Abstract
Background and objectives: In medullary sponge kidney (MSK)—a common malformative renal condition in patients with calcium nephrolithiasis—hypercalciuria, incomplete distal renal tubular acidosis, and hypocitraturia are common. Clinical conditions with concomitant hypercalciuria and/or incomplete distal renal tubular acidosis are almost invariably associated with bone disease, making osteopathy highly likely in MSK, too. Patients with MSK have never been investigated for osteopathy; neither has the potential effect of potassium citrate administration (CA) on their urinary metabolic risk factors and on bone mineralization.
Design, setting, participants, & measurements: These issues were retrospectively analyzed in 75 patients with MSK and primary stone risk factor (PSRF; hypercalciuria, hypocitraturia, hyperuricosuria, and/or hyperoxaluria) on an outpatient basis; 65 received CA (2.9 ± 0.8 g/d), whereas 10 received only general “stone clinic” suggestions. The 24-h urinary excretion of calcium, phosphate, oxalate, uric acid, and citrate; morning urine pH; serum biochemistry; and bone mineral density were investigated at baseline and at the end of follow-up (78 ± 13 and 72 ± 15 mo in groups A and B, respectively).
Results: CA led to a significant rise in urinary pH and citrate and decreased urinary calcium and phosphate (all P < 0.001). Patients with MSK and PSRF had reduced bone density. Bone density improved significantly in the group that was treated with oral CA.
Conclusions: Bone disease is very frequent in patients with MSK and concomitant PSRF. Long-term CA improves bone density. The concurrent effects of treatment on PSRF suggest that the subtle acidosis plays a pivotal role in bone disease and hypercalciuria in patients with MSK.
Medullary sponge kidney (MSK) is a malformative renal condition that is associated with (1) a high risk for nephrocalcinosis and renal stones; (2) a number of tubular functional anomalies (e.g., incomplete distal renal tubular acidosis [idRTA]) and hypocitraturia, defective urinary concentration, hypercalciuria); and (3) precalyceal cystic anomalies of the Bellini ducts (1). Approximately 3 to 5% of recurrent renal stone formers have MSK, although much larger proportions (up to 20%) have also been reported (1). The diagnosis is radiographic, and intravenous urography is still the diagnostic cornerstone. The most common presenting clinical sign is recurrent calcium nephrolithiasis. The frequent association of hypercalciuria, idRTA, and hypocitraturia, in conjunction with urinary stasis in the papillary duct ectasias, may trigger the formation of calcium phosphate and/or calcium oxalate stones.
Although MSK has been described in patients with various developmental disorders that involve other organs (1), it is considered a single-organ (kidney) disorder in the majority of cases. In other clinical conditions, both the hypercalciuria and the dRTA are almost invariably associated with bone disease, however, presumably making osteopathy highly likely in patients with MSK. Oddly enough, patients with MSK have never been investigated for any kind of bone disease; neither has the potential effect of alkali citrate administration on their urinary metabolic risk factors or any influence on bone mineralization. Hence, we investigated these issues in a retrospective analysis of a group of incident case patients who were observed at our institution, the results of which are reported here.
Materials and Methods
In the past decade, more than 10% of calcium stone formers who were followed up at the renal stone outpatients clinic at the Verona Ospedale Civile Maggiore University Hospital had MSK. The condition was diagnosed during the workup for recurrent calcium nephrolithiasis on the strength of typical MSK pictures at intravenous urography or, more recently, urographic computed tomography scan and exclusion of other causes of nephrocalcinosis. To be eligible for a diagnosis of MSK, patients had to have both kidneys involved, with typical nephrocalcinosis and/or cystic features at the papillary level in at least two papillae in each kidney. Mandatory for the diagnosis of MSK is the demonstration of papillary precaliceal ectasias documented on films taken at least 10 min after injection of the contrast medium, without compression maneuvers and without signs of obstruction.
Our routine diagnostic workup for recurrent calcium stone formers includes the following laboratory tests in serum: Calcium, phosphate, sodium, potassium, chloride, magnesium, osmolarity, intact molecule parathyroid hormone (Immulite; DPC, Los Angeles, CA), 25-hydroxyvitamin D3 (HPLC Eureka kit; Eureka, Ancona, Italy). The workup also includes creatinine clearance (Cockcroft-Gault formula) and urine volume, pH, sodium, chloride, potassium, citrate (by citrate lyase), calcium, phosphate, magnesium, uric acid, and oxalate (by oxalate decarboxylase) in two 24-h urine collections obtained with a 4- to 6-wk interval between them, while on the usual diet (and without any urinary tract infections), considering the mean values of the collections. Hypercalciuria was defined as a 24-h urine calcium level of >300 mg in men or 250 mg in women; hyperuricosuria was defined as a 24-h uric acid level >750 mg in men or >700 mg in women; hypocitraturia was defined as <350 mg/24 h; and hyperoxaluria was defined as >40 mg/24 h.
A pH electrode is used to measure pH on a fresh morning urine spot sample (in the absence of urinary tract infections). Blood gas analysis is performed for patients with a morning urine pH >5.5 and/or hypokalemia (<3.5 mEq/L) and/or hypocitraturia to rule out overt dRTA. The criteria used at our clinic to qualify patients for overt dRTA are metabolic acidosis (blood pH <7.30 and bicarbonate <18 mmol/L) together with a spot urine pH >5.5. Patients whose tests do not reveal overt dRTA are not generally tested for idRTA.
A food frequency questionnaire asking about diet in the previous year is administered only at the initial evaluation. Although our protocol includes bone densitometry, not all patients actually take the test for logistic reasons (approximately 30% of them have no baseline densitometric study). Bone mineral density (BMD; g/cm2) is measured on a level with the vertebrae (L1 through L4), femoral neck, and trochanter using dual-energy x-ray absorptiometry (DEXA; Hologic QDR 4500 fan beam densitometer with software 8.21, Waltham, MA). BMD data are presented here as total T score and Z score.
Treatment with oral potassium citrate (2 to 4 g/d, approximately 10 to 20 mmol/d divided into two or three doses) is generally recommended at our clinic for patients with MSK and at least one primary stone risk factor ([PSRF]; hypercalciuria, hypocitraturia, hyperuricosuria, and hyperoxaluria). The initial dosage is 2 g/d; if tolerated, then the potassium citrate dosage is increased stepwise in patients who initially fail to achieve the target citraturia level (>450 mg/24 h), adding 1 g at a time until the desired citrate level is reached, provided that the urine pH in a 24-h collection is <7.5. Patients are followed up once a month until the treatment dosage has been fine-adjusted, then once every 6 mo.
We retrospectively earmarked patients who had MSK and PSRF and had adhered or not to the suggested treatment for at least 1 yr. Thus, 65 patients who had MSK and PSRF and were treated with potassium citrate for >1 yr form the basis of this study (group A); 48 of them also had two DEXA assessments conducted with an interval of at least 1 yr between them. Ten patients who had MSK and PSRF and did not accept or were not compliant with the suggested treatment constitute the control group (group B). They had a baseline DEXA and recently underwent a second evaluation. All group A and B patients were recurrent stone formers.
All patients with MSK received general “stone clinic” recommendations concerning diet and water intake. In particular, we advised patients to follow a balanced diet that was rich in fruit and vegetables and a regular calcium content and to consume protein 1 g/kg and restrict sodium consumption. Although at follow-up visits no formal evaluation of dietary intakes was performed, patients were asked about their compliance with the suggestions and reinforced on adhering to them.
A statistical analysis was performed on the laboratory and DEXA data obtained during the diagnostic workup and the latest available assessments during the follow-up. The results are given as mean ± SD. Paired t test was used to compare data. Linear relations between continuous variables were evaluated by Pearson correlation.
Results
Demographic and general clinical data are shown in Table 1. All patients had hypercalciuria, and >80% had hypocitraturia. None had classic dRTA or primary or secondary forms of hyperparathyroidism. On average, the number of affected papillae per kidney was 5 ± 3, similar in both groups.
Table 1.
Clinical characteristics of patients with MSK at baseline
| Characteristic | Values |
|
|---|---|---|
| Group A | Group B | |
| Patients (n [% female]) | 65 (71) | 10 (70) |
| Age (yr; mean ± SD) | 26.2 ± 9.0 | 27.7 ± 5.4 |
| Hypercalciuria (n [%]) | 65 (100) | 10 (100) |
| Hypocitraturia (n [%]) | 54 (83) | 8 (80) |
| Hypercalciuria without hypocitraturia (n [%]) | 11 (17) | 2 (20) |
| Hypocitraturia without pH>5.5 (n [%]) | 8 (12) | 0 (0) |
| Hyperoxaluria (n [%]) | 3 (5) | 0 (0) |
| Hyperuricosuria (n [%]) | 4 (6) | 1 (10) |
Group A, patients who had MSK with PSRF and were on citrate; group B, patients who had MSK with PSRF and were not on citrate.
All of the patients with MSK had a creatinine clearance >70 ml/min per 1.73 m2. At baseline, none of the female patients with MSK were in menopause, and none of the patients of either gender had previously taken steroids for >30 d or had hyperthyroidism (based on T3, T4, and thyroid-stimulating plasma levels), chronic bowel disorders, diseases that affect bone remodeling (e.g., neoplasms, myeloma, diabetes, chronic infection, stages 3 through 5 chronic kidney disease, liver disorders), or a history of fractures or bone surgery. All patients were carefully questioned about whether they had ever adopted a low-calcium diet, but all denied such a possibility. Furthermore, food frequency questionnaires showed normal nutritional intakes for all patients. None was taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers or had taken diuretics, vitamin D supplements, or diphosphonates either previously or during the follow-up.
In group A, the average follow-up on citrate therapy was 78 ± 13 mo (range 12 to 96), with a mean potassium citrate dosage of 2.9 ± 0.8 g/d (13.7 ± 3.8 mmol/d). Serum and urine laboratory test findings at baseline and at the end of the treatment follow-up period are shown in Table 2. Compared with the situation before treatment, potassium citrate therapy led to a significant and sustained rise in urinary pH, citrate, and potassium and a decrease in the urinary anion gap. The urinary potassium increase by a mean of 1 g/d, which neared the average daily potassium intake, confirms good patient compliance with the treatment. Potassium citrate administration reduced the urinary calcium and phosphate excretion rates. During treatment, diuresis increased and sodium excretion decreased, suggesting good patient compliance with the general recommendations. Blood potassium levels rose, whereas chloride concentrations dropped slightly, both probably reflecting a less acidic systemic acid-base balance. Serum phosphate levels increased significantly. Both before and during the treatment, there was a strong negative correlation between citraturia and calciuria (r = −0.78 and r = −0.75, respectively; both P < 0.005). In group B, at the end of the follow-up (72 ± 15 mo; range 14 to 91 mo), urinary volume increased; although urinary sodium and potassium changes were NS, the ratio Na/K decreased significantly (Table 2), confirming patient compliance with general suggestions.
Table 2.
Serum and urine parameters at baseline and after at least 12 mo of follow-up
| Parameter | Group A |
Group B |
||
|---|---|---|---|---|
| Baseline | Follow-up Evaluation | Baseline | Follow-up Evaluation | |
| Serum | ||||
| creatinine clearance (ml/min) | 123 ± 41 | 134 ± 56 | 126 ± 14 | 128 ± 36 |
| calcium (mg/dl) | 9.08 ± 0.31 | 9.21 ± 0.40 | 9.32 ± 0.24 | 9.29 ± 0.41 |
| phosphorus (mg/dl) | 2.75 ± 0.33 | 3.49 ± 0.22b | 2.93 ± 0.38 | 2.85 ± 0.43 |
| magnesium (mmol/L) | 0.88 ± 0.09 | 0.91 ± 0.11 | 0.86 ± 0.12 | 0.89 ± 0.14 |
| potassium (mEq/L) | 3.50 ± 0.32 | 4.29 ± 0.39b | 3.42 ± 0.49 | 3.64 ± 0.29 |
| chloride (mEq/L) | 105 ± 4 | 102 ± 3b | 106 ± 4 | 104 ± 5 |
| osmolarity (mOsm/L) | 281 ± 9 | 286 ± 10 | 284 ± 9 | 285 ± 8 |
| PTH (pmol/L) | 4.13 ± 1.98 | 4.07 ± 1.77 | 4.48 ± 1.66 | 4.67 ± 1.90 |
| 25(OH)D3 (nmol/L) | 73.4 ± 17.3 | 71.0 ± 13.2 | 69.4 ± 23.7 | 73.0 ± 19.1 |
| 24-h urine | ||||
| volume (ml) | 2150 ± 231 | 2319 ± 401b | 1950 ± 279 | 2245 ± 251a |
| calcium (mg) | 359 ± 110 | 192 ± 97d | 333 ± 98 | 311 ± 76 |
| phosphorus (mg) | 975 ± 61 | 706 ± 78d | 1023 ± 84 | 893 ± 68c |
| calcium/creatinine (mg/mg) | 0.23 ± 0.15 | 0.15 ± 0.10d | 0.24 ± 0.17 | 0.21 ± 0.13 |
| sodium (mEq) | 212 ± 49 | 177 ± 65d | 207 ± 53 | 165 ± 71 |
| potassium (mEq) | 63 ± 24 | 89 ± 28d | 66 ± 28 | 75 ± 21 |
| chloride (mEq) | 158 ± 69 | 164 ± 57 | 163 ± 58 | 168 ± 44 |
| Na/K | 3.41 ± 0.64 | 2.13 ± 0.55d | 3.19 ± 0.56 | 2.29 ± 0.41d |
| pH | 6.44 ± 0.33 | 6.91 ± 0.52d | 6.23 ± 0.39 | 6.31 ± 0.30 |
| anion gap (mEq/L) | +55 ± 19 | +43 ± 22c | +57 ± 18 | +35 ± 21a |
| citrate (mg) | 288 ± 65 | 553 ± 44d | 267 ± 55 | 287 ± 39 |
| oxalate (mg) | 41 ± 23 | 35 ± 12 | 39 ± 26 | 38 ± 23 |
Data are mean ± SD. Group A, patients who had MSK with PSRF and were on citrate; group B, patients who had MSK with PSRF and were not on citrate.
Statistical significance versus the respective baseline (aP < 0.05, bP < 0.005, cP < 0.001, dP = 0.000).
Table 3 shows the densitometric data obtained before and after at least 12 mo of treatment (on average 6.5 and 6.0 yr after baseline evaluation in groups A and B, respectively). Patients with MSK had at baseline a DEXA profile of osteopenia-osteoporosis: 59% had a T score between −1.0 and −2.5 (osteopenia) and 12% had <−2.5 (osteoporosis). In group A, at baseline, a negative correlation was observed between calciuria and L1 through L4 BMD (r = − 0.68; P < 0.001) and total hip BMD (r = −0.54; P < 0.001); a positive correlation was shown between citraturia and total hip BMD (r = 0.56; P < 0.001) and L1 through L4 BMD (r = 0.42; P < 0.01). In group B, no statistically significant correlation was observed, probably because of the low number of patients.
Table 3.
BMD at baseline and after at least 12 mo follow-up
| Parameter | Group A (n = 49) |
Group B (n = 10) |
||
|---|---|---|---|---|
| Baseline | Follow-up Evaluation | Baseline | Follow-up Evaluation | |
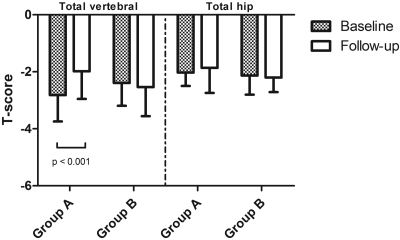
| Total vertebral (L1 through L4) T score | −2.82 ± 0.92 | −1.98 ± 0.97b | −2.39 ± 0.80 | −2.54 ± 1.02 |
| Total vertebral (L1 through L4) Z score | −1.60 ± 0.40 | −1.38 ± 0.54a | −1.45 ± 0.50 | −1.61 ± 0.60 |
| Total hip T score | −2.03 ± 0.47 | −1.86 ± 0.88 | −2.13 ± 0.67 | −2.20 ± 0.51 |
| Total hip Z score | −1.59 ± 0.54 | −1.21 ± 0.41b | −1.65 ± 0.74 | −1.71 ± 0.62 |
| No. (%) with osteoporosis | 6 (12) | 3 (6) | 1 (10) | 2 (20) |
| No. (%) with osteopenia | 29 (59) | 18 (37) | 6 (60) | 7 (70) |
Group A, patients who had MSK with PSRF and were on citrate; group B, patients who had MSK with PSRF and were not on citrate.
P < 0.05,
P < 0.001 versus baseline.
All of the DEXA parameters improved significantly in the group that was treated with oral potassium citrate (Table 3, Figure 1). On the contrary, a trend (not statistically significant) to further demineralization was observed in group B. In group A, variations (baseline to end of follow-up) in calciuria were correlated with variations in L1 through L4 BMD (r = −0.58; P < 0.001) and total hip BMD (r = −0.49; P < 0.01); changes in citraturia were correlated with changes in total hip BMD (r = 0.48; P < 0.001) and with changes in L1 through L4 BMD (r = 0.41; P < 0.01).
Figure 1.
T scores at baseline and at the end of follow-up in group A and B patients.
Discussion
The main outcome of this retrospective analysis is the unprecedented observation of frequent bone disease in renal stone formers with MSK and concomitant PSRF. The most frequent causes of osteopenia-osteoporosis were ruled out in this series of patients with MSK, and all women were menstruating regularly. Osteopenia and osteoporosis have been frequently reported in idiopathic and hypercalciuric renal stone patients. Although in idiopathic and hypercalciuric renal stone patients osteopenia and osteoporosis are generally thought to have a multifactorial and partly iatrogenic cause, because many of them probably adopted a low-calcium diet (2), a negative calcium balance as a result of a limited nutritional intake of calcium does not seem to have played a role in our patients with MSK, however, because they all reported adopting no nutritional restrictions and the dietary questionnaire did not disclose any reduced intake. In idiopathic renal calcium stone formers, a negative calcium balance as a result of renal hypercalciuria might also be involved in the pathogenesis of osteopenia-osteoporosis, although a high primary bone turnover has also been suggested, in which case an increased cytokine synthesis may play a part (3).
All of our patients with MSK had hypercalciuria, excreting a mean 333 to 359 mg of calcium in 24 h, and calciuria was particularly high (>500 mg/d) in some cases. If it is of renal origin, then hypercalciuria could certainly contribute to an abnormally low bone density in patients with MSK. We did not investigate the origin of hypercalciuria in our patients with MSK. The possibility exists that it was due to a renal calcium-handling defect, which might be one of the many renal electrolyte- and water-handling impairments described in MSK (1,4). Conversely, it has been claimed that calcium excretion in patients with MSK is unlikely to be increased directly by a derangement that mainly affects the collecting ducts (5). It has also been reported that some patients with MSK have absorptive hypercalciuria (6), but they are a minority (7).
Osther et al. (8) suggested that a defective urinary acidification might play an important role in the mechanism of hypercalciuria in patients with MSK, and Higashihara et al. (9) showed in a small study that correcting acidosis with bicarbonate reduced calciuria in their patients with MSK. Taking these concepts together, it may be that hypercalciuria in patients with MSK reflects an abnormally high bone turnover as a result of another tubular defect: the disrupted acidification seen in many patients with MSK.
Although it has never been suggested that idRTA causes bone demineralization in renal stone patients as a result of bone buffering of acids, we believe that such a mechanism should be considered in patients with MSK in light of the following findings. Weger et al. (10) found a 44% prevalence of idRTA in men and 20% prevalence in premenopausal women, who would otherwise have received a diagnosis of primary osteoporosis. One study showed that, in a group of children with posterior urethral valves with or without dRTA, those with idRTA were shorter in height than those without acidification defects but not as short as children with classic, complete dRTA (11). That overt dRTA causes growth retardation, rickets, hypercalciuria, and nephrocalcinosis as a result of chronic systemic metabolic acidosis is widely known, but the observation that children with idRTA are also shorter than control subjects (11) supports Weger's claim (10) that such an incomplete dRTA may affect bone metabolism. Case reports involving children have described an association among MSK, growth retardation, and overt or incomplete dRTA (12,13).
Incomplete dRTA typically causes no overt systemic acidosis but may cause recurrent positive acid loads in periods of increased protein intake or catabolic stress triggering alkali release from the bone and thus leading to a greater bone reabsorption (14); in fact, increased osteoblast and osteoclast activation has been described in idRTA (15), and the G protein–coupled proton sensor OGR1 was recently found to work as a H+-sensing receptor in osteoblasts (16). Conversely, even normal individuals with normal renal acidifying activity have shown a close stoichiometry between positive H+ balance and negative calcium balance during the ingestion of excessive acid loads (17), which has prompted the view that incoming H+ titrates bone carbonates, thereby protecting serum bicarbonate concentrations at the expense of a slow bone dissolution.
Incomplete and overt dRTA are reportedly very frequent in patients with MSK (33 to 40%) (8,18–20), although some have found only a 2.9% prevalence of the overt form (21). According to Daudon et al. (22), who analyzed a large series of stones from patients with MSK, their mainly carbapatite and brushite composition and morphology also support the idea that distal tubule acidification defects are very common in MSK.
None of our patients had overt dRTA, and we unfortunately have no direct proof that our patients with MSK had idRTA, because no acidification tests were performed. The association of hypocitraturia and a morning urine pH of >5.5 in >78% of these patients nonetheless points strongly to such a possibility. Hypocitraturia alone has also been suggested as a surrogate for ammonium chloride testing for the diagnosis of idRTA (23). The relationships between calciuria, citraturia, and BMD at baseline and the effect of potassium citrate treatment—reduction in calciuria, increase in citraturia, and improvement in BMD—support the conviction that hypercalciuria is at least partially associated with the acidification defect and is of bone origin in MSK. Thus, a subtle acidosis seems to be the causative factor behind osteopenia-osteoporosis in patients with MSK.
Potassium citrate treatment is tantamount to administering bicarbonate, because citrate is metabolized to become bicarbonate in the liver, thereby inducing a systemic alkalinization that has been shown to improve growth in children with dRTA (24). In postmenopausal women, despite no apparent acidification defect, the administration of potassium citrate at approximately the same dosage as we used induced a significant increase in urinary citrate with a parallel significant reduction in the urinary calcium and bone reabsorption markers, together with an improvement in bone mass (25). In patients with idiopathic calcium urolithiasis, treatment with potassium citrate can reverse bone loss (26,27).
As concerns the cause of the distal tubular acidification defect, it could be either primary or secondary to the renal stone disease in patients with MSK. Regardless of whether the subtle acidosis that is observed in MSK is primary, our data suggest that, when established, it has a strong and very prevalent impact on bone mineralization and on the urinary stone risk profile.
This study has a number of limitations, including the retrospective design and the use of a nonrandomly chosen control group, the incomplete data set (as concerns DEXA data for 25 to 30% of the patients investigated), and the lack of a precise diagnosis of idRTA and hypercalciuria classification. We followed the same diagnostic and therapeutic protocol for all patients with MSK, so we are confident that no bias was introduced. Furthermore, the two groups are very similar in reference to demographic and biochemical characteristics. We do not think that our data on the prevalence of reduced BMD in patients with MSK could have been biased by the fact that only 70 to 75% of them had a baseline densitometric assessment, because the only reason that the others were not assessed had to do with logistic issues. The high prevalence of women among our case groups, a finding not previously observed in other studies of patients with MSK, could suggest a selection bias. Although we have no explanation for such an observation, because none of them was in menopause and had followed low-calcium (including vegetarian) diets, we are confident that this skewed gender composition did not introduce any bias in the determination of bone disease prevalence in patients with MSK. Finally, the biologic congruence of the data supports our idea that they reflect a real pathophysiologic derangement in MSK disease.
The activity of the patients' stone disease clearly improved in parallel with the rising citraturia and urine pH and falling calciuria (A.F. and G.G. et al., manuscript in preparation). Although we might have expected potassium citrate treatment to be very effective in reducing the number of stone relapses among patients with MSK, the unprecedented good news is that it also has a very important impact on BMD in these patients, who—according to our data—are at increased risk for fractures.
Disclosures
None.
Acknowledgments
This study was partly supported by a donation to Prof. G. Gambaro from the Banca Popolare di Verona.
We thank Dr. L. Delle Carbonare, University of Verona, for critical reading of this article and helpful suggestions.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A: Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): A Padua Medical School discovery in the 1930s. Kidney Int 69: 663–670, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Zerwekh JE: Bone disease and idiopathic hypercalciuria. Semin Nephrol 28: 133–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacifici R: Idiopathic hypercalciuria and osteoporosis: Distinct clinical manifestations of increased cytokine-induced bone resorption? J Clin Endocrinol Metab 82: 29–31, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Gambaro G, Fabris A, Citron L, Tosetto E, Anglani F, Bellan F, Conte M, Bonfante L, Lupo A, D'Angelo A: An unusual association of contralateral congenital small kidney, reduced renal function and hyperparathyroidism in sponge kidney patients: On the track of the molecular basis. Nephrol Dial Transplant 20: 1042–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cameron S: Medullary sponge kidney. In: Oxford Textbook of Clinical Nephrology, 3rd Ed., edited by Davison AM, Cameron JS, Grunfeld J-P, Ponticelli C, Ritz E, Winearls CG, van Ypersele C.Oxford, Oxford University Press, 2004, pp 2495–2501 [Google Scholar]
- 6.O'Neill M, Breslau NA, Pak CY: Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 245: 1233–1236, 1981 [PubMed] [Google Scholar]
- 7.Maschio G, Tessitore N, D'Angelo A, Fabris A, Corgnati A, Oldrizzi L, Loschiavo C, Lupo A, Valvo E, Gammaro L, Rugiu C: Medullary sponge kidney and hyperparathyroidism: A puzzling association. Am J Nephrol 2: 77–84, 1982 [DOI] [PubMed] [Google Scholar]
- 8.Osther PJ, Mathiasen H, Hansen AB, Nissen HM: Urinary acidification and urinary excretion of calcium and citrate in women with bilateral medullary sponge kidney. Urol Int 52: 126–130, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Higashihara E, Nutahara K, Niijima T: Renal hypercalciuria and metabolic acidosis associated with medullary sponge kidney: Effect of alkali therapy. Urol Res 16: 95–100, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Weger M, Deutschmann H, Weger W, Kotanko P, Skrabal F: Incomplete renal tubular acidosis in ‘primary’ osteoporosis. Osteoporos Int 10: 325–329, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Sharma AP, Sharma RK, Kapoor R, Kornecki A, Sural S, Filler G: Incomplete distal renal tubular acidosis affects growth in children. Nephrol Dial Transplant 22: 2879–2885, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kasap B, Soylu A, Oren O, Türkmen M, Kavukçu S: Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 165: 648–651, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sluysmans T, Vanoverschelde JP, Malvaux P: Growth failure associated with medullary sponge kidney, due to incomplete renal tubular acidosis type 1. Eur J Pediatr 146: 78–80, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Barzel US, Jowsey J: The effects of chronic acid and alkali administration on bone turnover in adult rats. Clin Sci 36: 517–524, 1969 [PubMed] [Google Scholar]
- 15.Green J, Kleeman CR: Role of bone in regulation of systemic acid–base balance. Kidney Int 39: 9–26, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Frick KK, Krieger NS, Nehrke K, Bushinsky DA: Metabolic acidosis increases intracellular calcium in bone cells through activation of the proton receptor OGR1. J Bone Miner Res 24: 305–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litzow JR, Lemann J, Jr, Lennon EJ: The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest 46: 280–286, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granberg PO, Lagergren C, Theve NO: Renal function studies in medullary sponge kidney. Scand J Urol Nephrol 5: 177–180, 1971 [DOI] [PubMed] [Google Scholar]
- 19.Lahme S, Bichler K-H, Lang F, Feil G, Strohmaier WL, Radjiaipour M: Metabolic evaluation of patients suffering from medullary sponge kidney In: 8th European Symposium on Urolithiasis, Parma, Italy, edited by Borghi L.Cosenza, Italy, 1999, pp 599–601 [Google Scholar]
- 20.Higashihara E, Nutahara K, Tago K, Ueno A, Niijima T: Medullary sponge kidney and renal acidification defect. Kidney Int 25: 453–459, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Ginalski JM, Portmann L, Jaeger P: Does medullary sponge kidney cause nephrolithiasis? AJR Am J Roentgenol 155: 299–302, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Daudon M, Cohen-Solal F, Lacour B, Jungers P: Urinary stones and urinary tract abnormalities: Is the stone composition independent of the anatomical abnormality? Prog Urol 13: 1320–1329, 2003 [PubMed] [Google Scholar]
- 23.Lemann J, Jr, Worcester EM, Gray RW: Hypercalciuria and stones. Am J Kidney Dis 17: 386–391, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Caldas A, Broyer M, Dechaux M, Kleinknecht C: Primary distal tubular acidosis in childhood: Clinical study and long-term follow-up of 28 patients. J Pediatr 121: 233–241, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R: Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol 17: 3213–3222, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Pak CY, Peterson RD, Poindexter J: Prevention of spinal bone loss by potassium citrate in cases of calcium urolithiasis. J Urol 168: 31–34, 2002 [PubMed] [Google Scholar]
- 27.Vescini F, Buffa A, La Manna G, Ciavatti A, Rizzoli E, Bottura A, Stefoni S, Caudarella R: Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J Endocrinol Invest 28: 218–222, 2005 [DOI] [PubMed] [Google Scholar]