Abstract
Background and objectives: A close linkage between chronic kidney disease (CKD) and cardiovascular disease (CVD) has been demonstrated. Coronary artery calcification (CAC) is considered to be the causal link connecting them. The aim of the study is to determine the relationship between level of kidney function and the prevalence of CAC.
Design, setting, participants, & measurements: Autopsy subjects known to have coronary artery disease and a wide range of kidney function were studied. Patients without CKD were classified into five groups depending on estimated GFR (eGFR) and proteinuria: eGFR ≥60 ml/min/1.73 m2 without proteinuria; CKD1/2: eGFR ≥60 ml/min/1.73 m2 with proteinuria; CKD3: 60 ml/min/1.73 m2 >eGFR ≥30 ml/min/1.73 m2; CKD4/5: eGFR <30 ml/min/1.73 m2; and CKD5D: on hemodialysis. Intimal and medial calcification of the coronary arteries was evaluated. Risk factors for CVD and uremia were identified as relevant to CAC using logistic regression analysis.
Results: Intimal calcification of plaques was present in all groups, but was most frequent and severe in the CKD5D group and less so in the CKD4/5 and CKD3 groups. Risk factors included luminal stenosis, age, smoking, diabetes, calcium-phosphorus product, inflammation, and kidney function. Medial calcification was seen in a small number of CKD4/5 and CKD5D groups. Risk factors were use of calcium-containing phosphate binders, hemodialysis treatment, and duration.
Conclusions: It was concluded that CAC was present in the intimal plaque of both nonrenal and renal patients. Renal function and traditional risks were linked to initimal calcification. Medial calcification occurred only in CKD patients.
Cardiovascular disease (CVD) is the main cause of morbidity and mortality in patients with end-stage renal disease (ESRD) (1,2) or chronic kidney disease (CKD) (3–7). The mechanisms underlying this increased cardiovascular risk are not clearly understood. In the general population, traditional risk factors for CVD have been well characterized (8), and these are also present in CKD (3–6,9). The mechanisms involved in the connection between CKD and CVD are probably numerous (3–6). Vascular calcification, such as coronary artery calcification (CAC) (10,11), is considered to be the causal link between them.
Vascular calcification is common in physiologic and pathologic conditions such as aging, diabetes, dyslipidemia, genetic diseases, and diseases with disturbances of calcium metabolism (12–14). In CKD patients, vascular calcification is even more common, developing early and contributing to the markedly increased cardiovascular risk. Pathomorphologically, atherosclerosis (plaque-forming degenerative changes of the aorta and of large elastic arteries) and arteriosclerosis (concentric medial thickening and hyalinosis of muscular arteries) can be distinguished. Increased knowledge about the mechanisms of calcification together with improved imaging techniques have provided evidence that vascular calcification should be divided into two distinct entities according to the specific site of calcification within the vascular wall: plaque calcification, involving patchy calcification of the intima in the vicinity of lipid or cholesterol deposits, and calcification of the media in the absence of such lipid or cholesterol deposits, known as Mönckeberg-type atherosclerosis (12–14). These two types of calcification may vary in terms of the type of vessel affected, the location along the arterial tree (proximal versus distal), clinical presentation, and treatment and prognosis (12–14). In the general population and in patients with CKD, electron-beam computed tomography (EBCT) has proven CAC as a potent predictor of cardiac events (15–18). Both the prevalence and intensity of CAC are increased in patients with CKD (19–27). Several studies have been undertaken to investigate whether calcification occurs in the intima or media of the coronaries and whether the morphologic details of calcified plaques differ between renal and nonrenal patients (12–14,24). Causal elements for either type of CAC have not been definitively determined (12–14).
Autopsy studies are limited in terms of patient selection, but have a major advantage in terms of being able to distinguish intimal from medial calcification. Therefore, our primary goal is to determine whether, among autopsy subjects known to have CAD, there exists a direct relationship between level of kidney function and the prevalence of intimal or medial calcification.
Materials and Methods
Study Population
During the 6-yr period between 1991 and 1997, 699 subjects were autopsied at the National Cardiovascular Center, Osaka, Japan (28). Given our goal of examining the coronary arteries, we limited autopsy cases to those with significant coronary artery disease (CAD), resulting in 123 remaining cases. To permit a pathologic study of the coronary arteries, we excluded six cases in which serum creatinine had not been measured. Thus, a final total of 117 autopsy subjects were reviewed histopathologicaly and immunohistochemicaly.
CAD included myocardial infarction and stable and unstable angina pectoris. Myocardial infarction was diagnosed using either electrocardiograms or echocardiograms, and angina pectoris was diagnosed based on both symptoms and electrocardiograms. Cerebral vascular disease was diagnosed using clinical history and computerized tomography findings. Peripheral vascular disease was diagnosed based on symptoms, an ankle/brachial pressure index of less than 0.9, or prior lower limb revascularization procedures. Thoracic or abdominal aortic aneurysm was diagnosed using clinical history and computed tomography. Smoking habits were recorded in two categories: current or former smokers, and nonsmokers. We possessed extensive laboratory data on all the study subjects, including serum creatinine, cholesterol, glucose, and urinalysis results. To create our dataset we chose the most representative data from the 3 mo before death. All laboratory values and medical information was obtained by chart review.
To define the presence of CKD, we applied the Modification of Diet in Renal Disease (MDRD) Study equation for evaluating Japanese CKD patients (29–31); eGFR (ml/min/1.73m2) = 0.741 × 175 × (serum creatinine, mg/dl)−1.154 × (age, years)−0.203 × (0.742 for women).
One-hundred seventeen study subjects were classified into five groups based on eGFR and proteinuria, as follows: without CKD: eGFR over 60 ml/min/1.73 m2 without proteinuria; CKD1/2: eGFR over 60 ml/min/1.73 m2 with proteinuria; CKD3: eGFR below 60 ml/min/1.73 m2 but above 30 ml/min/1.73 m2; CKD4/5: eGFR below 30 ml/min/1.73 m2; and CKD5D: patients on maintenance hemodialysis therapy.
In the CKD5D group, the renal diseases that caused ESRD included nine cases of chronic glomerulonephritis, 11 cases of diabetic nephropathy, and three cases of nephrosclerosis. The mean duration (± SD) of hemodialysis therapy was 43 ± 60 mo.
Causes of death among all subjects were coronary heart disease in 67% of cases, cerebrovascular disease in 9%, aortic aneurysm in 9%, infection in 10%, and other causes in the remaining 5%.
Pathologic Examination of Coronary Arteries
In accordance with the regulations of our pathology department, the all autopsy examination was performed within 3 h after patient's death. The coronary arteries were perfusion-fixed for 2 h with neutral buffered formalin at 100 mmHg and then studied by subserial sectioning at 4 mm intervals. These sections were sliced 5 μm thick on glass slides and stained. We studied all sections with or without significant stenosis of the proximal and distal right coronary artery (RCA), left main coronary artery, proximal and distal left anterior descending (LAD) coronary artery, and proximal and distal left circumflex (LCX) coronary artery. Apparent stenosis of a coronary artery with more than 75% narrowing of the luminal area was considered significant.
We used the American Heart Association histologic classification of atherosclerosis (32) to identify type V lesions that manifested as calcified plaques. Histologic calcification of the intima and media was detected using Hematoxylin and eosin (HE) staining and osteopontin immunostaining. To determine the extent of calcification, coronary artery sections were evaluated using a 5-point scale: grade 0, no calcification; grade 1, calcification <25% in cross-sectional area; grade 2, calcification >25% and <50% in cross-sectional area; grade 3, calcification >50% and <75% in cross-sectional area; grade 4, calcification >75% in cross-sectional area. To detect inflammation, immunostaining for CD68-positive macrophages was performed.
Statistical Analyses
Values are represented as means ± SD with a p-value of <0.05 as significance. To compare the variables, one-way ANOVA and chi-squared tests were used. The association between risk factors and calcification of coronary arteries was determined by logistic regression analysis. Degree of luminal stenosis (%), smoking, diabetes, age (years), serum calcium level (mg/dl), serum phosphate level (mg/dl), calcium-phosphorus product (mg2/dl2), use of calcium-containing phosphate binders, hemodialysis, kidney function (eGFR, ml/min/1.73 m2), C-reactive protein (CRP) (mg/dl), and use of vitamin D and warfarin were included in univariate models. All statistical analyses were performed with Stat View version 5.0 (SAS Institute Inc).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree on the manuscript as written.
Results
Patient Characteristics
Table 1 shows the baseline characteristics. The mean age was higher in the CKD3 and CKD4/5 groups. Hypertension was evident in the CKD4/5 and CKD5D groups. Diabetes was infrequent in the patients without CKD. Incidence of smoking, CAD, CVD, peripheral vascular disease, and aortic aneurysm were not different among the groups. Serum albumin concentration was lower in the CKD4/5 and CKD5D groups. CRP data were higher in the CKD4/5 and CKD5D groups. The prescription of phosphate binders was more frequent in CKD5D groups. The use of statins, vitamin D, and warfarin, which may affect vascular calcification, was not different.
Table 1.
Patients' clinical and biochemical characteristicsa
| Patient Characteristics | Patients without CKD | Patients with CKD1/2 | Patients with CKD3 | Patients with CKD4/5 | Patients with CKD5D |
|---|---|---|---|---|---|
| Number of subjects | 15 | 15 | 48 | 16 | 23 |
| Age (years) | 63 ± 13 | 69 ± 10 | 70 ± 11c | 73 ± 9c | 66 ± 12 |
| Gender (% male) | 80 | 73 | 63 | 81 | 78 |
| eGFR (ml/min/1.73m2) | 111 ± 22 | 72 ± 10b | 48 ± 11c,e | 15 ± 6c,e,g | — |
| Body mass index (kg/m2) | 22 ± 5 | 22 ± 3 | 21 ± 3 | 21 ± 2 | 21 ± 4 |
| Comorbid conditions (% yes) | |||||
| Hypertension | 47 | 60 | 77 | 100b,d | 91b,d |
| Diabetes | 13 | 67b | 46b | 63b | 52b |
| Dyslipidemia | 33 | 47 | 40 | 19 | 38 |
| Smoking | 53 | 60 | 52 | 63 | 78 |
| Coronary artery disease (single vessel disease) | 20 | 20 | 23 | 6 | 26 |
| Coronary artery disease (double vessel disease) | 20 | 13 | 25 | 19 | 35 |
| Coronary artery disease (triple vessel disease) | 60 | 67 | 52 | 75 | 39 |
| Cerebrovascular disease | 40 | 53 | 38 | 56 | 18 |
| Peripheral vascular disease | 7 | 13 | 15 | 25 | 27 |
| Aortic aneurysm | 27 | 20 | 21 | 25 | 27 |
| Laboratory parameters | |||||
| Total protein (g/dl) | 6.6 ± 0.7 | 6.2 ± 1.1 | 6.4 ± 0.9 | 6.0 ± 0.7c | 6.5 ± 0.8h |
| Serum albumin (g/dl) | 3.8 ± 0.6 | 3.5 ± 0.8 | 3.7 ± 0.5 | 3.4 ± 0.5c | 3.4 ± 0.6c |
| Hematocrit (%) | 41 ± 5 | 38 ± 5 | 36 ± 7c | 32 ± 7b,d,f | 31 ± 6b,e,g |
| Serum phosphorus (mg/dl) | 3.4 ± 0.9 | 2.7 ± 0.9 | 3.4 ± 1.1c | 3.9 ± 0.9e | 4.8 ± 1.6b,e,g,h |
| Serum calcium (mg/dl) | 9.3 ± 0.4 | 9.1 ± 0.6 | 9.2 ± 1.0 | 9.1 ± 0.5 | 9.9 ± 1.6f |
| Calcium-phosphorus product (mg2/dl2) | 32 ± 9 | 24 ± 9 | 31 ± 11d | 36 ± 8e | 47 ± 17b,e,g,h |
| Total cholesterol (mg/dl) | 161 ± 40 | 186 ± 30 | 166 ± 49 | 210 ± 57b | 177 ± 46h |
| Glucose (mg/dl) | 142 ± 58 | 156 ± 63 | 156 ± 87 | 160 ± 67 | 136 ± 63 |
| CRP (mg/dl) | 1.0 ± 1.3 | 0.4 ± 0.2 | 1.6 ± 2.2 | 2.6 ± 2.8b,d | 3.7 ± 4.8b,d,f |
| Medications (% yes) | |||||
| Calcium-containing phosphate binders | 0 | 0 | 0 | 6 | 43c,e,g,i |
| Aluminum-containing phosphate binders | 0 | 0 | 0 | 0 | 9b,d,f,h |
| Calcium channel blockers | 53 | 80 | 67 | 81 | 52 |
| Angiotensin-converting enzyme inhibitors | 20 | 27 | 23 | 13 | 39 |
| hb blockers | 7 | 13 | 15 | 25 | 39 |
| Statins | 13 | 7 | 10 | 19 | 17 |
| Vitamin D | 0 | 7 | 4 | 0 | 17 |
| Warfarin | 7 | 33 | 23 | 19 | 22 |
Data refer to the mean ± SD.
Versus without CKD:
P <0.05,
P <0.01.
versus CKD1/2:
P <0.05,
P <0.01.
Versus CKD3:
P <0.05,
P <0.01.
Versus CKD4/5:
P <0.05,
P <0.01.
CKD, chronic kidney disease; eGFR, estimated GFR; CRP, C-reactive protein.
Histopathology of Coronary Arteries
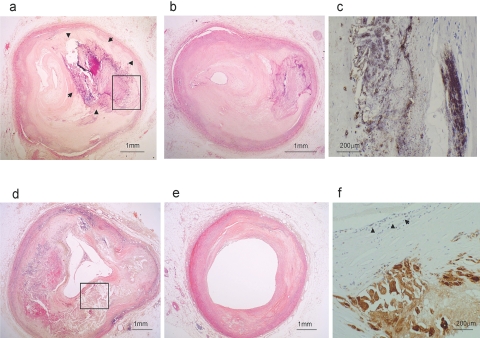
Table 2 shows the degree of luminal stenosis of coronary arteries, and the stenosis was severe in CKD4/5 group. Figure 1 shows representative sections of coronary arteries. In the CKD5D group, severe stenosis in the proximal segment was composed of an atheromatous plaque containing a large area of intimal calcification (Figure 1A), and distal segment showed also severe stenosis with calcified plaque (Figure 1B). In a patient without CKD we observed a large necrotic core with minute foci of calcification at proximal segments (Figure 1D), without severe stenosis of distal segments (Figure 1E). Using immunohistochemical staining, osteopontin-positive calcified plaque was found in the atheromatous plaque of the intima at the proximal segment in the CKD5D group (Figure 1C), and an accumulation of CD68-positive macrophages was seen in an atheromatous plaque with lymphocyte infiltration (Figure 1F).
Table 2.
The degree of luminal stenosis of coronary arterya
| Patients without CKD | Patients with CKD1/2 | Patients with CKD3 | Patients with CKD4/5 | Patients with CKD5D | |
|---|---|---|---|---|---|
| Right coronary artery, proximal (%) | 50 ± 10 | 62 ± 28 | 71 ± 27 | 69 ± 23 | 69 ± 28 |
| Right coronary artery, distal (%) | 53 ± 12 | 58 ± 34 | 57 ± 26 | 76 ± 21c | 56 ± 30d |
| Left main coronary artery (%) | 48 ± 8 | 58 ± 24 | 56 ± 22 | 57 ± 20 | 52 ± 21 |
| Left anterior descending coronary artery, proximal (%) | 63 ± 25 | 71 ± 23 | 71 ± 24 | 77 ± 18 | 73 ± 21 |
| Left anterior descending coronary artery, distal (%) | 95 ± 7 | 65 ± 34 | 65 ± 28 | 76 ± 15 | 65 ± 21 |
| Left circumflex coronary artery, proximal (%) | 77 ± 3 | 67 ± 22 | 68 ± 25 | 80 ± 19b | 64 ± 27d |
| Left circumflex coronary artery, distal (%) | 50 ± 14 | 58 ± 28 | 60 ± 28 | 74 ± 20b,c | 55 ± 36d |
Data refer to the mean ± SD
Versus CKD1/2:
P <0.05.
versus CKD3:
P <0.05.
Versus CKD4/5:
P <0.05.
Figure 1.
Coronary artery pathologic findings (A) A representative left anterior descending coronary artery proximal segment of CKD5D case with acute myocardial infarction. Large calcification of intimal atheromatous plaque (surrounded by arrow) (HE). (B) Distal segment of the same case as (A). Severe stenosis with calcified hard plaque (HE). (C) Osteopontin-positive calcified plaque (crop area from A) (immunohistochemistry, DAB). (D) RCA proximal segment of case without CKD. Large necrotic core with minute foci of calcification (HE). (E) Distal segment of the same case as (D) shows no stenosis (HE). (F) Significant CD68-positive macrophages accumulation in atheromatous plaque with lymphocyte (arrow) infiltration (crop area from D) (immunohistochemistry, DAB).
Intimal Plaque Calcification and Risk Factors
Intimal calcification was most evident in groups CKD5D, CKD4/5, and CKD3 (Table 3).
Table 3.
Coronary artery intimal plaque calcification
| The Percentage of Patients with Intimal Calcification (%) |
|||||
|---|---|---|---|---|---|
| Patients without CKD | Patients with CKD1/2 | Patients with CKD3 | Patients with CKD4/5 | Patients with CKD5D | |
| Right coronary artery, proximal | 20 | 47 | 58a | 56a | 78a |
| Right coronary artery, distal | 7 | 27 | 29 | 44b | 78b,c,d |
| Left main coronary artery | 33 | 53 | 50 | 50 | 96b |
| Left anterior descending coronary artery, proximal | 40 | 40 | 73b,c | 69b,c | 87b,c |
| Left anterior descending coronary artery, distal | 7 | 27 | 23 | 25 | 91b,c,d,e |
| Left circumflex coronary artery, proximal | 53 | 40 | 58 | 88a,c | 83a,c |
| Left circumflex coronary artery, distal | 27 | 13 | 33 | 44a,c | 61a,c,d |
Versus without CKD:
P <0.05,
P <0.01. Versus CKD1/2:
P <0.05.
Versus CKD3:
P <0.05. Versus CKD4/5:
P <0.05.
Table 4 shows the intimal calcification score in each segment of the coronary arteries. Calcification was higher in the CKD5D group than in the other groups. The scores were also high in the CKD4/5 group, in the proximal and distal RCA lesions and proximal LCX coronary artery lesion.
Table 4.
Coronary artery intimal calcification scorea
| Patients without CKD | Patients with CKD1/2 | Patients with CKD3 | Patients with CKD4/5 | Patients with CKD5D | |
|---|---|---|---|---|---|
| Right coronary artery, proximal | 0.4 ± 0.4 | 0.8 ± 1.0 | 1.0 ± 0.9b | 1.0 ± 1.0b | 2.1 ± 1.6c,e,f,g |
| Right coronary artery, distal | 0.1 ± 0.3 | 0.4 ± 0.7 | 0.4 ± 0.6 | 0.8 ± 0.8b | 1.8 ± 1.4c,e,f,g |
| Left main coronary artery | 0.7 ± 1.0 | 0.6 ± 0.6 | 0.9 ± 0.9 | 1.0 ± 1.0 | 2.2 ± 1.0c,e,f,h |
| Left anterior descending coronary artery, proximal | 0.7 ± 1.0 | 0.6 ± 0.8 | 1.5 ± 1.1e | 1.6 ± 1.1b,d | 2.9 ± 1.1c,e,f,g |
| Left anterior descending coronary artery, distal | 0.1 ± 0.3 | 0.8 ± 1.1 | 0.6 ± 0.9 | 0.7 ± 1.0 | 1.9 ± 1.0c,e,f,g |
| Left circumflex coronary artery, proximal | 0.6 ± 0.6 | 0.7 ± 0.8 | 1.1 ± 0.9d | 1.7 ± 1.0c,d,e | 2.4 ± 1.4c,e,f,g |
| Left circumflex coronary artery, distal | 0.4 ± 0.9 | 0.5 ± 0.9 | 0.6 ± 0.8 | 1.1 ± 1.1 | 1.6 ± 1.5c,e,f |
Data refer to the mean ± SD
Versus without CKD:
P <0.05,
P <0.01. Versus CKD1/2:
P <0.05,
P <0.01.
Versus CKD3:
P <0.01. Versus CKD4:
P <0.05,
P <0.01.
Coronary arterial sections were evaluated regarding extent of calcification using a 5-point scale: grade 0, no calcification; grade 1, calcification <25% of cross-sectional area; grade 2, calcification > 25% and <50% of cross-sectional area; grade 3, calcification >50% and <75% of cross-sectional area; grade 4, calcification >75% of cross-sectional area.
Univariate analysis was performed to assess the presence of intimal calcification (Table 5). Stenosis, hemodialysis, and kidney function seemed to be linked to intimal calcification in almost all cases. Smoking was linked to calcification of proximal lesions, while calcium-phosphorus product or phosphate binder use was linked to calcification of distal lesions. Diabetes, age, and inflammation seemed to be linked to calcification of both of proximal and distal lesions. The use of vitamin D was not linked to calcification, but warfarin administration tended to relate the coronary calcification.
Table 5.
Univariate risk factors for the presence of intimal plaque calcification in coronary arteries based on logistic regression analysis
| Risk Factors | Right Coronary Artery, Proximal |
Right Coronary Artery, Distal |
||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Luminal stenosis (%) | 1.03 (1.01 to 1.05) | <0.001 | 1.02 (1.01 to 1.04) | <0.05 |
| Smoking | 2.67 (1.15 to 6.19) | <0.05 | 1.22 (0.50 to 2.98) | 0.66 |
| Diabetes | 1.64 (0.74 to 3.60) | 0.22 | 2.70 (1.14 to 6.36) | <0.05 |
| Age (years) | 1.02 (0.98 to 1.06) | 0.41 | 1.07 (1.02 to 1.12) | <0.01 |
| Serum calcium (mg/dl) | 1.30 (0.84 to 2.00) | 0.24 | 2.44 (1.22 to 4.89) | <0.05 |
| Serum phosphorus (mg/dl) | 0.88 (0.64 to 1.20) | 0.42 | 1.09 (0.78 to 1.51) | 0.63 |
| Calcium-phosphorus product (mg2/dl2) | 1.00 (0.97 to 1.03) | 0.99 | 1.02 (0.99 to 1.06) | 0.24 |
| Calcium-containing phosphate binder | 3.04 (0.61 to 15.10) | 0.17 | 6.32 (1.26 to 31.75) | <0.05 |
| Hemodialysis | 2.81 (0.95 to 8.32) | 0.07 | 4.70 (1.51 to 14.66) | <0.01 |
| eGFR (ml/min/1.73 m2) | 0.99 (0.98 to 1.01) | 0.26 | 0.97 (0.95 to 0.99) | <0.0005 |
| CRP (mg/dl) | 1.10 (0.94 to 1.29) | 0.23 | 1.14 (1.01 to 1.30) | <0.05 |
| Vitamin D | 0.25 (0.05 to 1.33) | 0.11 | 1.32 (0.18 to 9.85) | 0.78 |
| Warfarin | 1.60 (0.60 to 4.05) | 0.36 | 1.81 (0.64 to 5.14) | 0.26 |
| Risk Factors | Left Main Coronary Artery |
Left Anterior Descending Coronary Artery, Proximal |
||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Luminal stenosis (%) | 1.01 (0.99 to 1.04) | 0.18 | 1.03 (1.01 to 1.05) | <0.01 |
| Smoking | 0.87 (0.35 to 2.17) | 0.76 | 1.44 (0.54 to 3.84) | 0.46 |
| Diabetes | 1.32 (0.57 to 3.07) | 0.52 | 2.08 (0.79 to 5.49) | 0.14 |
| Age (years) | 1.03 (0.99 to 1.08) | 0.12 | 1.06 (1.01 to 1.11) | <0.05 |
| Serum calcium (mg/dl) | 0.81 (0.52 to 1.26) | 0.35 | 1.28 (0.79 to 2.01) | 0.32 |
| Serum phosphorus (mg/dl) | 0.98 (0.69 to 1.37) | 0.89 | 1.12 (0.76 to 1.64) | 0.58 |
| Calcium-phosphorus product (mg2/dl2) | 0.99 (0.96 to 1.02) | 0.56 | 1.01 (0.97 to 1.05) | 0.55 |
| Calcium-containing phosphate binders | 10.04 (0.40 to 87.52) | 0.98 | 9.80 (0.30 to 82.40) | 0.98 |
| Hemodialysis | 14.2 (1.8 to 111.5) | <0.05 | 7.53 (0.95 to 59.6) | 0.06 |
| eGFR (ml/min/1.73 m2) | 0.97 (0.95 to 0.99) | <0.0005 | 0.99 (0.97 to 1.00) | 0.09 |
| CRP (mg/dl) | 1.64 (1.04 to 2.60) | <0.05 | 1.32 (0.93 to 1.88) | 0.12 |
| Vitamin D | 1.02 (0.18 to 5.86) | 0.99 | 0.61 (0.11 to 3.56) | 0.58 |
| Warfarin | 2.84 (0.87 to 9.22) | 0.08 | 4.00 (0.86 to 18.57) | 0.07 |
| Risk Factors | Left Anterior Decending Coronary Artery, Distal |
Left Circumflex Coronary Artery, Proximal |
||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Luminal stenosis (%) | 1.01 (0.99 to 1.03) | 0.19 | 1.03 (1.01 to 1.05) | <0.01 |
| Smoking | 0.68 (0.26 to −1.77) | 0.43 | 1.35 (0.54 to 3.35) | 0.52 |
| Diabetes | 1.61 (0.65 to 4.00) | 0.30 | 2.50 (1.00 to 6.25) | 0.05 |
| Age (years) | 1.04 (0.99 to 1.09) | 0.07 | 1.03 (0.99 to 1.08) | 0.16 |
| Serum calcium (mg/dl) | 1.80 (0.93 to 3.49) | 0.08 | 1.22 (0.78 to 1.93) | 0.38 |
| Serum phosphorus (mg/dl) | 1.37 (0.91 to 1.98) | 0.14 | 1.24 (0.85 to 1.79) | 0.27 |
| Calcium-phosphorus product (mg2/dl2) | 1.05 (0.99 to 1.09) | 0.07 | 1.03 (0.99 to 1.07) | 0.19 |
| Calcium-containing phosphate binders | 12.12 (1.41 to 104.05) | <0.05 | 3.60 (0.43 to 29.86) | 0.24 |
| Hemodialysis | 9.53 (2.44 to 37.2) | <0.005 | 2.88 (0.78 to 10.6) | 0.12 |
| eGFR (ml/min/1.73 m2) | 0.97 (0.95 to 0.99) | <0.005 | 0.97 (0.95 to 0.99) | <0.001 |
| CRP (mg/dl) | 1.10 (0.94 to 1.28) | 0.23 | 1.01 (0.87 to 1.18) | 0.88 |
| Vitamin D | 0.45 (0.05 to 4.51) | 0.50 | 0.71 (0.12 to 4.14) | 0.71 |
| Warfarin | 1.25 (0.45 to 3.47) | 0.68 | 2.02 (0.62 to 6.61) | 0.24 |
| Risk Factors | Left Circumflex Coronary Artery, Distal |
|
|---|---|---|
| RR (95% CI) | P value | |
| Risk factors | ||
| Luminal stenosis (%) | 1.02 (1.01 to 1.04) | <0.05 |
| Smoking | 0.63 (0.26 to 1.52) | 0.30 |
| Diabetes | 5.10 (2.05 to 12.7) | <0.0005 |
| Age (years) | 1.02 (0.98 to 1.06) | 0.29 |
| Serum calcium (mg/dl) | 1.27 (0.80 to 2.01) | 0.30 |
| Serum phosphorus (mg/dl) | 1.29 (0.91 to 1.83) | 0.15 |
| Calcium-phosphorus product (mg2/dl2) | 1.03 (0.99 to 1.07) | 0.13 |
| Calcium-containing phosphate binders | 3.25 (0.78 to 13.50) | 0.06 |
| Hemodialysis | 3.84 (1.01 to 8.80) | <0.05 |
| eGFR (ml/min/1.73 m2) | 0.98 (0.96 to 0.99) | <0.01 |
| CRP (mg/dl) | 1.12 (0.97 to 1.30) | 0.14 |
| Vitamin D | 1.24 (0.17 to 9.20) | 0.84 |
| Warfarin | 1.53 (0.57 to 4.10) | 0.40 |
Multivariate analysis for the presence of intimal calcification was performed based on the logistic regression analysis (Table 6). The significant variables were kidney function, luminal stenosis, and age.
Table 6.
Multivariate risk factors for the presence of intimal plaque calcification in each coronary artery segment based on logistic regression analysis
| Right Coronary Artery, Proximal |
Right Coronary Artery, Distal |
Left Main Coronary Artery |
||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Luminal stenosis (%) | 1.05 (1.02 to 1.08) | <0.001 | 1.01 (0.96 to 1.03) | 0.64 | 02 (1.00 to 1.05) | 0.06 |
| eGFR (ml/min/1.73 m2) | 0.98 (0.95 to 1.01) | 0.15 | 0.96 (0.93 to 0.99) | <0.005 | 0.97 (0.94 to 0.99) | <0.05 |
| Smoking | 4.19 (1.16 to 15.07) | <0.05 | 2.07 (0.93 to 9.90) | 0.36 | 0.55 (0.15 to 2.06) | 0.38 |
| Diabetes | 2.33 (0.70 to 7.75) | 0.17 | 1.26 (0.33 to 4.88) | 0.73 | 0.99 (0.28 to 3.63) | 0.99 |
| Age (years) | 1.05 (1.01 to 1.11) | <0.05 | 1.09 (1.01 to 1.17) | <0.05 | 1.04 (0.98 to 1.10) | 0.17 |
| Calcium-phosphorus product (mg2/dl2) | 0.99 (0.95 to 1.05) | 0.97 | 1.01 (0.96 to 1.07) | 0.60 | 0.96 (0.91 to 1.01) | 0.12 |
| CRP (mg/dl) | 1.07 (0.90 to 1.28) | 0.45 | 1.05 (0.88 to 1.26) | 0.59 | 1.13 (0.87 to 1.48) | 0.36 |
| Left Anterior Descending Coronary Artery, Proximal |
Left Anterior Descending Coronary Artery, Distal |
|||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Luminal stenosis (%) | 06 (1.02 to 1.11) | <0.005 | 1.02 (0.99 to 1.05) | 0.20 |
| eGFR (ml/min/1.73 m2) | 0.96 (0.93 to 0.99) | <0.05 | 0.98 (0.96 to 0.99) | <0.05 |
| Smoking | 0.95 (0.21 to 4.31) | 0.94 | 0.63 (0.14 to 2.88) | 0.55 |
| Diabetes | 6.92 (1.03 to 46.41) | <0.05 | 0.91 (0.24 to 3.39) | 0.88 |
| Age (years) | 1.05 (0.99 to 1.11) | 0.12 | 98 (0.92 to 1.06) | 0.63 |
| Calcium-phosphorus product (mg2/dl2) | 1.02 (0.95 to 1.09) | 0.56 | 1.02 (0.97 to 1.08) | 0.45 |
| CRP (mg/dl) | 1.07 (0.85 to 1.34) | 0.59 | 1.03 (0.82 to 1.30) | 0.80 |
| Left Circumflex Coronary Artery, Proximal |
Left Circumflex Coronary Artery, Distal |
|||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Luminal stenosis (%) | 1.04 (1.01 to 1.07) | <0.05 | 1.03 (1.00 to 1.06) | 0.08 |
| eGFR (ml/min/1.73 m2) | 0.97 (0.95 to 0.99) | <0.05 | 0.99 (0.96 to 1.02) | 0.37 |
| Smoking | 1.60 (0.38 to 6.67) | 0.52 | 1.34 (0.27 to 6.56) | 0.72 |
| Diabetes | 3.10 (0.67 to 13.95) | 0.14 | 5.06 (1.05 to 24.38) | <0.05 |
| Age (years) | 1.02 (0.97 to 1.08) | 0.41 | 1.01 (0.95 to 1.08) | 0.79 |
| Calcium-phosphorus product (mg2/dl2) | 03 (0.97 to 1.10) | 0.36 | 1.06 (0.99 to 1.13) | 0.08 |
| CRP (mg/dl) | 0.93 (0.77 to 1.11) | 0.40 | 0.92 (0.70 to 1.21) | 0.55 |
Medial Calcification and Risk Factors
We evaluated calcification in media of coronary arteries in all subjects. Medial calcification was present in CKD4/5 and CKD5D groups, in proximal lesions of four cases and distal lesions of three cases among 39 cases. Medial calcification of both proximal and distal lesions was seen in the segments with or without intimal calcification, and the continuous deposit of intimal calcification into the media was not observed. The area of medial calcification occurred <25% of the total medial area in the proximal lesions of three patients and in the distal lesions of two patients. One patient showed medial calcification >75% of the total medial area in both proximal and distal lesions, who had severe CAD and more than 20 yr duration of hemodialysis.
We performed univariate analysis to assess the presence of medial calcification. The only significant variable associated with medial calcification in proximal lesions was the use of calcium-containing phosphate binders (RR 21.0, 95% CI 1.9 to 236.1, P <0.05), while for distal lesions significant variables were hemodialysis treatment (RR 5.1, 95% CI 1.2 to 39.4, P < 0.05) and hemodialysis duration (RR 1.05, 95% CI 1.01 to 1.15, P < 0.05).
Discussion
We evaluated the pathologic findings of coronary arteries focusing on locality and severity of calcification in autopsy cases known to have CAD and a wide range of kidney function. Our results showed that: (1) calcification of coronary arteries was deposited intimal plaque mainly in all cases but also medial layer among cases of CKD4/5 and CKD5D; (2) intimal plaque calcification was more severe in patients who had received hemodialysis than in those who did not; (3) intimal calcification was more intense in patients with an eGFR below 30 ml/min/1.73 m2 than in CKD patients with an eGFR over 60 ml/min/1.73 m2 or in patients without CKD; (4) intimal calcification was associated with both traditional cardiovascular and uremic risk factors; and (5) medial calcification was present in CKD4/5 and hemodialysis cases, with incidence increased by the presence of uremic risk factors.
Calcification may arise in all types of arteries, both large elastic vessels and smaller muscular ones. The location and degree of vascular calcification depend on physiologic and pathologic conditions (12–14). Calcification develops earlier than otherwise might in the absence of severe CKD, and contributes to the markedly increased cardiovascular risk observed in this particular population (12–27).
The CAC is thought to occur in two types in the vessel wall. There is still debate about whether these two types of calcification are distinct entities or not (12–14). London et al. found that typical plain x-ray aspects in CKD patients showed either a patchy distribution, which is thought to be characteristic of intimal calcification in association with atherosclerosis, or a pipeline-like distribution attributed to medial calcification (33,34). However, in many patients with ESRD these two processes seem to develop in parallel (12). Although the entire vascular tree may calcify, only some segments develop atherosclerosis, including the coronary arteries, the aorta, and the arteries of the abdomen and lower extremities (12). In contrast, for example, the arteries of the upper extremities, appear relatively or entirely resistant to the atheromatous process (12).
Intimal and medial calcification is thought to differ with regard to their clinical relevance. Whereas intimal calcification appears to contribute to plaque vulnerability, possibly in a biphasic manner, medial calcification contributes to vascular stiffness, which increases pulse-wave velocity and decreases diastolic BP and increases systolic BP (35). Hemodialysis patients with predominantly intimal calcification have a higher relative risk of mortality than those with predominantly medial calcification (34). However, presently available noninvasive imaging techniques cannot provide a clear-cut distinction between intimal and medial calcification. Only microscopic analysis of vessel samples from surgery or autopsy allows that distinction of localization of calcification. Most adult patients with CKD suffer from both intimal and medial calcification.
Given the above, we chose to analyze autopsied cases with known CAD and a wide range of kidney function to distinguish the presence of both intimal and medial calcification and determine which risk factors contribute to each type of calcification. We found that in patients with known CAD, CAC occurred at plaque-forming degenerative intimal changes. The degree of intimal plaque calcification correlated with the degree of plaque-forming degenerative changes that is with the degree of luminal stenosis. Intimal calcification was also related to renal function. Using EBCT, Tomiyama et al. found that traditional and nontraditional risk factors correlated with calcification in prehemodialysis patients (27). Garlannd et al. reported that in patients with stages 3, 4, and 5 CKD without cardiovascular disease, traditional cardiovascular risk factors and serum calcium levels were associated with CAC, while no association was shown with eGFR (25). The range of kidney function was wider in our study than in those mentioned above (25,27), enabling us to evaluate the association between CAC and kidney function. Indeed, no association was found in several segments in our study in CKD3, CKD4/5, or CKD5D patients. These findings showed that intimal calcification had already started before kidney function reached CKD3, and after reaching CKD3 the degree of calcification developed significantly. The finding of Go et al. that eGFR below 45 ml/min was a risk factor for cardiovascular mortality (36) may be associated with the presence of intimal calcification of CKD3 patients.
We demonstrated that medial calcification occurs in CKD4/5 and CKD5D patients with known CAD, in coronary sections with and without significant stenosis or intimal calcification. The medial calcification was not due to the continuous deposition of intimal calcification into the media. This finding was more evident in hemodialysis patients without significant stenosis or intimal calcification (unpublished data). This observation may support the hypothesis that mechanism of both types of calcification would be different. We cannot conclude that no association was present between medial calcification and atheromatous plaque formation. Medial calcification was associated with hemodialysis therapy, but not with stenosis of arteries. We identified the different risk factors for intimal and medial calcification; for medial calcification, these included uremic risk factors.
One limitation of our study is that we analyzed only autopsy subjects known to have CAD, most of whom died of cardiovascular diseases, and did not examine any subjects without CAD. Our autopsy study was necessarily performed using a chart review to obtain each individual's clinical history, potentially introducing a degree of ascertainment bias. A second source of potential bias in this study is the use of estimated GFR. At higher GFR levels, the value may be underestimated, an issue common to all populations.
In conclusion, we have demonstrated the presence and associations of CAC in patients with CAD and a wide variation in kidney function. Traditional and nontraditional risk factors were applicable to CAC in patients with CAD and CKD. Kidney function, smoking, diabetes, calcium-phosphorus metabolism, and aging were risk factors for intimal calcification. Medial calcification was strongly suspected to relate to uremic risks. We should attempt to control both types of risk factors to prevent the onset of CAC before stage 3 CKD.
Disclosures
None.
Acknowledgments
The authors thank the members of the Department of Pathology, National Cardiovascular Center, for their technical support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Coronary Calcification in Chronic Kidney Disease: Morphology, Mechanisms and Mortality,” on pages 1883–1885.
References
- 1.US Renal Data System. Patient mortality and survival in ESRD. Am J Kidney Dis 34: S74–S86, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Nakai S, Masakane I, Akiba T, Shigematsu T, Yamagata K, Watanabe Y, Iseki K, Itami N, Shinoda T, Morozumi K, Shoji T, Marubayashi S, Morita O, Kimata N, Shoji T, Suzuki K, Tsuchida K, Nakamoto H, Hamano T, Yamashita A, Wakai K, Wada A, Tsubakihara Y: Overview of dialysis treatment in Japan as of 31 December 2006. Ther Apher Dial 12: 428–456, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W: The cardiovascular disease continuum validated: Clinical evidence of improved patient outcomes. part II: Clinical trial evidence (acute coronary syndromes through renal disease) and future directions. Circulation 114: 2871–2891, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Levey AS, Schoolwerth AC, Coresch J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey M, Pfeffer M, Raji L, Spinosa DJ, Wilson PW: American Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention: Kidney disease as a risk factor for development of cardiovascular diseases: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Hostetter TH: Chronic kidney disease predicts cardiovascular disease. N Engl J Med 351: 1344–1346, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lamerie N: for the European Uremic Toxin Work Group: Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S12–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Dawber TR, Kagan A: Factors of risk in the development of coronary artery disease: Six-year follow-up experience. The Framingham study. Ann Intern Med 55: 33–50, 1960 [DOI] [PubMed] [Google Scholar]
- 9.Tomita J, Kimura G, Inoue T, Inenaga T, Sanai T, Kawano Y, Nakamura S, Baba S, Matsuoka H, Omae T: Role of systolic blood pressure in determining prognosis of hemodialyzed patients. Am J Kidney Dis 25: 405–412, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 11.McCullough PA, Sandberg KR, Dumler F, Yanez JE: Determinants of coronary vascular calcification in patients with chronic kidney disease and end-stage renal disease: A systemic review. J Nephrol 17: 205–215, 2004 [PubMed] [Google Scholar]
- 12.Drueke TB: Arterial intima and media calcification: Distinct entities with different pathogenesis or all the same? Clin J Am Soc Nephrol 3: 1583–1584, 2008 [DOI] [PubMed] [Google Scholar]
- 13.McCullough PA, Agrawal V, Danielewicz E, Abela GS: Accelerated atherosclerotic calcification and Monckebergs's sclerosis: A continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol 3: 1585–1598, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Amann K: Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3: 1599–1605, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS: Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathological correlative study. Circulation 92: 2157–2162, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregorie J, Fitzpatrick LA, Schwartz RS: Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using no decalcifying methodology. J Am Coll Cardiol 31: 126–133, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Detrano R, Hsiai T, Wang S, Puentes G, Fallavollita J, Shields P, Sranford W, Wolfkiel C, Georgiou D, Budoff M, Reed J: Prognostic value of coronary calcification and angiographic stenosis in patients undergoing coronary angiography. J Am Coll Cardiol 27: 285–290,1996 [DOI] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358: 1336–1345, 2008 [DOI] [PubMed] [Google Scholar]
- 19.McCullough PA, Soman S: Cardiovascular calcification in patients with chronic renal failure. Are we on target with this risk factor? Kidney Int 66: S18–S24, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Bursztyn M, Motro M, Grossman E, Shemesch J: Accelerated coronary calcification in mildly reduced kidney function of high-risk hypertensives: 3-year prospective observation. J Hypertens 21: 1953–1959, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fox CS, Larson MG, Keyers MJ, Clouse ME, Culleton B, O'Donnell CJ: Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: The Framingham Heart Study. Kidney Int 66: 2017–2021, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mehrotra R, Budoff M, Christenson P, Ipp E, Takasu J, Gupta A, Norris K, Adler S: Determinants of coronary artery calcification in diabetes with and without nephropathy. Kidney Int 66: 2022–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Gross ML, Meyer HP, Ziebart H, Rieger P, Wenzel U, Amann K, Berger I, Adamczak M, Schirmacher P, Ritz E: Calcification of coronary intima and media: Immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol 2: 121–134, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Garland JS, Holden RM, Groome P, Lam M, Nolan RL, Morton AR, Pickett W: Prevalence and associations of coronary artery calcification in patients with stage 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis 52: 849–858, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RD, Hedayati SS: Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int 73: 615–621, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Tomiyama C, Higa A, Dalboni MA, Cendorogio M, Draibe SA, Cuppari L, Carvalho AB, Neto EM, Canziani ME: The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant 21: 2464–2471, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kuroda S, Nishida N, Uzu T, Takeji M, Nishimura M, Fujii T, Nakamura S, Inenaga T, Yutani C, Kimura G: Prevalence of renal artery stenosis in autopsy patients with stroke. Stroke 31: 61–65, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Green T, Eknoyan G, Levy A: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 30.National-Kidney-Foundation.K/DOQI: Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 31.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S: Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis 50: 927–937, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW: A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 15: 1512–1531, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Bellasi A, Raggi P: Techniques and technologies to assess vascular calcification. Semin Dial 20: 129–133, 2007 [DOI] [PubMed] [Google Scholar]
- 34.London GM, Guerin AP, Marchais S, Metvier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Jhonson RC, Leopold JA, Loscalzo J: Vascular calcification: Pathobiological mechanisms and clinical implications. Circ Res 99: 1044–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]