Abstract
Background and objectives: Acute kidney injury (AKI) is associated with adverse outcomes in critically ill patients. The influence of preexisting chronic kidney disease (CKD) on AKI outcomes is unclear.
Design, setting, participants, & measurements: We analyzed data from a prospective observational cohort study of AKI in critically ill patients who received nephrology consultation: the Program to Improve Care in Acute Renal Disease. In-hospital mortality rate, length of stay, and dialysis dependence were compared in patients with and without a prior history of CKD, defined by an elevated serum creatinine, proteinuria, and/or abnormal renal ultrasound within a year before hospitalization. We hypothesized that patients with AKI and prior history of CKD would have lower mortality rates, shorter lengths of stay, and higher rates of dialysis dependence than patients without prior history of CKD.
Results: Patients with AKI and a prior history of CKD were older and underwent nephrology consultation earlier in the course of AKI. In-hospital mortality rate was lower (31 versus 40%, P = 0.04), and median intensive care unit length of stay was 4.6 d shorter (14.7 versus 19.3 d, P = 0.001) in patients with a prior history of CKD. Among dialyzed survivors, patients with prior CKD were also more likely to be dialysis dependent at hospital discharge. Differences in outcome were most evident in patients with lower severity of illness.
Conclusions: Among critically ill patients with AKI, those with prior CKD experience a lower mortality rate but are more likely to be dialysis dependent at hospital discharge. Future studies should determine optimal strategies for managing AKI with and without a prior history of CKD.
Acute kidney injury (AKI) is associated with adverse outcomes, particularly in critically ill patients. In-hospital mortality rates range from 30 to 80%, depending on the clinical setting and definitions used. Several studies have shown that underlying chronic kidney disease (CKD) markedly increases the risk of AKI and that the risk increases proportional to the CKD stage (1–4). Additionally, data from the United States Renal Data System showed that the percentage of incident ESRD patients who experienced an episode of AKI in the previous 2 yr has doubled over the past 10 yr (4). Among hospitalized Medicare beneficiaries, patients with CKD have a 10-fold higher incidence of AKI. It is unclear whether patients with AKI and prior CKD experience a different disease course and outcomes than those without prior CKD, because data from several studies are conflicting (5–7). Most studies have suggested that patients with prior CKD are more likely to remain dialysis dependent after an episode of dialysis-requiring AKI (5,8,9). The association between prior CKD and mortality has been variably described. Recent studies suggest that prior CKD may be associated with lower mortality in dialyzed intensive care unit (ICU) patients (5,6,10). In this analysis, we explored whether prior CKD modified the association between AKI and outcomes (mortality, ICU and hospital length of stay, and dialysis dependence) in critically ill patients enrolled in a prospective observational multicenter cohort study (11). We hypothesized that patients with AKI who had a prior history of CKD would have lower mortality rates, shorter lengths of stay, and higher rates of dialysis dependence than patients with AKI with no prior history of CKD.
Materials and Methods
The Program to Improve Care in Acute Renal Disease (PICARD) is an observational study from five academic medical centers (University of California San Diego [UCSD], Cleveland Clinic Foundation, Maine Medical Center, Vanderbilt University, and University of California San Francisco [UCSF]) that aimed to identify demographic, process of care, and clinical factors that were associated with favorable and adverse outcomes after AKI among ICU patients who underwent nephrology consultation. It enrolled patients from February 1999 to August 2001. A detailed description of PICARD inclusion and exclusion criteria, data elements, data collection, and management strategies are described elsewhere (11).
Definitions
Acute kidney injury was defined as an increase in serum creatinine ≥0.5 mg/dl and baseline serum creatinine <1.5 mg/dl or an increase in serum creatinine ≥1.0 mg/dl and baseline serum creatinine ≥1.5 mg/dl and <5.0 mg/dl, as described previously (12). Patients with a baseline serum creatinine ≥5.0 mg/dl or AKI responsive to volume resuscitation were not considered for study inclusion. For each patient, the date on which he or she met criteria for AKI was identified and designated the AKI start date. The serum creatinine concentration at this point served as the AKI start creatinine.
CKD status was determined at time of enrollment for each patient by evaluating available clinical and laboratory data and history from the patient or surrogate. This information was reviewed for all 618 patients. Patients were considered to have CKD if they had evidence of elevated serum creatinine (>1.5 mg/dl), proteinuria (>300 mg/d), or abnormal renal ultrasound (abnormal renal echogenicity or kidney size or presence of cysts) within a year before the index hospitalization. The specific serum creatinine concentration before hospitalization was not recorded. Patients were classified as AKI with a prior history of CKD if they met criteria for CKD as defined above (28% of cohort). All remaining patients were considered as AKI without a prior history of CKD. Severity of illness scores were calculated for each day of hospitalization (13).
Outcomes
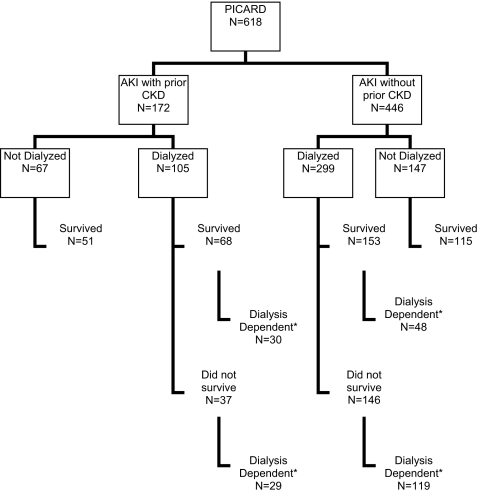
Outcomes included in-hospital mortality rate, provision of dialysis, dialysis dependence (evaluated only in patients who survived hospitalization and defined as receiving dialysis within the last 3 d of hospitalization), and ICU and hospital lengths of stay. Dialysis decisions for each patient were at the discretion of individual nephrologists based on standard indications of solute, volume, and acid/base management. Figure 1 shows a flow chart for patients included in this analysis.
Figure 1.
Flow chart for patients in PICARD comparing AKI with and without preexisting CKD. *Dialysis dependence was defined as receiving dialysis within the last 3 d of death or discharge.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median with interquartile range and compared using either the t test or Wilcoxon rank sum test where appropriate. Categorical variables were expressed as proportions and compared with the χ2 test. Logistic regression was used for multivariable analyses, adjusting for comorbidities and the components of the nonrenal sequential organ failure assessment (SOFA) score at AKI start (platelet count, bilirubin level, administered dose of epinephrine, administration of dobutamine, the Pao2/Fio2 ratio, and Glasgow coma scale score). Model discrimination was assessed using the area under the receiver operating characteristic curve (14,15). Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test. The Hosmer-Lemeshow test compares model performance (observed versus expected) across deciles of risk to test whether the model is biased (i.e., performs differentially at the extremes of risk). A nonsignificant value for the Hosmer-Lemeshow χ2 suggests an absence of such bias (14,15). Two-tailed P < 0.05 was considered significant. Statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
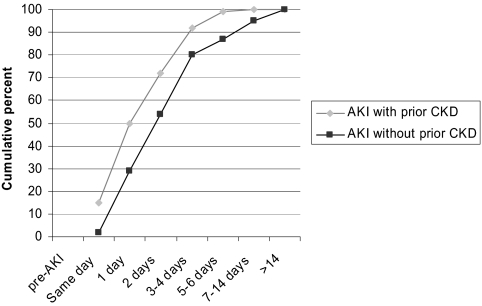
The most common cause of AKI in both those with and without prior CKD was a nephrotoxic medication (26% in both groups). The characteristics of the PICARD cohort at the time of AKI diagnosis for those with and without prior CKD are presented in Table 1. Patients with prior CKD were older, with higher rates of hypertension, diabetes, heart failure, and coronary artery disease, and tended to have lower severity of illness (manifested by a lower SOFA score, excluding the SOFA renal component) at time of AKI diagnosis. Patients with prior CKD also received nephrology consultation earlier in the AKI course (Figure 2).
Table 1.
Patient baseline characteristics at time of AKI start (n = 618)
| Variable | With Prior CKD(n = 172) | Without Prior CKD(n = 446) | P Value |
|---|---|---|---|
| Age (yr) | 65.6 | 57.1 | <0.0001 |
| Male (%) | 58 | 59 | 0.81 |
| Dry weight (kg) | 80.4 | 82.2 | 0.37 |
| Race/ethnicity (%) | 0.79 | ||
| White | 79 | 80 | |
| African American | 12 | 7 | |
| Hispanic | 5 | 7 | |
| Asian/Pacific Islander | 3 | 4 | |
| Other/mixed race | 2 | 1 | |
| Surgery before/on day of ICU admission (%) | 35 | 39 | 0.33 |
| Hypertension (%) | 70 | 46 | <0.0001 |
| Diabetes mellitus (%) | 44 | 23 | <0.0001 |
| Liver disease (%) | 14 | 23 | 0.01 |
| Heart failure (%) | 51 | 19 | <0.0001 |
| Coronary artery disease (%) | 59 | 28 | <0.0001 |
| Systolic BP (mmHg) | 114 | 117 | 0.16 |
| Diastolic BP (mmHg) | 56 | 55 | 0.32 |
| MAP (mmHg) | 76 | 76 | 0.88 |
| Temperature (°C) | 37.0 | 36.8 | 0.02 |
| Median urine output (ml) | 1278 | 920 | 0.59 |
| Oliguria (≤400 ml/day) | 26 | 30 | 0.33 |
| Creatinine (mg/dL)a | 3.8 | 3.3 | 0.002 |
| BUN (mg/dL) | 60 | 77 | <0.0001 |
| pH | 7.35 | 7.36 | 0.77 |
| Potassium (mM) | 4.6 | 4.7 | 0.40 |
| Bicarbonate (mM) | 21.2 | 21.8 | 0.19 |
| Leukocyte count (1000/mm3) | 15 | 12 | 0.0008 |
| Hemoglobin (g/dL) | 10.3 | 9.9 | 0.03 |
| SOFA score | 8.1 | 9.3 | 0.0002 |
| Days between AKI diagnosis and nephrology consultation | 0.9 | 3.2 | <0.0001 |
BUN, blood urea nitrogen.
Creatinine at time of AKI start is the creatinine that qualified patient for AKI as defined by PICARD enrollment criteria (increase in serum creatinine ≥0.5 mg/dl and baseline serum creatinine <1.5 mg/dl or an increase in serum creatinine ≥1.0 mg/dl and baseline serum creatinine ≥1.5 mg/dl and <5.0 mg/dl.
Figure 2.
Time between AKI start and nephrology consultation based on prior history of CKD.
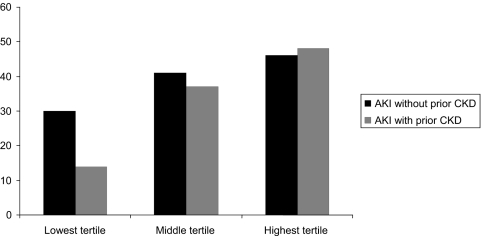
Table 2 shows the outcomes in the overall cohort (n = 618). Patients with prior CKD had lower in-hospital mortality rates. Figure 3 shows mortality rates for those with and without prior history of CKD based on tertile of nonrenal SOFA score. The difference in in-hospital mortality for patients with prior CKD was most pronounced in patients with the lowest severity of illness. After adjustment for comorbidities and nonrenal SOFA score, only Glascow coma score (odds ratio [OR], 1.29; 95% confidence interval [CI], 1.07 to 1.54) and platelet count (OR, 1.20; 95% CI, 1.00 to 1.43) remained significantly associated with mortality (area under the model's receiver operating characteristic curve was 0.66, and the model was well calibrated; Hosmer-Lemeshow χ2, P = 0.91). Individual comorbidities were not significantly associated with mortality. The median ICU length of stay was 4.6 d shorter for those with prior CKD; the difference remained significant after adjustment for comorbidities and nonrenal SOFA score (P < 0.001). Total length of hospitalization and the provision of dialysis during the AKI course were similar in both groups.
Table 2.
Outcomes in those with and without prior CKD
| Variable | With Prior CKD (n = 172) | Without Prior CKD (n = 446) | P Value |
|---|---|---|---|
| In-hospital mortality (%) | 31 | 40 | 0.04 |
| Provision of dialysis (%) | 61 | 67 | 0.16 |
| ICU length of stay (d) | 14.7 | 19.3 | 0.001 |
| Hospital length of stay (d) | 31.3 | 34.8 | 0.19 |
Figure 3.
Mortality in tertiles of nonrenal SOFA scores based on prior history of CKD.
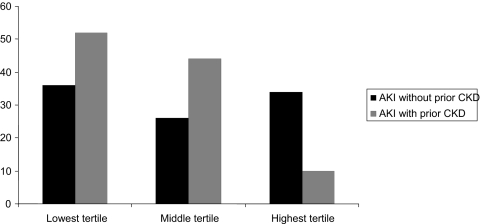
Although death was less common, dialysis dependence was more frequent among patients with prior CKD. Among the 219 dialyzed survivors, patients with prior CKD were more likely to be dialysis dependent at discharge (45 versus 32%, P = 0.06). The association of prior CKD with dialysis dependence stratified by nonrenal SOFA score is shown in Figure 4.
Figure 4.
Dialysis dependence in tertiles of nonrenal SOFA scores based on prior history of CKD.
Discussion
Our knowledge of the link between AKI and CKD continues to evolve. It is evident that CKD influences the risk of developing AKI, and recent studies suggest that AKI per se may contribute to CKD progression and incidence of ESRD (4). However, the degree to which CKD modifies the association between AKI and outcomes remains unclear. Prior studies have used a variety of definitions for AKI and CKD, rendering comparisons across studies difficult. The initial inpatient serum creatinine concentration has often been considered a “baseline” even when it is substantially higher than the true baseline, potentially misclassifying patients with CKD. A key aspect of this study is the delineation of CKD based on data (history, clinical, and laboratory) available before hospitalization rather than the serum creatinine concentration at the start of hospitalization (where “baseline” serum creatinine could reflect AKI in evolution).
Several previous studies have shown different mortality rates based on CKD status in patients with AKI. Two studies showed lower mortality rates associated with prior CKD, both at ICU discharge and at 90 d (5,6). Conversely, another study found no difference in mortality rate by baseline CKD status at 28 d, 90 d, and 1 yr (7). High serum creatinine concentrations at hospital admission have also been associated with lower mortality and shorter lengths of stay in critically ill patients, although is it unclear whether this finding relates to underlying CKD or other factors influencing serum creatinine (16). In our study, patients with prior CKD were less likely to die in-hospital despite older age and more extensive comorbidity. Concordant with prior studies, we also showed a higher rate of dialysis dependence in dialyzed survivors who had underlying CKD. Because severity of illness conditions the course and outcomes of AKI, the associations of baseline CKD status with death and dialysis dependence within each of tertile of severity are instructive. The apparent influence of CKD was in opposing directions for death and dialysis dependence. With regard to mortality, the “protective” association of prior CKD was most evident in the lowest tertile of severity of illness. In contrast, among survivors, those with prior CKD in the lowest tertile of severity of illness had the highest rates of dialysis dependence. Taken together, these findings support the notion that any protective innate characteristic that a critically ill patient may possess is unlikely to influence outcomes in the sickest patients because of the overwhelming nature of the underlying severity of illness.
Although it is difficult to ascertain a specific cause for these associations in an observational study, our data are consistent with previous observations. Several reasons could be postulated to explain these findings. Preclinical studies have shown that the presence of adaptive mechanisms such as high osmolar clearance per nephron and low fractional excretion of sodium seen in CKD can alter the course of AKI (17). Similarly, ischemic preconditioning seems to be protective for AKI (18). Whether these factors play a role in humans is unknown and will need further prospective evaluation. Of interest, there were no differences in median urine output, the proportion with oliguria, peak blood urea nitrogen, and serum creatinine in the two groups. Aside from patient characteristics, process of care elements may also influence outcomes in AKI (19). In our study, earlier identification of AKI among patients with prior CKD could have modified the process of care delivered to these patients, manifested by earlier nephrology consultation (by roughly 2 d). Whether earlier consultation in patients with CKD reflected heightened concern for kidney function or whether patients without prior CKD experienced more urgent unrelated problems, prompting a relative delay in nephrology consultation, is unknown. Additionally, because dialysis decisions were not based on specific protocols, it is likely that some patients did not receive dialysis because of perceived futility. The degree to which these factors influenced our findings is unclear.
This study has several important strengths. We used standardized definitions for AKI and CKD. We captured extensive data on comorbidity and severity of illness, which allowed us to adjust for these factors when considering outcomes such as mortality, provision of dialysis, and ICU and hospital lengths of study. The cohort was ethnically diverse and reasonably representative of critically ill patients in the United States. There are also important limitations to our analysis. As an observational study, results could be influenced by residual confounding for which we could not adjust. It is also possible that we misclassified some patients as having CKD, especially because the creatinine cut-off for defining CKD status of >1.5 mg/dl (a standard definition at the time of PICARD design) did not take into account age, gender, or race. Additionally, we did not specifically record individual parameters (elevated serum creatinine, the presence of proteinuria, or abnormal renal ultrasound) leading to designation of CKD or the time duration of CKD. However, the designation of CKD was multidimensional, and we were somewhat reassured that the prevalence of CKD in this study was comparable to other studies of similar cohorts with AKI (5,20–22). Last, the results of this study only apply to ICU patients who develop AKI and undergo nephrology consultation. Patients who have AKI for whom nephrology consultation is not requested may be different in important ways that reduce our findings generalizability.
The most clinically useful implications of this study relate to prognostic stratification for critically ill patients with AKI. The results suggest that expected outcomes for patients with AKI in the ICU vis-à-vis length of stay and dialysis requirements differ considerably by CKD status. Although mortality rates are high for both groups, patients with AKI and prior CKD who survived hospitalization were more likely to remain dialysis dependent. We suggest that, in critically ill patients, efforts should be made to determine a prior history of CKD at hospital admission. Renoprotective and therapeutic maneuvers need to consider the different trajectories of AKI patients with and without prior CKD for mortality and dialysis dependence. Nephrologists and other intensivists should be mindful of these findings, particularly when working with families and other physicians and surgeons in the midst of acute illness. Furthermore, future studies evaluating the influence of prior CKD on AKI should consider standardizing the concept of “baseline” creatinine to truly reflect underlying CKD status.
As more is learned about the interactions between AKI and CKD, we are hopeful that novel, optimal, and potentially unique management strategies for patients with and without prior CKD can be developed.
Disclosures
None.
Acknowledgments
This study was supported by the following research grants: NIH-NIDDK RO1-DK53412, RO1-DK53411, and RO1-DK53413. This study was presented in abstract form at the 2007 Meeting of the American Society of Nephrology; San Francisco, CA; November 2 through 5, 2007.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash KA, Hafeez S, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ: Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 320: 143–149, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, MacLeod AM: A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant 22: 2513–2519, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Groeneveld AB, Tran DD, van der Meulen J, Nauta JJ, Thijs LG: Acute renal failure in the medical intensive care unit: Predisposing, complicating factors and outcome. Nephron 59: 602–610, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T: Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit Care 9: R700–R709, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spurney RF, Fulkerson WJ, Schwab SJ: Acute renal failure in critically ill patients: Prognosis for recovery of kidney function after prolonged dialysis support. Crit Care Med 19: 8–11, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Noble JS, MacKirdy FN, Donaldson SI, Howie JC: Renal and respiratory failure in Scottish ICUs. Anaesthesia 56: 124–129, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Brar H, Olivier J, Lebrun C, Gabbard W, Fulop T, Schmidt D: Predictors of mortality in a cohort of intensive care unit patients with acute renal failure receiving continuous renal replacement therapy. Am J Med Sci 335: 342–347, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM; Program to Improve Care in Acute Renal Disease: Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT: Hospital-acquired renal insufficiency: A prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM: Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cartin-Ceba R, Afessa B, Gajic O: Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index. Crit Care Med 35: 2420–2423, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Cronin RE, de Torrente A, Miller PD, Bulger RE, Burke TJ, Schrier RW: Pathogenic mechanisms in early norepinephrine-induced acute renal failure: Functional and histological correlates of protection. Kidney Int 14: 115–125, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Bonventre JV: Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 11: 43–48, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Mehta RL, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual MT, Zhuang S, Kaplan RM, Chertow GM: Nephrology consultation in acute renal failure: Does timing matter? Am J Med 113: 456–461, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Metcalfe W, Simpson M, Khan IH, Prescott GJ, Simpson K, Smith WC, MacLeod AM; Scottish Renal Registry: Acute renal failure requiring renal replacement therapy: Incidence and outcome. QJM 95: 579–583, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hegarty J, Middleton RJ, Krebs M, Hussain H, Cheung C, Ledson T, Hutchison AJ, Kalra PA, Rayner HC, Stevens PE, O'Donoghue DJ: Severe acute renal failure in adults: Place of care, incidence and outcomes. QJM 98: 661–666, 2005 [DOI] [PubMed] [Google Scholar]