 1.37 | 1.48 | 1.55 | 1.69 | 2.13 | 2.13 | 2.66 |
| WAC | NP | NP | 1.10 | 1.18 | 1.24 | 1.35 | 1.71 | 1.71 | 2.13 | ||||
| TUMS | Calcium carbonate | 500 mg | GlaxoSmithKline | AWP | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| WAC | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | ||||
| TUMS Ultra | Calcium carbonate | 1000 mg | GlaxoSmithKline | AWP | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| WAC | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | ||||
| PhosLo | Calcium acetate | 667 mg | Braintree Laboratories, Fresenius Medical Care | AWP | 0.15 | 0.15 | 0.19 | 0.21 | 0.34 | 0.34 | 0.47 | 0.88 | 0.88 |
| WAC | 0.12 | 0.12 | 0.15 | NP | 0.27 | 0.27 | 0.38 | 0.70 | 0.70 | ||||
| Generic | Calcium acetate | 667 mg | Roxane | AWP | NM | NM | NM | NM | NM | NM | NM | NMb | 0.79 |
| WAC | NM | NM | NM | NM | NM | NM | NM | NMb | NP | ||||
| Eliphos | Calcium acetate | 667 mg | Cypress (Hawthorn) | AWP | NM | NM | NM | NM | NM | NM | NM | NMc | 0.56 |
| WAC | NM | NM | NM | NM | NM | NM | NM | NMc | 0.45 | ||||
NP, product was on the market but the price was not published in MediSpan's PriceChek PC; NM, product was not on the market.
Price as of July 1 in the year indicated. Data are from MediSpan's PriceChek PC (Indianapolis, IN: Wolters Kluwer Health, 2009) and are based on archived and current data as of July 1, 2009.
Generic calcium acetate capsules from Roxane received U.S. Food and Drug Administration approval on February 26, 2008.
Calcium acetate tablets (Eliphos) from Hawthorn (a division of Cypress) received U.S. Food and Drug Administration approval on November 24, 2008.
All costs were indexed to 2001, which was the base year of the study. Inflation adjustments to Medicare costs were based on quarter level Medicare Market-basket index tables, and the 2001 AWP and WAC prices were used for phosphate binder costs (Table 1). Based on increases in Medicare and medication costs from 2001 to 2004, costs were indexed to 2004 in an additional sensitivity analysis.
Statistical Evaluation
The mean (weighted, with follow-up time as the weight), median, SD, minimum, and maximum for PMPM Medicare allowable expenditures were reported for total, inpatient, outpatient, skilled nursing facility, and other costs; 95% confidence intervals (CIs) were noted for the base case scenario. The Wilcoxon rank sum test was used to compare costs between the two treatment groups. A multiple linear regression model was used to evaluate differences in inflation-adjusted PMPM costs between treatment groups after adjusting for age, sex, race, patient prestudy comorbid conditions, and dialysis duration (6). Log scale PMPM costs were used for the regression model because of the skewness in PMPM (9). P < 0.05 was considered significant. All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC).
Results
In all, 1895 dosed participants (961 sevelamer and 934 calcium) were included in the cost analysis. Characteristics were well balanced between groups, except for a significantly higher proportion of participants with baseline atherosclerotic heart disease in the calcium group than in the sevelamer group (37.3 versus 32.6%). Participant characteristics have been published previously (5). Total inflation-adjusted Medicare PMPM costs were lower for sevelamer-treated than for calcium-treated participants by a mean differential of $199 PMPM (mean, $5236 versus $5435; median, $4653 versus $4933; Table 2). The cost differential was mainly driven by the inpatient component, with mean (median) differences of $183 ($235) PMPM. Outpatient, skilled nursing facility, and other inflation-adjusted expenditures were similar. Linear regression analysis showed that the relative cost and 95% CI of total inflation-adjusted Medicare costs in sevelamer versus calcium participants were 0.95 and 0.92 to 1.00, respectively (P = 0.06). Unadjusted Medicare costs showed the same relationship.
Table 2.
Expenditures in sevelamer and calcium groups (dosed cohort with 90-day follow-up rulea)
| Raw PPPM Costs ($) |
Inflation-Adjusted PPPM Costs ($) |
|||||
|---|---|---|---|---|---|---|
| Calcium(n = 961) | Sevelamer(n = 934) | Pb | Calcium(n = 961) | Sevelamer(n = 934) | Pb | |
| Total | 0.06 | 0.06 | ||||
| Mean ± SDc | 5724 ± 2863 | 5529 ± 2663 | 5435 ± 2768 | 5236 ± 2550 | ||
| Median | 5179 | 4905 | 4933 | 4653 | ||
| Min-Max | 809–101,446 | 1078–42,117 | 809–100,169 | 1071–42,286 | ||
| Inpatient | 0.05 | 0.05 | ||||
| Mean ± SDc | 1729 ± 2270 | 1544 ± 2027 | 1644 ± 2189 | 1461 ± 1937 | ||
| Median | 1196 | 965 | 1144 | 909 | ||
| Min-Max | 0–101,446 | 0–39,855 | 0–100,169 | 0–40,015 | ||
| Outpatient | 0.14 | 0.15 | ||||
| Mean ± SDc | 3711 ± 1115 | 3730 ± 1052 | 3521 ± 1064 | 3535 ± 1000 | ||
| Median | 3364 | 3441 | 3234 | 3297 | ||
| Min-Max | 0–12,095 | 1078–16,284 | 0–11,697 | 1071–15,972 | ||
| SNF | 0.19 | 0.19 | ||||
| Mean ± SDc | 110 ± 405 | 80 ± 307 | 105 ± 391 | 76 ± 296 | ||
| Median | 0 | 0 | 0 | 0 | ||
| Min-Max | 0–7152 | 0–6,173 | 0–6966 | 0–6166 | ||
| Other | 0.07 | 0.06 | ||||
| Mean ± SDc | 175 ± 319 | 174 ± 334 | 166 ± 306 | 164 ± 317 | ||
| Median | 37 | 25 | 36 | 24 | ||
| Min-Max | 0–4707 | 0–3423 | 0–4556 | 0–3322 | ||
Estimated costs of phosphate binders are not included in this table.
Max, maximum value; Min, minimum value; SNF, skilled nursing facility.
Early-termination patients followed an additional 90 d after early termination.
Wilcoxon rank sum test.
Weighted mean with follow-up time as the weight.
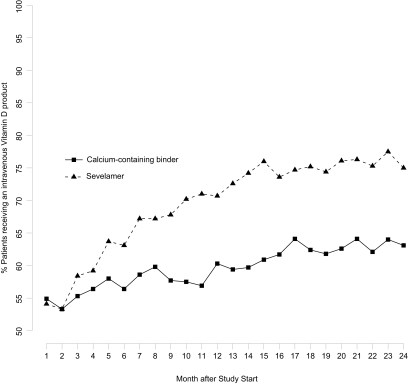
Inpatient and outpatient cost categories were evaluated for clinically (≥$5 PMPM) and statistically significant cost differences between groups (Table 3). Calcium-treated participants incurred higher Medicare PMPM costs for surgery-related care, specialist physicians, ambulance services, and outpatient erythropoiesis-stimulating agents than did sevelamer participants. Sevelamer-treated participants incurred much higher costs ($82 PMPM) for outpatient intravenous vitamin D therapy. Given this observation, we further evaluated vitamin D use over the course of the study. Figure 1 shows that, of participants who survived at least 2 yr (∼70%), the percentage of sevelamer patients receiving vitamin D increased from 54.1 to 75.0% (20.9 percentage points) from study start to 24 mo; the percentage of calcium patients receiving vitamin D increased only from 54.9 to 63.1% (8.2 percentage points).
Table 3.
Mean per member per month Medicare allowable costsa by selected cost categories (dosed cohort with 90-d follow-up ruleb)
| Medical Definitionc | PMPM Costs ($) |
Pd | ||
|---|---|---|---|---|
| Calcium | Sevelamer | Difference | ||
| Part B | ||||
| Inpatient: primary surgeon | 37.03 | 29.46 | −7.57 | 0.09 |
| Inpatient: hospital visit, specialist | 59.70 | 49.82 | −9.88 | 0.04 |
| Outpatient: consults, nephrologist | 0.47 | 0.29 | −0.18 | 0.04 |
| Ambulance | 195.24 | 157.00 | −38.24 | 0.06 |
| Outpatient | ||||
| Hemodialysis | 1538.40 | 1549.20 | 10.80 | 0.27 |
| Erythropoietic agents | 740.14 | 713.42 | −26.72 | 0.54 |
| Vitamin D sterols | 194.64 | 276.25 | 81.61 | <0.01 |
| Outpatient surgery | 89.74 | 82.94 | −6.81 | 0.54 |
| Radiology | 72.90 | 64.73 | −8.18 | 0.23 |
| Supplies | 69.53 | 63.84 | −5.69 | 0.81 |
| Inpatient | ||||
| Medical | 706.54 | 614.62 | −91.92 | 0.21 |
| Surgical | 723.62 | 658.73 | −64.90 | 0.03 |
| Other | 9.63 | 12.96 | 3.32 | 0.04 |
| SNF and home health care | ||||
| Total | 110.05 | 80.40 | −29.64 | 0.19 |
SNF, skilled nursing facility.
Not adjusted for inflation.
Early-termination patients followed an additional 90 d after early termination.
For the other category, between-group differences were not clinically or statistically different.
Wilcoxon rank sum test.
Figure 1.
Percentage of patients who survived at least 2 yr and received an intravenous vitamin D product in each month.
The mean weighted daily sevelamer dose per patient was 6695 ± 3210 mg. In the calcium group, 660 (70.7%) participants were started on calcium acetate only, 267 (28.6%) on calcium carbonate only, and 7 (0.8%) on both. The weighted mean daily elemental calcium acetate and calcium carbonate doses per patient were 5289 ± 2706 and 5007 ± 3355 mg, respectively. Using 2001 as the base year, mean PMPM AWP and WAC for sevelamer were $308.49 and $246.75 and were $27.09 and $21.74 for calcium phosphate binders, respectively. Including binder costs, the mean total inflation-adjusted PMPM medical costs were $5544 for the sevelamer group and $5463 for the calcium group using AWP; costs were $5483 for the sevelamer group and $5458 for the calcium group using WAC. Overall, the cost differences favored calcium (−$81; 95% CI, −$321 to $157 PMPM for AWP; −$25; 95% CI, −$256 to $213 PMPM for WAC; Table 4).
Table 4.
Base analysis (2001) evaluating inflation-adjusted PMPM costs with 95% CIs in dosed cohorta
| Inflation-Adjusted Costs ($) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | SNF | Other | Phosphate Binders |
Total |
|||
| AWP | WAC | AWP | WAC | |||||
| Calcium | ||||||||
| Mean | 1644 | 3521 | 105 | 166 | 27 | 22 | 5463 | 5458 |
| 95% CI | (1503, 1784) | (3453, 3590) | (79, 130) | (146, 186) | (26, 28) | (21, 23) | (5285, 5640) | (5279, 5635) |
| Sevelamer | ||||||||
| Mean | 1461 | 3535 | 76 | 164 | 308 | 247 | 5544 | 5483 |
| 95% CI | (1338, 1584) | (3471, 3598) | (57, 95) | (144, 184) | (299, 318) | (239, 254) | (5383, 5706) | (5321, 5644) |
| Difference | ||||||||
| Mean | 183 | −14 | 29 | 2 | −281 | −225 | −81 | −25 |
| 95% CI | (−1, 367) | (−106, 78) | (−3, 59) | (−25, 29) | (−291, −271) | (−233, −217) | (−321, 157) | (−256, 213) |
Drug costs were estimated costs and were not directly collected in the study.
SNF, skilled nursing facility.
Early-termination patients followed an additional 90 d after early termination.
In sensitivity analyses, costs were also calculated for the randomized Medicare cohort (52 additional patients overall). Patients were followed in a 90-d follow-up and to study completion (4,6). We assumed that patients who discontinued the study early remained on the same medications and doses as at the time they discontinued. For patients who were randomized but not administered a study drug, we used the median drug dose and cost for their randomization group. Cost differences between groups were smaller for the randomized than for the dosed cohorts (Table 5). The differences became larger when 2004 costs were considered, again favoring the calcium group.
Table 5.
Sensitivity analysis evaluating inflation-adjusted PMPM costs in two patient follow-up cohorts in 2001 and three patient cohorts in 2004
| Inflation-Adjusted Costs ($) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | SNF | Other | Phosphate Binders |
Total |
|||
| AWP | WAC | AWP | WAC | |||||
| 2001 | ||||||||
| Cohort | ||||||||
| Randomized (90 da) | ||||||||
| Calcium | 1660 | 3533 | 106 | 164 | 27 | 22 | 5490 | 5485 |
| Sevelamer | 1476 | 3530 | 79 | 163 | 308 | 246 | 5556 | 5494 |
| Difference | 184 | 3 | 27 | 1 | −281 | −224 | −66 | −9 |
| Randomized (end of 2004b) | ||||||||
| Calcium | 1727 | 3544 | 120 | 175 | 27 | 21 | 5593 | 5587 |
| Sevelamer | 1591 | 3509 | 87 | 162 | 304 | 243 | 5653 | 5592 |
| Difference | 136 | 35 | 33 | 13 | −277 | −222 | −60 | −5 |
| 2004 | ||||||||
| Cohort | ||||||||
| Dosed (90 da) | ||||||||
| Calcium | 1841 | 3944 | 116 | 183 | 42 | 33 | 6125 | 6116 |
| Sevelamer | 1636 | 3959 | 84 | 180 | 371 | 297 | 6231 | 6157 |
| Difference | 205 | -16 | 32 | 2 | −329 | −264 | −106 | −41 |
| Randomized (90 da) | ||||||||
| Calcium | 1859 | 3957 | 117 | 180 | 47 | 37 | 6160 | 6150 |
| Sevelamer | 1653 | 3954 | 87 | 179 | 376 | 301 | 6249 | 6174 |
| Difference | 206 | 3 | 30 | 1 | −329 | −264 | −89 | −24 |
| Randomized (end of 2004b) | ||||||||
| Calcium | 1934 | 3969 | 132 | 193 | 45 | 36 | 6273 | 6264 |
| Sevelamer | 1782 | 3930 | 96 | 178 | 370 | 296 | 6356 | 6282 |
| Difference | 152 | 39 | 36 | 14 | −325 | −260 | −83 | −18 |
Drug costs were estimated costs and were not directly collected in the study.
SNF, skilled nursing facility.
Early-termination patients followed an additional 90 d after early termination.
Early-termination patients followed to the end of 2004.
Discussion
Hyperphosphatemia is an inevitable consequence of severe chronic kidney disease; higher serum phosphorus levels have been associated with increased mortality (10), and phosphate binder therapy is a well-established management tool used to control serum phosphate in dialysis patients. A recent observational study showed that phosphate binder treatment was, in general, associated with decreased mortality risk compared with no binder treatment (11). The DCOR study preceded this study; DCOR was a large-scale randomized clinical trial designed to evaluate whether the non–calcium-, non–aluminum-, non–magnesium-containing binder, sevelamer hydrochloride, was superior to calcium-containing binders using the hard endpoints of mortality and hospitalization. DCOR results showed no difference in overall mortality, but suggested a significantly lower hospitalization rate and number of hospital days for patients using sevelamer versus calcium carbonate (6). In this study, laboratory goals were set for serum phosphorous, but dose changes and laboratory monitoring reflected usual clinical practice (4). Medication adherence was not assessed, and use of other bone-related medications, such as vitamin D, was not restricted. Thus, data from this study reflected a more pragmatic approach than many efficacy trials, which is a superior design for assessing pharmacoeconomic outcomes.
Sevelamer is appreciably more expensive than either calcium acetate or calcium carbonate. However, if sevelamer reduces hospitalizations or other medical services compared with calcium-containing binders, it is important to understand whether the increased cost of therapy is offset by decreased medical costs. U.S. ESRD patients are entitled to Medicare benefits; >90% of hemodialysis patients were covered by Medicare in 2005 (12). In this study, 93% of the original DCOR cohort met our strict definition of Medicare-as-primary-payer status (5), and we performed a comprehensive assessment of medical services and costs for these patients. Sevelamer and calcium groups were well balanced regarding baseline characteristics; the only exception was a higher percent of calcium patients with atherosclerotic heart disease at baseline.
Evaluating dosed patients and following them for 90 d after early study discontinuation showed total and inpatient average PMPM Medicare costs $199 and $183 less, respectively, in the sevelamer than in the calcium group. Annualizing costs showed that Medicare costs were $2388 less for sevelamer-treated than for calcium-treated patients. Lower hospitalization costs mainly drove the differential between treatment groups. Linear regression analysis, adjusted for baseline patient characteristics, showed a trend toward reduced total costs in the sevelamer group compared with the calcium binder group.
Outpatient costs were similar for the sevelamer and calcium groups despite outpatient intravenous vitamin D costs $82 PMPM (annualized, $972) higher for sevelamer-treated than for calcium-treated patients. The increase over time in the percentage of sevelamer patients using intravenous vitamin D was greater than for calcium patients.
However, to determine sevelamer versus calcium binder effects on medical expenditures, the costs of the binders must be incorporated. At the time of this study, Medicare did not cover the cost of most long-term oral prescription drugs. Thus, we could not directly incorporate Medicare allowable costs for phosphate binders. We used Medi-Span AWP and WAC pricing data (8) for Phoslo, TUMS, and Renagel in 2001 (study base year) and 2004 to assess a reasonable range of possible costs, with AWP and WAC representing important pricing benchmarks that the Centers for Medicare & Medicaid Services uses to determine reimbursement under Medicare Part D.
Today, because most hemodialysis patients are eligible for prescription benefits under Medicare Part D and most national Part D prescription drug plans include calcium acetate and sevelamer on their formularies (13,14), our study has implications for overall Medicare-related costs. Our analyses showed that, overall, medical PMPM costs were greater for sevelamer-treated than for calcium-treated patients, once the costs of the phosphate binders were included. This is primarily because of the cost differential between Renagel and calcium binders from 2001 to 2004 (Table 1). From 2004 to 2009, both Renagel and PhosLo costs continued to outpace Medicare inflation rates (Table 1), and the cost differential between the two agents grew larger. Thus, given no changes in practice patterns and the current availability of generic calcium acetate (Table 1), we would expect a larger total PMPM cost differential in 2009 than in 2004, favoring the calcium-treated group.
Importantly, our study was simply a cost-minimization analysis and not a cost-effectiveness analysis, in which a cost per clinical outcome (e.g., hospitalization averted) or quality measure (e.g., quality-of-life years gained) would have been calculated. Earlier studies have evaluated the cost effectiveness of sevelamer versus calcium-containing phosphate binders with disparate conclusions (7,15). Using a complex cost-effectiveness model, Huybrechts et al. (15,16) predicted a favorable cost-effectiveness ratio of ∼$2200 per life-year gained (discounted). However, this study was published before DCOR and other trial results were available; thus, medical and patient outcome inputs and medical cost inputs were all based on estimates and multiple model assumptions. Manns et al. (7) performed a comprehensive cost-effectiveness analysis using model inputs for mortality and hospitalization from the primary analysis of the DCOR trial (4) and quality-of-life data from other sources. They found that sevelamer was associated with a cost per quality-of-life years gained ranging from CAN$77,600 to CAN$278,000, depending on whether or not dialysis costs were included. In general, these amounts surpass what would be considered good value for the cost. These authors also showed that sevelamer use would have to reduce hospitalization rates by 30% to offset the additional medication cost (7). However, their analysis used health care and medication cost estimates, because directly linked costs were not available.
Using actual hospitalization costs, we determined that the hospitalization rate for sevelamer patients would need to be reduced by an additional 1.5 to 4.9% (using 2001 as the base year) or 2.8 to 5.5% (using 2004 as the base year) over the apparent reduction in hospitalization seen in the DCOR study (5,6) to offset the higher costs of sevelamer. This assumes that other health care costs (outpatient and skilled nursing facility) remain the same.
Our study removes most cost assumptions, compared with previous studies, because information on both Medicare health care services and costs was collected simultaneously. Phosphate binder costs could be estimated based on dosing information available from the DCOR trial. Sevelamer-treated patients experienced less hospitalization and lower inpatient and total Medicare costs than calcium-treated patients. In our study, inpatient costs were reduced by 11.1% in the sevelamer group, using 2001 as the base year. Both groups had similar outpatient costs, mainly because of increased use of intravenous vitamin D in sevelamer patients. However, once phosphate binder costs were incorporated, patients receiving sevelamer were more costly than those receiving calcium binders. The cost differential was low in 2001 (study base year) but increased by 2004 because drug costs (on an annual percentage basis) rose more rapidly than did Medicare allowable rates for medical services (Table 5).
Our analysis has some limitations. The premise that sevelamer, compared with calcium binders, may reduce medical costs is based on DCOR secondary findings showing a relative reduction in hospitalizations in sevelamer-treated compared with calcium-treated patients. Thus, potential cost savings from averted hospitalization or other medical services should be considered more speculative than definitive. The 95% CI of costs in Table 4 show the uncertainty around the cost measurements, reflecting the uncertainty around the effect benefit. Drug costs were based on prescribed dose, and 100% patient adherence to each phosphate binder was assumed. Medication adherence data were not collected in the DCOR study, nor were patients urged to take their phosphate binders beyond what would be done in the usual practice setting. Adherence to phosphate-binding agents is notoriously low (17,18); Tomasello et al. (17) showed that 65% of older and 80% of younger patients repeatedly do not adhere. This has implications for our analysis in that drug costs incorporated represented the maximum possible cost and not the actual cost based on patient adherence. The difference in actual medical costs between the two groups might be smaller than is shown in our analyses. Also, indirect medical costs, such as lost work time, were not accounted for in this analysis. We included dialysis costs, which may not be directly related to phosphate binder therapy, but inpatient dialysis costs could not be separated from other inpatient costs.
In addition, the DCOR population was limited to patients with Medicare as primary payer so costs could be evaluated, and these results may not be directly applicable to hemodialysis patients with other types of insurance. Non-Medicare spending for ESRD patients is rising at faster rates than Medicare spending, and cost results may be dramatically different within employer group health plans. However, because Medicare pays for the health care of most U.S. hemodialysis patients (19), these data are applicable to most U.S. hemodialysis patients. Because peritoneal dialysis patients were not studied, these data may not be applicable to this population. Finally, this cost analysis may not be directly applicable to health care systems outside the United States.
In conclusion, our ability to link Centers for Medicare & Medicaid Services data with information from the DCOR clinical study allowed a direct assessment of the effect of sevelamer versus calcium binders on both clinical and economic outcomes. Sevelamer was associated with reduced Medicare inpatient and total costs compared with calcium binders; however, when the costs of binders were incorporated, PMPM costs favored calcium-treated patients. The cost differential widened from 2001 to 2004, as increases in drug costs outpaced increases in Medicare health care service expenditures.
Disclosures
W.L.S.P. is employed by the University of Minnesota and affiliated with the Chronic Disease Research Group. E.W. and J.L. are employed by the Chronic Disease Research Group; Q.F. was previously employed by the Chronic Disease Research Group. The Chronic Disease Research Group receives grant funding from Genzyme and has received funding from Abbott. W.L.S.P. has received honoraria from Abbott and served on its Advisory Board.
Acknowledgments
Some of the data presented in this paper were presented at the 2006 American Society of Nephrology Meeting held in San Diego, CA. This study was supported by a research contract with Genzyme, Inc. (Cambridge, MA). The contract provides for the authors to have final deter. mination of manuscript content. The findings and discussion do not represent the U.S. Renal Data System or the National Institutes of Health. The authors thank Chronic Disease Research Group colleagues Anne Shaw and Shane Nygaard for manuscript preparation and Nan Booth, MSW, MPH, for manuscript editing. The authors also thank Stephen Schondelmeyer, PharmD, PhD, for procuring drug pricing information and for advice on incorporating that information into this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–201, 2003 [PubMed] [Google Scholar]
- 2.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Li S, St Peter WL, Ebben J, Roberts T, Ma JZ, Manning W: Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12: 2465–2473, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK: Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72: 1130–1137, 2007 [DOI] [PubMed] [Google Scholar]
- 5.St Peter WL, Liu J, Weinhandl ED, Fan Q: Linking Centers for Medicare & Medicaid Services data with prospective DCOR trial data: methods and data comparison results. Hemodial Int 12: 480–491, 2008 [DOI] [PubMed] [Google Scholar]
- 6.St Peter WL, Liu J, Weinhandl E, Fan Q: A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: a secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis 51: 445–454, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Manns B, Klarenbach S, Lee H, Culleton B, Shrive F, Tonelli M: Economic evaluation of sevelamer in patients with end-stage renal disease. Nephrol Dial Transplant 22: 2867–2878, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Medispan: PriceChek PC®: A drug database by MediSpan (Indianapolis, IN), a division of Wolters Kluwer Health, Inc., based on archived and current data as of July 1, 2009. Available at http://www.medispan.com/index.aspx Accessed September 14, 2009
- 9.Collins AJ, Li S, Ebben J, Ma JZ, Manning W: Hematocrit levels and associated Medicare expenditures. Am J Kidney Dis 36: 282–293, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Isakova T, Gutierrez OM, Chang Y, Shah A, Tamez H, Smith K, Thadhani R, Wolf M: Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20: 388–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Renal Data System: 2007 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 13.St Peter WL: Potential impact of Medicare Part D in the end-stage renal disease population. Adv Chronic Kidney Dis 15: 140–146, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Medicare Drugs Awareness and Education Initiative: Changing formularies over time. Available at: http://www.kidneydrugcoverage.org/pdf/ChangesinFormulariesOverTime11–15–07.pdf Accessed September 14, 2009
- 15.Huybrechts KF, Caro JJ, Wilson DA, O'Brien JA: Health and economic consequences of sevelamer use for hyperphosphatemia in patients on hemodialysis. Value Health 8: 549–561, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Huybrechts KF, Caro JJ, London GM: Modeling the implications of changes in vascular calcification in patients on hemodialysis. Kidney Int 67: 1532–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Tomasello S, Dhupar S, Sherman RA: Phosphate binders, K/DOQI guidelines, and compliance: The unfortunate reality. Dial Transplant 33: 236–240, 2004 [Google Scholar]
- 18.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Renal Data System: 2008 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]